The Influence of Epitaxial Crystallization on the Mechanical Properties of Polyamide 66/Reduced Graphene Oxide Nanocomposite Injection Bar
Abstract
:1. Introduction
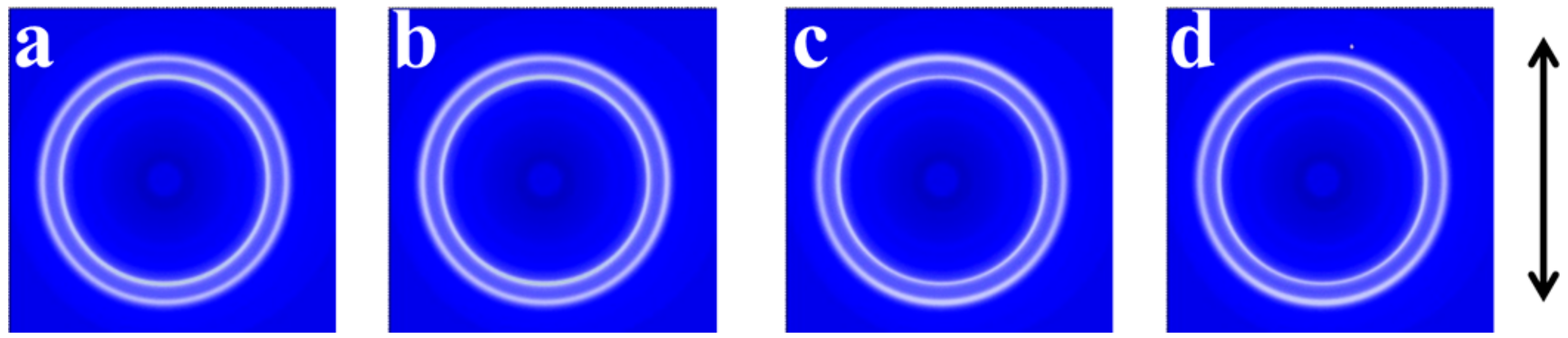
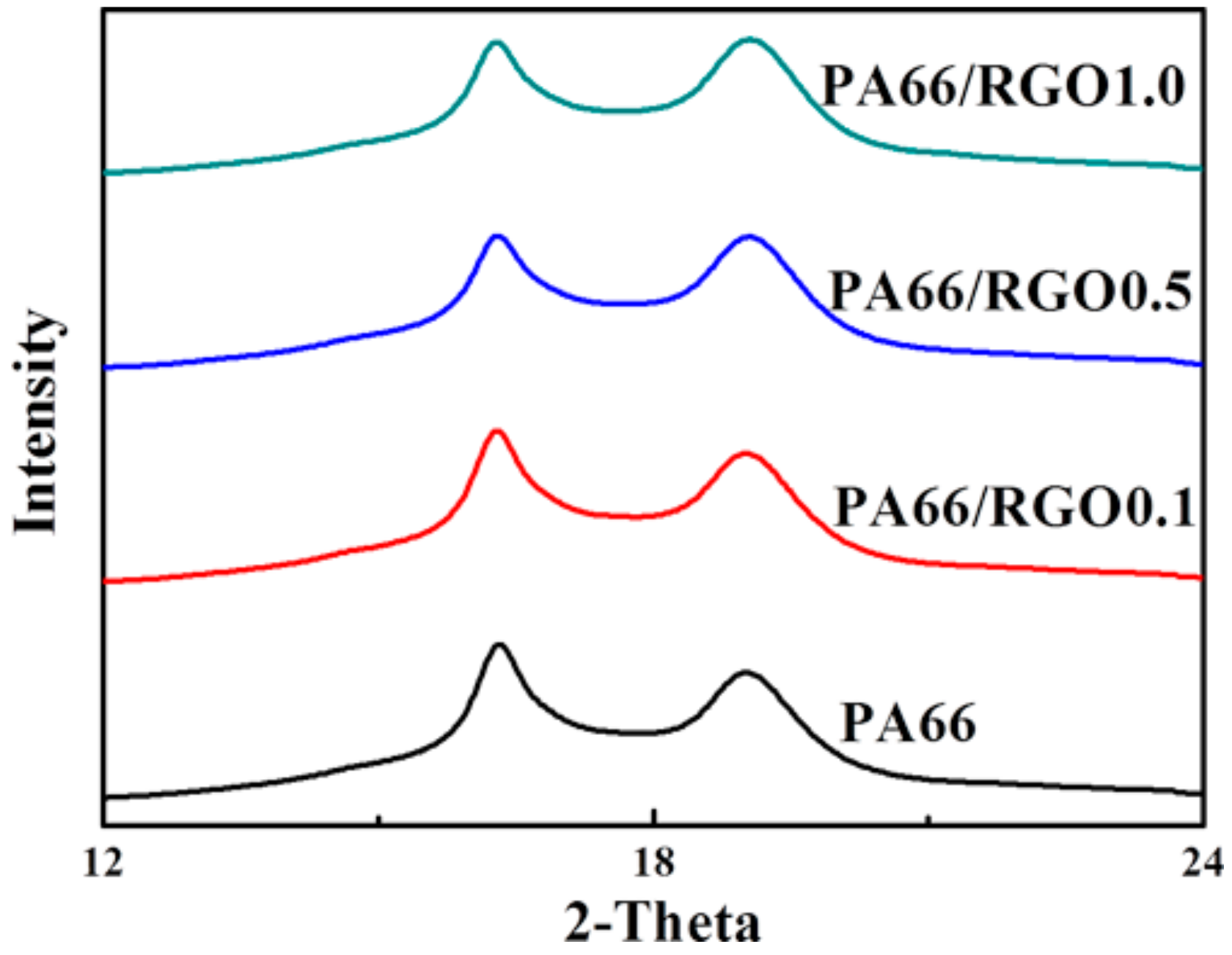
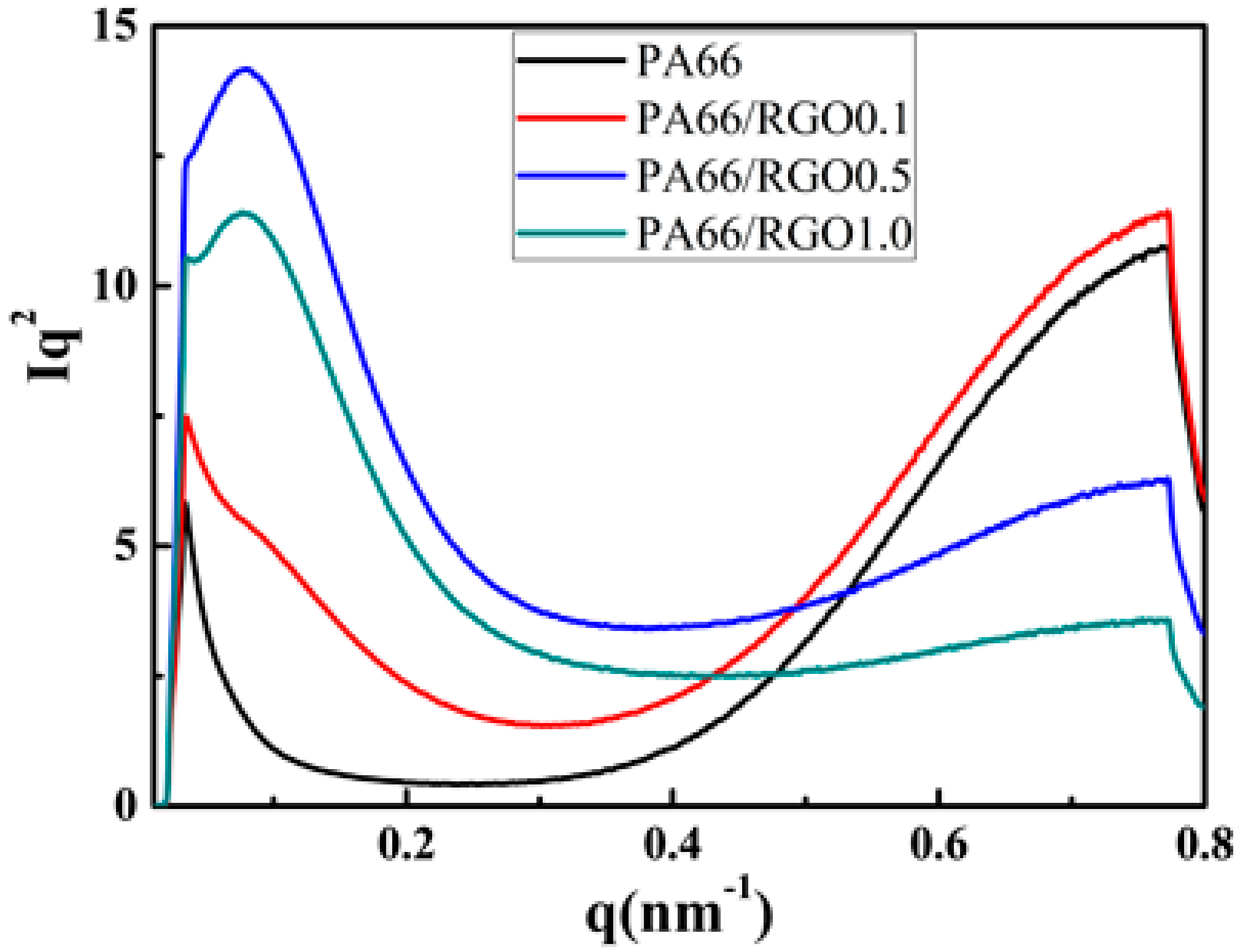
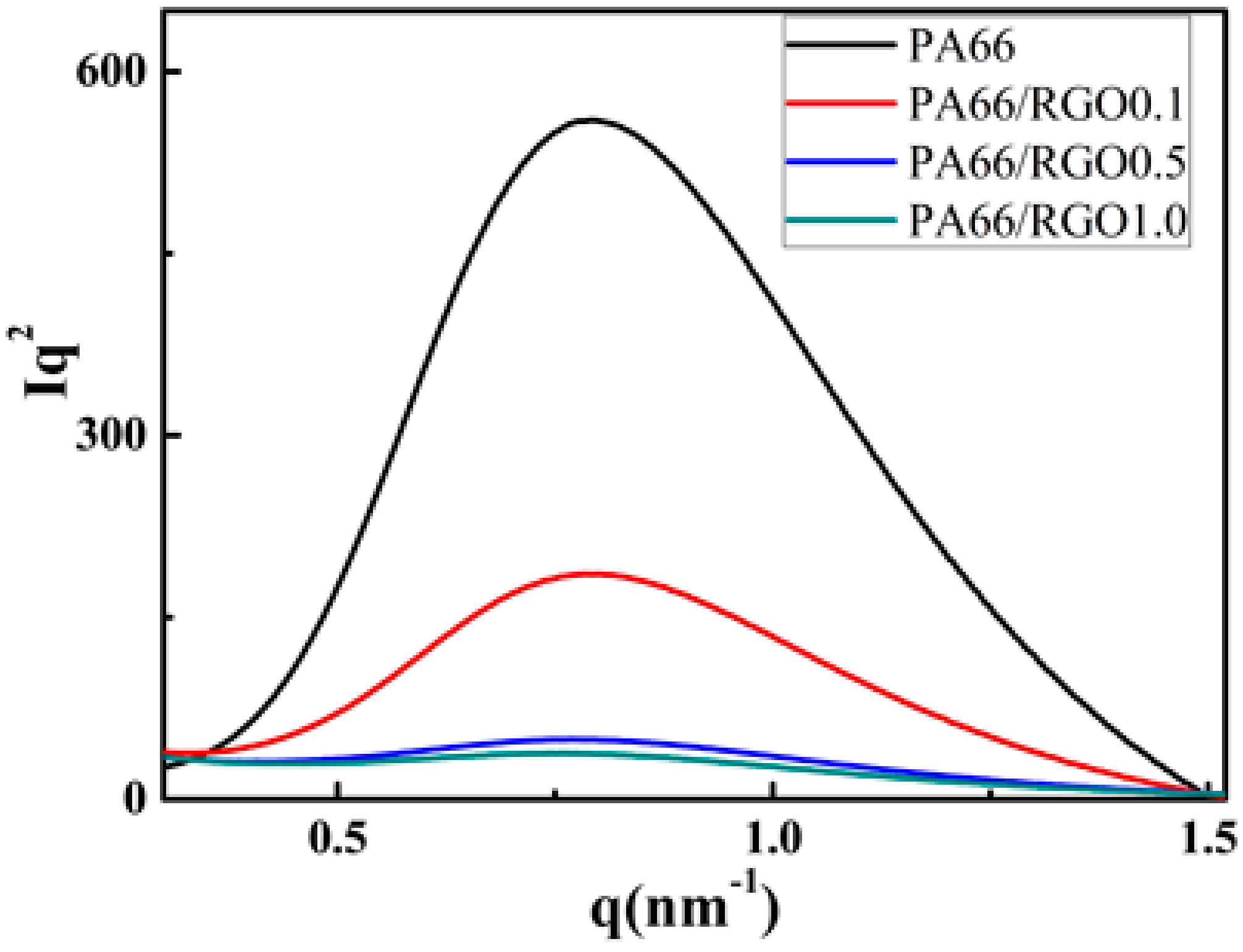
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Analytical Methods
4. Conclusions
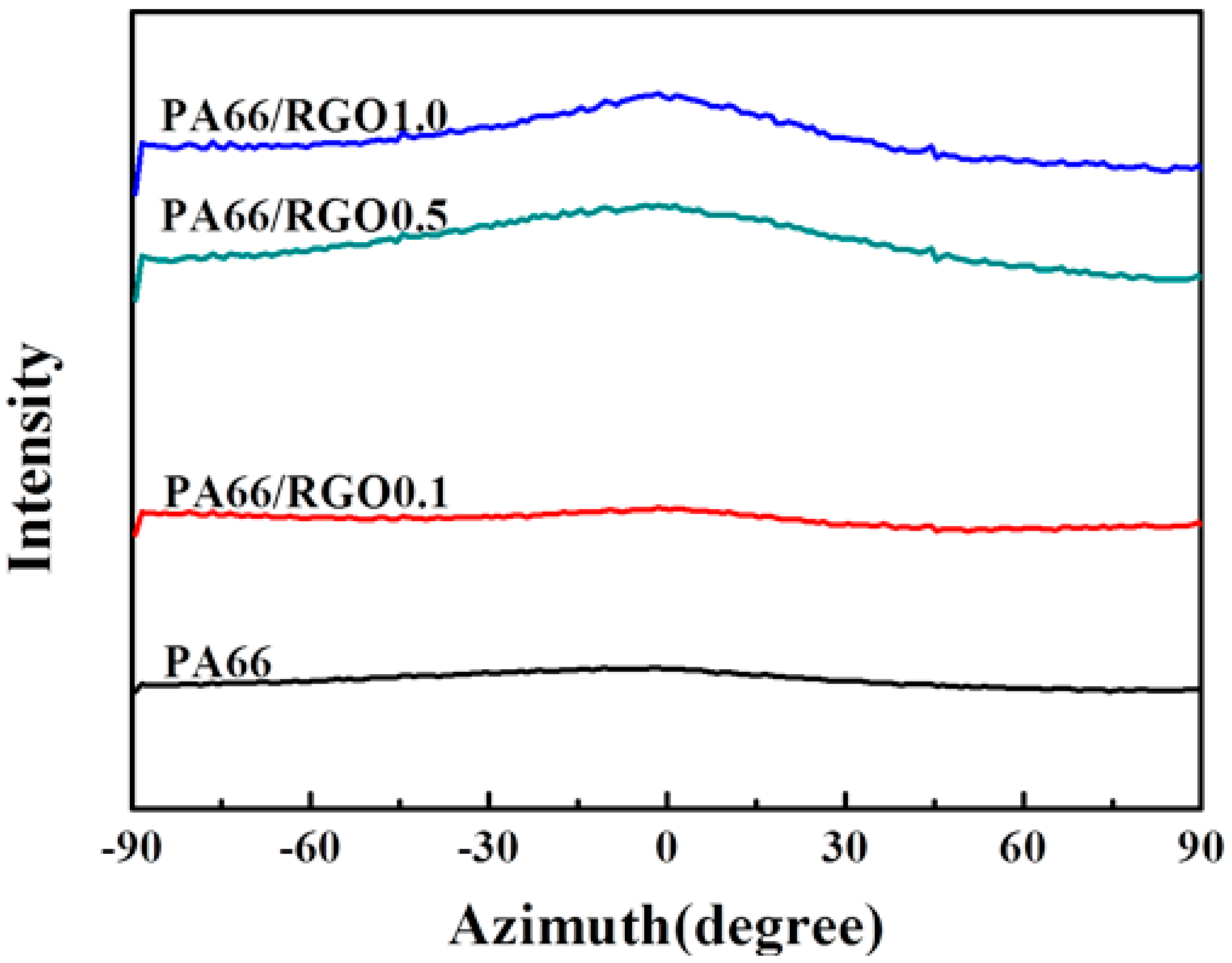
- Platy RGO is a nucleation agent to assist the crystallization of PA66 but does not influence the crystal structure, and can enhance the orientation degree of PA66 chains in the flow direction.
- The crystal of PA66/RGO nanocomposites includes the non-epitaxial crystal and the epitaxial crystal; the size of the epitaxial crystal is much greater than that of the non-epitaxial crystal.
- The orientation degree of PA66/RGO nanocomposites is determined by the proportion of epitaxial crystals present when platy RGO is added; the non-epitaxial crystal orientation of the PA66 matrix is unchanged.
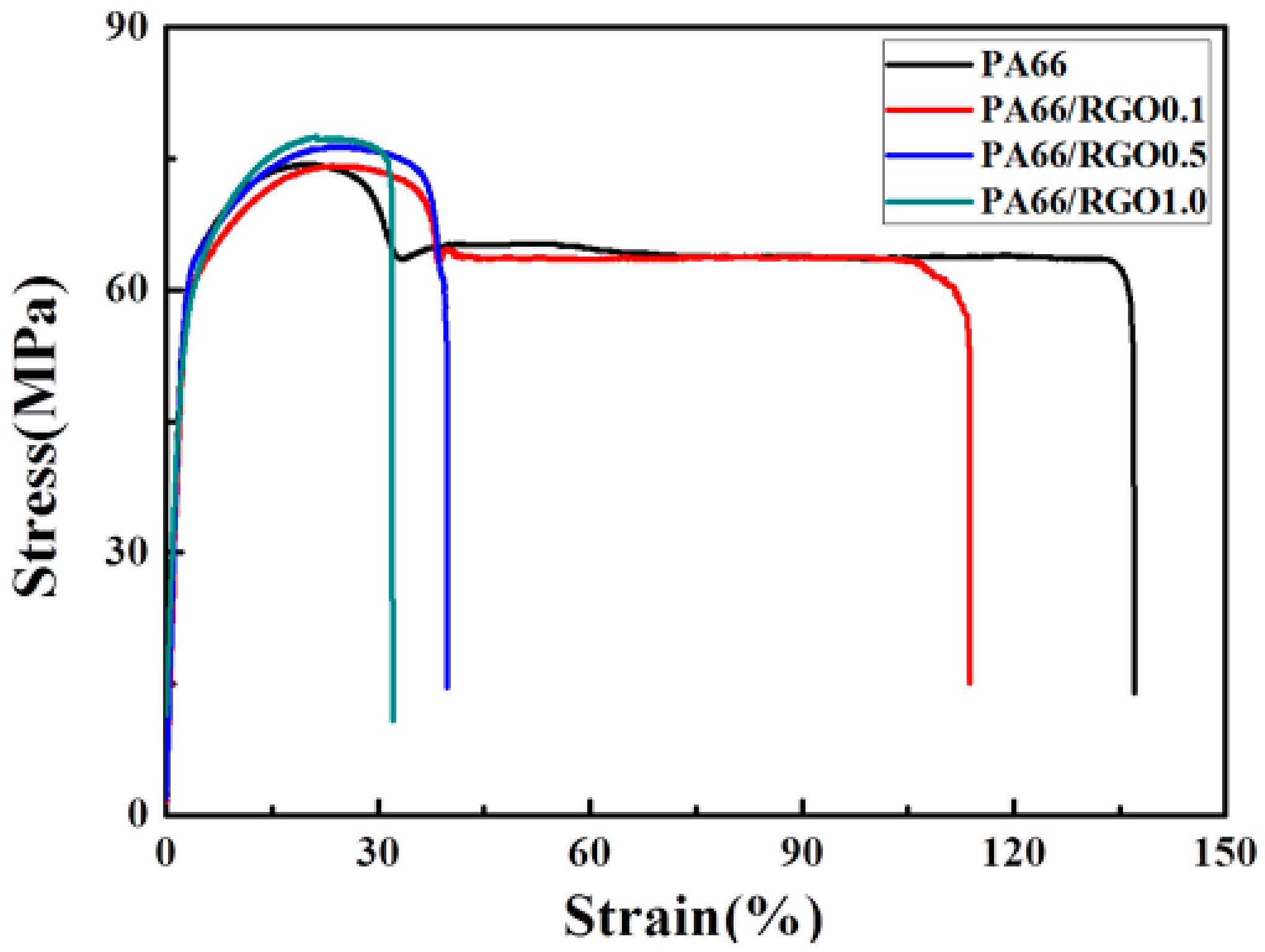
- The presence of fewer epitaxial crystals can improve the mechanical properties of a polymer. The tensile strength of hydrophilic PA66/RGO nanocomposites is increased by 4%.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gray, D.H.; Hu, S.L.; Juang, E.; Gin, D.L. Highly ordered polymer-inorganic nanocomposites via monomer self-assembly: In situ condensation approach. Adv. Mater. 1997, 9, 731–736. [Google Scholar] [CrossRef]
- Rahmat, M.; Hubert, P. Carbon nanotube-polymer interactions in nanocomposites: A review. Compos. Sci. Technol. 2011, 72, 72–84. [Google Scholar] [CrossRef]
- Takenaka, Y.; Miyaji, H.; Hoshino, A.; Tracz, A.; Jeszka, J.K.; Kucinska, I. Interface structure of epitaxial polyethylene crystal grown on HOPG and MoS2 substrates. Macromolecules 2004, 37, 9667–9669. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, L.; Cai, W.; Kodjie, S.L.; Tenneti, K.K. Nanohybrid shish-kebabs: Periodically functionalized carbon nanotubes. Adv. Mater. 2005, 17, 1198–1202. [Google Scholar] [CrossRef]
- Petermann, J.; Broza, G.; Rieck, U.; Kawaguchi, A. Epitaxial interfaces in semi-crystalline polymers and their applications. J. Mater. Sci. 1987, 22, 1477–1481. [Google Scholar] [CrossRef]
- Petermann, J.; Xu, Y. Mechanical properties of epitaxially crystallized 1,4-polybutadiene on uniaxially oriented isotactic polypropylene. Colloid Polym. Sci. 1991, 269, 455–459. [Google Scholar] [CrossRef]
- Gross, B.; Peterman, J. Synergisms of mechanical properties in blends of semi-crystalline polymers. J. Mater. Sci. 1984, 19, 105–112. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Y.J.; Dai, J.; Lang, M.D.; Huang, X.Y. An efficient way to functionalize graphene sheets with presynthesized polymer via ATNRC chemistry. J. Polym. Sci. Pol. Chem. 2011, 49, 1582–1590. [Google Scholar] [CrossRef]
- Yang, B.-X.; Pramoda, K.P.; Xu, G.Q.; Goh, S.H. Mechanical reinforcement of polyethylene using polyethylene-grafted multiwalled carbon nanotubes. Adv. Funct. Mater. 2007, 17, 2062–2069. [Google Scholar] [CrossRef]
- Cao, Y.W.; Lai, Z.L.; Feng, J.C.; Wu, P.Y. Graphene oxide sheets covalently functionalized with block copolymers via click chemistry as reinforcing fillers. J. Mater. Chem. 2011, 21, 9271–9278. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martinez, G. Importance of covalent linkages in the preparation of effective reduced graphene oxide-poly(vinyl chloride) nanocomposites. Macromolecules 2011, 44, 2685–2692. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, Z.; Dufresne, A.; Huang, J.; Wei, M.; An, L. Structure and properties of new thermoforming bionanocomposites based on chitin whisker-graft-polycaprolactone. J. Appl. Polym. Sci. 2009, 112, 2830–2837. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Wang, J.; Wan, L.; Liu, F.; Zheng, H.; Chen, R.; Xu, C. Preparation and properties of graphene nanosheets-polystyrene nanocomposites via in situ emulsion polymerization. Chem. Phys. Lett. 2010, 484, 247–253. [Google Scholar] [CrossRef]
- Sun, T.; Garces, J.M. High-performance polypropylene-clay nanocomposites by in-situ polymerization with metallocene/clay catalysts. Adv. Mater. 2002, 14, 128. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, B.-H.; Choi, S.-W.; Kim, S.; Kim, H.-J. The effect of types of maleic anhydride-grafted polypropylene (MAPP) on the interfacial adhesion properties of bio-flour-filled polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1473–1482. [Google Scholar] [CrossRef]
- Imase, T.; Ohira, A.; Okoshi, K.; Sano, N.; Kawauchi, S.; Watanabe, J.; Kunitake, M. AFM study of two-dimensional epitaxial arrays of poly(γ-l-glutamates) with long n-alkyl side chains on graphite. Macromolecules 2003, 36, 1865–1869. [Google Scholar] [CrossRef]
- Ning, N.; Fu, S.; Zhang, W.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Realizing the enhancement of interfacial interaction in semicrystalline polymer/filler composites via interfacial crystallization. Prog. Polym. Sci. 2012, 37, 1425–1455. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Ikada, Y.; Tsuji, H.; Puiggali, J.; Lotz, B. Epitaxial crystallization and crystalline polymorphism of polylactides. Polymer 2000, 41, 8909–8919. [Google Scholar] [CrossRef]
- Pashley, D.W. The nucleation, growth, structure and epitaxy of thin surface films. Adv. Phys. 1965, 14, 327–416. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Navarro, C.; Meyer, J.C.; Sundaram, R.S.; Chuvilin, A.; Kurasch, S.; Burghard, M.; Kern, K.; Kaiser, U. Atomic structure of reduced graphene oxide. Nano Lett. 2010, 10, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Quaresimin, M.; Schulte, K.; Zappalorto, M.; Chandrasekaran, S. Toughening mechanisms in polymer nanocomposites: From experiments to modelling. Compos. Sci. Technol. 2016, 123, 187–204. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Verdejo, R.; Bernal, M.M.; Romasanta, L.J.; Lopezmanchado, M.A. Graphene filled polymer nanocomposites. J. Mater. Chem. 2011, 21, 3301–3310. [Google Scholar] [CrossRef]
- Glover, A.J.; Cai, M.; Overdeep, K.R.; Kranbuehl, D.E.; Schniepp, H.C. In situ reduction of graphene oxide in polymers. Macromolecules 2011, 44, 9821–9829. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Wang, B.J.; Li, Y.G.; Weng, G.S.; Jiang, Z.Q.; Chen, P.; Wang, Z.B.; Gu, Q. Reduced graphene oxide enhances the crystallization and orientation of poly(epsilon-caprolactone). Compos. Sci. Technol. 2014, 96, 63–70. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Zhang, J.; Li, H.; Chen, P.; Wang, Z.; Gu, Q. Noncovalent method for improving the interaction between reduced graphene oxide and poly(ε-caprolactone). Ind. Eng. Chem. Res. 2013, 52, 15824–15828. [Google Scholar] [CrossRef]
- Sano, M.; Sasaki, D.Y.; Kunitake, T. Polymerization-induced epitaxy: Scanning tunneling microscopy of a hydrogen-bonded sheet of polyamide on graphite. Science 1992, 258, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Chen, X.; Hsuan, Y.G.; Li, C.Y. Reduced graphene oxide-induced polyethylene crystallization in solution and nanocomposites. Macromolecules 2011, 45, 993–1000. [Google Scholar] [CrossRef]
- Cao, Z.; Song, P.; Fang, Z.; Xu, Y.; Zhang, Y.; Guo, Z. Physical wrapping of reduced graphene oxide sheets by polyethylene wax and its modification on the mechanical properties of polyethylene. J. Appl. Polym. Sci. 2012, 126, 1546–1555. [Google Scholar] [CrossRef]
- Araujo, E.M.; Araujo, K.D.; Paz, R.A.; Gouveia, T.R.; Barbosa, R.; Ito, E.N. Polyamide 66/brazilian clay nanocomposites. J. Nanomater. 2009, 2009, 136856. [Google Scholar] [CrossRef]
- Song, L.; Hu, Y.; He, Q.; You, F. Study on crystallization, thermal and flame retardant properties of nylon 66/organoclay nanocomposites by in situ polymerization. J. Fire Sci. 2008, 26, 475–492. [Google Scholar] [CrossRef]
- Qin, H.L.; Su, Q.S.; Zhang, S.M.; Zhao, B.; Yang, M.S. Thermal stability and flammability of polyamide 66/montmorillonite nanocomposltes. Polymer 2003, 44, 7533–7538. [Google Scholar] [CrossRef]
- Bunn, C.W.; Garner, E.V. The crystal structures of two polyamides (‘Nylons’). Proc. R. Soc. A Math. Phys. 1947, 189, 39–68. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S.T. The structure of suspended grapheme sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, D.; Starkweather Jr, H.W. Hydrogen bonding in nylon 66 and model compounds. J. Polym. Sci. Pol. Phys. 1985, 23, 537–555. [Google Scholar] [CrossRef]
- Lim, L.T.; Britt, I.J.; Tung, M.A. Sorption and transport of water vapor in nylon 6,6 film. J. Appl. Polym. Sci. 1999, 71, 197–206. [Google Scholar] [CrossRef]
- Yao, G.; Duan, T.; An, M.; Xu, H.; Tian, F.; Wang, Z. The influence of epitaxial crystallization on the mechanical properties of a high density polyethylene/reduced graphene oxide nanocomposite injection bar. RSC Adv. 2017, 7, 21918–21925. [Google Scholar] [CrossRef]
- Xu, J.Z.; Chen, C.; Wang, Y.; Tang, H.; Li, Z.M.; Hsiao, B.S. Graphene nanosheets and shear flow induced crystallization in isotactic polypropylene nanocomposites. Macromolecules 2011, 21, 53–63. [Google Scholar] [CrossRef]
- Fukumaru, T.; Fujigaya, T.; Nakashima, N. Mechanical reinforcement of polybenzoxazole by carbon nanotubes through noncovalent functionalization. Macromolecules 2013, 46, 4034–4040. [Google Scholar] [CrossRef]
- Yuan, M.; Turng, L.S. Microstructure and mechanical properties of microcellular injection molded polyamide-6 nanocomposites. Polymer 2005, 46, 7273–7292. [Google Scholar] [CrossRef]
- Trifan, D.S.; Terenzi, J.F. Extents of hydrogen bonding in polyamides and polyurethanes. J. Polym. Sci. 1958, 28, 443–445. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, D.; Lin, C.T.; Li, J.; Lin, Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 2010, 132, 9274. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, E.; An, M.; Yao, G.; Tian, F.; Wang, Z. The Influence of Epitaxial Crystallization on the Mechanical Properties of Polyamide 66/Reduced Graphene Oxide Nanocomposite Injection Bar. Crystals 2017, 7, 384. https://doi.org/10.3390/cryst7120384
Chi E, An M, Yao G, Tian F, Wang Z. The Influence of Epitaxial Crystallization on the Mechanical Properties of Polyamide 66/Reduced Graphene Oxide Nanocomposite Injection Bar. Crystals. 2017; 7(12):384. https://doi.org/10.3390/cryst7120384
Chicago/Turabian StyleChi, Enyi, Minfang An, Guibin Yao, Feng Tian, and Zongbao Wang. 2017. "The Influence of Epitaxial Crystallization on the Mechanical Properties of Polyamide 66/Reduced Graphene Oxide Nanocomposite Injection Bar" Crystals 7, no. 12: 384. https://doi.org/10.3390/cryst7120384





