Unexpected Au Alloying in Tailoring In-Doped SnTe Nanostructures with Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussions
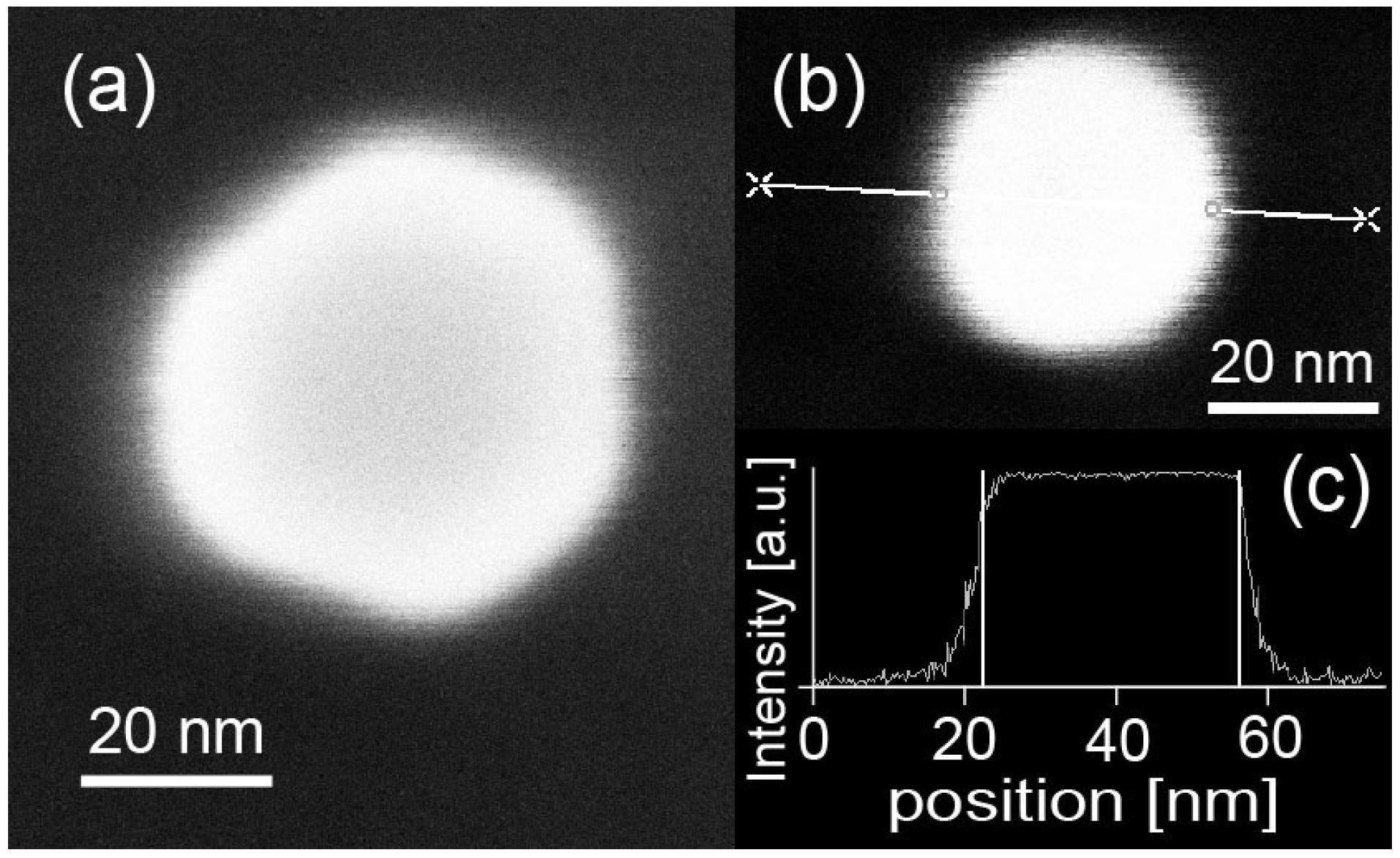
2.1. Preparation of Dispersedly-Deposited Gold Nanoparticles on Substrates
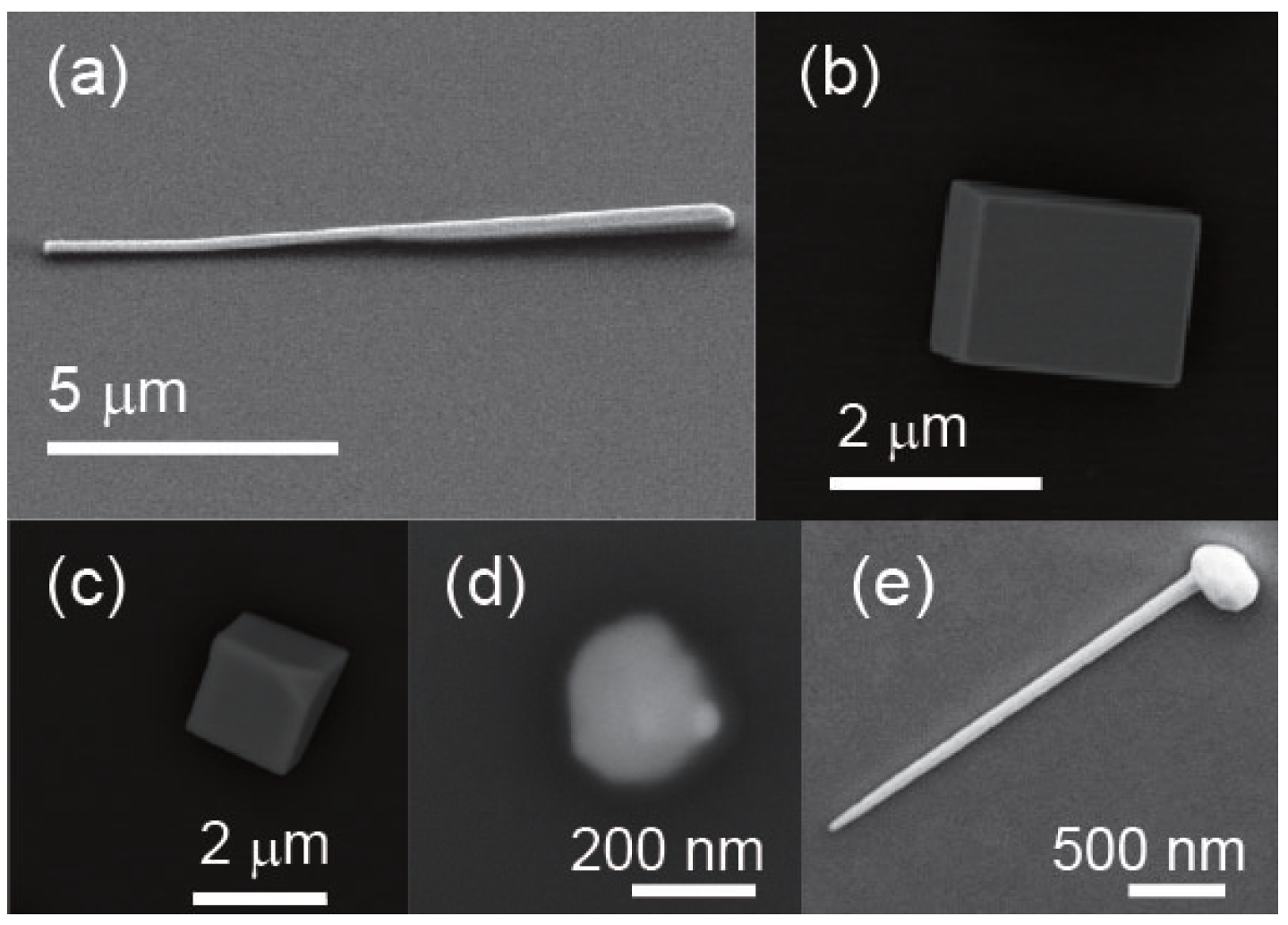
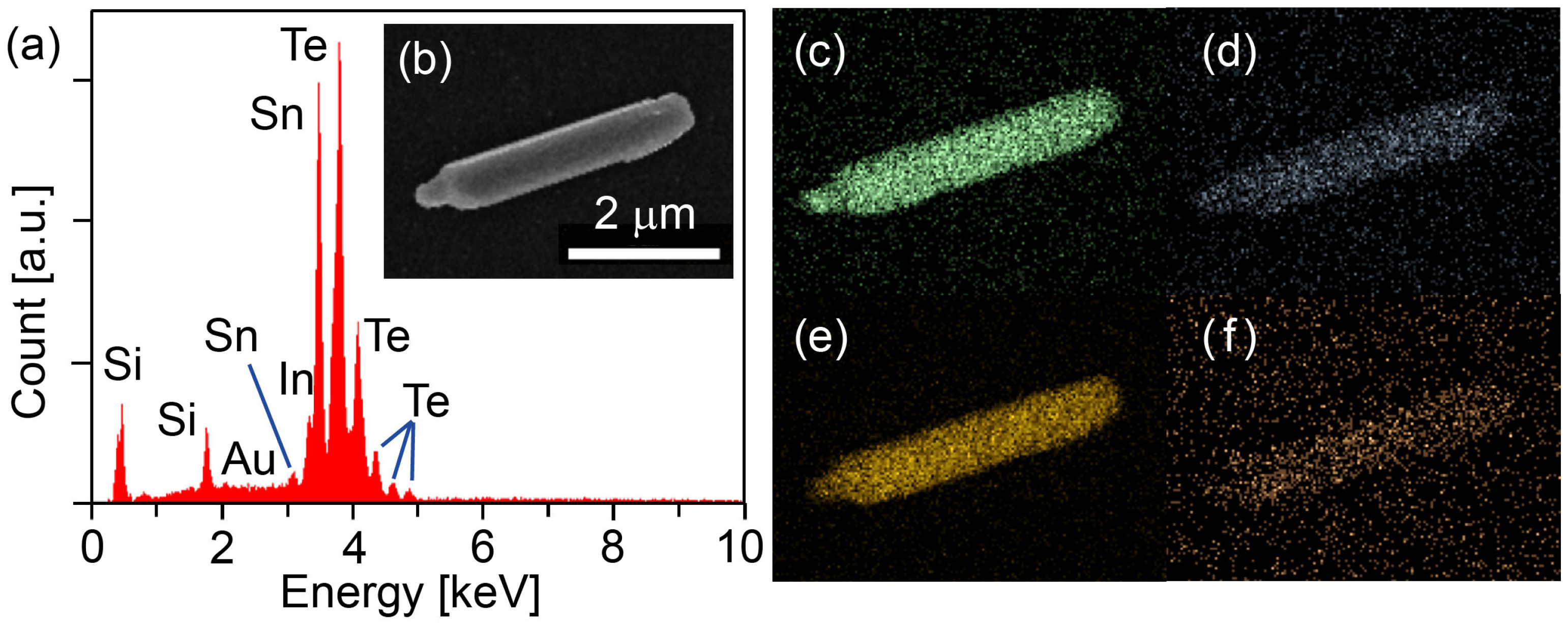
2.2. In-SnTe Nanostructures Grown under the V–L–S Mechanism with the Use of Gold Nanoparticles
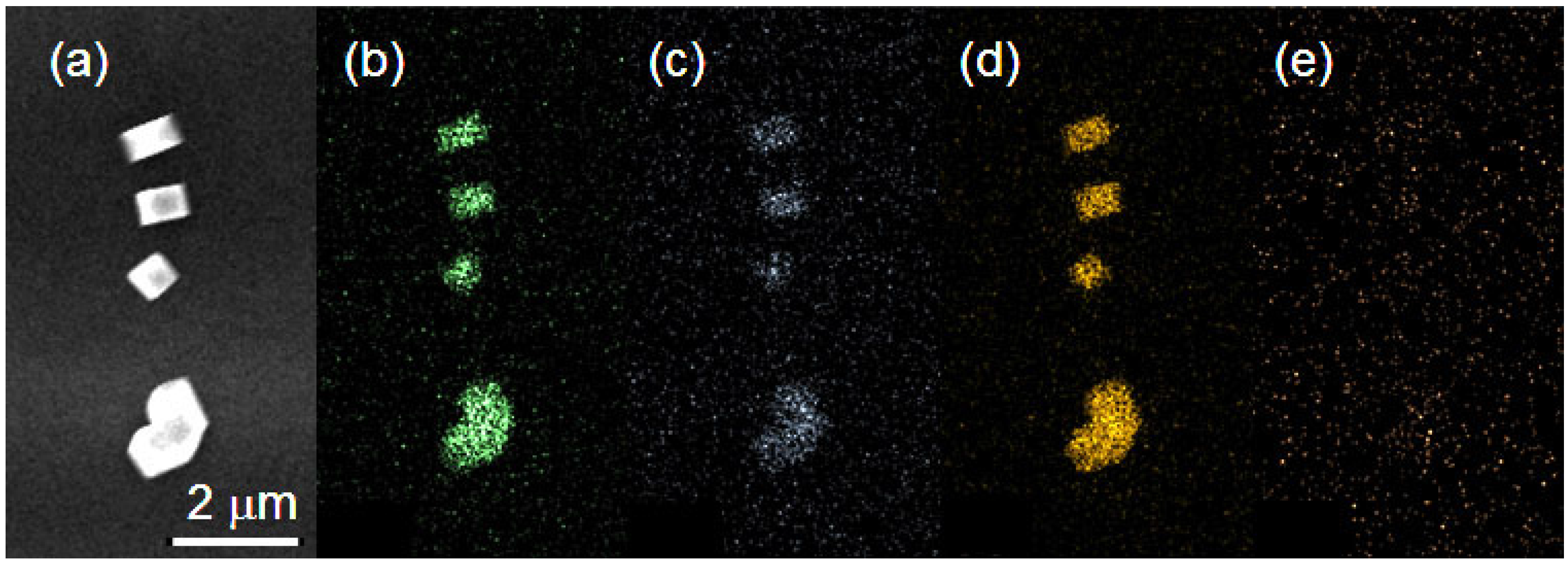
2.3. In-SnTe Nanostructures Grown under the V–S Mechanism without Gold Nanoparticles
3. Materials and Methods
3.1. Bulk Polycrystalline In-SnTe Growth
3.2. Growth of In-SnTe Nanostructures by Vapor Transport
3.3. SEM Topography and EDX Mapping Investigation of In-SnTe Nanostructures
4. Conclusions and Outlook
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TI | topological insulator |
| TCI | topological crystalline insulator |
| TSC | topological superconductor |
| MF | Majorana fermion |
| SOI | spin-orbit interaction |
| In-SnTe | indium-doped tin telluride |
| VTG | vapor transport growth |
| V–L–S | vapor–liquid–solid |
| V–S | vapor–solid |
| GNP | gold nanoparticle |
| SEM | scanning electron microscope |
| FEG-SEM | field-emission-gun scanning electron microscope |
| EDX | energy dispersive X-ray |
| IPA | isopropyl alcohol |
| PMMA | polymethyl methacrylate |
References
- Hasan, M.Z.; Kane, C.L. Colloquium: Topological insulators. Rev. Mod. Phys. 2010, 82, 3045–3067. [Google Scholar] [CrossRef]
- Qi, X.-L.; Zhang, S.-C. Topological insulators and Superconductors. Rev. Mod. Phys. 2011, 83, 1057–1110. [Google Scholar] [CrossRef]
- Ando, Y. Topological insulator materials. J. Phys. Soc. Jpn. 2013, 82, 102001. [Google Scholar] [CrossRef]
- Fu, L. Topological crystalline insulators. Phys. Rev. Lett. 2011, 106, 106802. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Lin, H.; Liu, U.; Duan, W.; Bansil, A.; Fu, L. Topological crystalline insulators in SnTe material class. Nat. Commun. 2012, 3, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Ren, Z.; Sato, T.; Nakayama, K.; Souma, S.; Takahashi, T.; Segawa, K.; Ando, Y. Experimental realization of a topological crystalline insulator in SnTe. Nat. Phys. 2012, 8, 800–803. [Google Scholar] [CrossRef]
- Hulm, J.K.; Jones, C.K.; Deis, D.W.; Fairbank, H.A.; Lawless, P.A. Superconducting interactions in tin telluride. Phys. Rev. 1968, 169, 388–394. [Google Scholar] [CrossRef]
- Mathur, M.P.; Deis, D.W.; Jones, C.K.; Carr, W.J., Jr. Superconductivity as a function of carrier density and magnetic spin concentration in the SnTe-MnTe system. J. Phys. Chem. Solids 1973, 34, 183–188. [Google Scholar] [CrossRef]
- Erickson, A.S.; Chu, J.-H.; Toney, M.F.; Geballe, T.H.; Fisher, I.R. Enhanced superconducting pairing interaction in indium-doped tin telluride. Phys. Rev. B 2009, 79, 024520. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Miura, O. High-pressure synthesis and Superconductivity of Ag-doped topological crystalline insulator SnTe (Sn1−xAgxTe with x = 0 − 0.5). J. Phys. Soc. Jpn. 2016, 85, 053702. [Google Scholar] [CrossRef]
- Haldolaarachchige, N.; Gibson, Q.; Xie, W.; Nielsen, M.B.; Kushwaha, S.; Cava, R.J. Anomalous composition dependence of the superconductivity in In-doped SnTe. Phys. Rev. B 2016, 93, 024520. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Bawden, L.; Cavendish, S.; Lees, M.R. Superconducting properties of the In-substituted topological crystalline insulator SnTe. Phys. Rev. B 2013, 87, 140507. [Google Scholar] [CrossRef]
- Zhong, R.D.; Schneeloch, J.A.; Shi, X.Y.; Xu, Z.J.; Zhang, C.; Tranquada, J.M.; Li, Q.; Gu, G.D. Optimizing the superconducting transition temperature and upper critical field of Sn1−xInxTe. Phys. Rev. B 2013, 88, 020505. [Google Scholar] [CrossRef]
- Novak, M.; Sasaki, S.; Kriener, M.; Segqawa, K.; Ando, Y. Unusual nature of fully gapped superconductivity in In-doped SnTe. Phys. Rev. B 2013, 88, 140502. [Google Scholar] [CrossRef]
- Sasaki, S.; Ren, Z.; Taskin, A.A.; Segawa, K.; Fu, L.; Ando, Y. Odd-parity pairing and topological superconductivity in a strongly spin-orbit coupled semiconductor. Phys. Rev. Lett. 2012, 109, 217004. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Kane, C.L. Superconducting proximity effect and Majorana fermions at the surface of a topological insulator. Phys. Rev. Lett. 2008, 100, 096407. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Takahashi, Y.; Fujimoto, S. Non-abelian topological order in s-wave superfluids of ultracold fermionic atoms. Phys. Rev. Lett. 2009, 103, 020401. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Berg, E. Odd-parity topological superconductors: Theory and application to CuxBi2Se3. Phys. Rev. Lett. 2010, 105, 097001. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, A.P.; Ryu, S.; Furusaki, A.; Ludwig, A.W.W. Classification of topological insulators and superconductors in three spatial dimensions. Phys. Rev. B 2008, 78, 195125. [Google Scholar] [CrossRef]
- Ando, Y.; Fu, L. Topological crystalline insulators and topological superconductors: From concepts to materials. Annu. Rev. Condens. Matter Phys. 2015, 6, 361–381. [Google Scholar] [CrossRef]
- Beenakker, C.; Kouwenhoven, L. A road to reality with topological superconductors. Nat. Phys. 2016, 12, 618–621. [Google Scholar] [CrossRef]
- Sasaki, S.; Mizushima, T. Superconducting doped topological materials. Physica C 2015, 514, 206–217. [Google Scholar] [CrossRef]
- Sato, M.; Ando, Y. Topological superconductors. arXiv 2016. [Google Scholar]
- Wilczek, F. Majorana returns. Nat. Phys. 2009, 5, 614–618. [Google Scholar] [CrossRef]
- Alicea, J. New directions in the pursuit of Majorana fermions in solid state systems. Rep. Prog. Phys. 2012, 75, 076501. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Ando, Y. Superconducting Sn1−xInxTe Nanoplates. Cryst. Growth Des. 2015, 15, 2748–2752. [Google Scholar] [CrossRef]
- Hor, Y.S.; Williams, A.J.; Checkelsky, J.G.; Roushan, P.; Seo, J.; Xu, Q.; Zandbergen, H.W.; Yazdani, Q.; Ong, N.P.; Cava, R.J. Superconductivity in CuxBi2Se3 and its implications for pairing in the undoped topological insulator. Phys. Rev. Lett. 2010, 104, 057001. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Kriener, M.; Segawa, K.; Yada, K.; Tanaka, Y.; Sato, M.; Ando, Y. Topological superconductivity in CuxBi2Se3. Phys. Rev. Lett. 2011, 107, 217001. [Google Scholar] [CrossRef] [PubMed]
- Kriener, M.; Segawa, K.; Sasaki, S.; Ando, Y. Anomalous suppression of the superfluid density in the CuxBi2Se3 superconductor upon progressive Cu intercalation. Phys. Rev. B 2012, 86, 180505. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Shao, J.; Zuo, M.; Pi, L.; Tan, S.; Zhang, C.; Zhang, Y. Superconductivity with Topological Surface State in SrxBi2Se3. J. Am. Chem. Soc. 2015, 137, 10512–10515. [Google Scholar] [CrossRef] [PubMed]
- Saghir, M.; Lees, M.R.; York, S.J.; Balakrishnan, G. Synthesis and characterization of nanomaterials of the topological crystalline insulator SnTe. Cryst. Growth Des. 2014, 14, 2009–2013. [Google Scholar] [CrossRef]
- Safdar, M.; Wang, Q.; Mirza, M.; Wang, Z.; He, J. Crystal shape engineering of topological crystalline insulator SnTe microcrystals and nanowires with huge thermal activation energy Gap. Cryst. Growth Des. 2014, 14, 2502–2509. [Google Scholar] [CrossRef]
- Li, Z.; Shao, S.; Li, N.; McCall, K.; Wang, J.; Zhang, S.X. Single crystalline nanostructures of topological crystalline insulator SnTe with distinct facets and morphologies. Nano Lett. 2013, 13, 5443–5448. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Jung, Y.; Disa, A.S.; Walker, F.J.; Ahn, C.H.; Cha, J.J. Synthesis of SnTe nanoplates with {100} and {111} surfaces. Nano Lett. 2014, 14, 4183–4188. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.; Wang, Q.; Mirza, M.; Wang, Z.; Xu, K.; He, J. Topological Surface Transport Properties of Single-Crystalline SnTe Nanowire. Nano Lett. 2013, 13, 5344–5349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, F.; Li, J.; Wang, Z.; Zhan, X.; He, J. Low-dimensional topological crystalline insulators. Small 2015, 11, 4613–4624. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xie, Y.; Cha, J.J. Revealing surface states in In-doped SnTe Nanoplates with low bulk mobility. Nano Lett. 2015, 15, 3827–3832. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Fidler, A.F.; He, K.; Su, D.; Chen, G.; Lin, Q.; Pietryga, J.M.; Klimov, V.I. Shape-controlled narrow-gap SnTe nanostructures: From nanocubes to nanorods and nanowires. J. Am. Chem. Soc. 2015, 137, 15074–15077. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, H.J.; Ratchford, J.B.; Goldthorpe, I.A.; McIntyre, P.C.; Chidsey, C.E.D. Vertically oriented germanium nanowires grown from gold Colloids on silicon substrates and subsequent gold removal. Nano Lett. 2007, 7, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.H.; Gao, Q.; Tan, H.H.; Jagadish, C.; Kim, Y.; Zou, J.; Smith, L.M.; Jackson, H.E.; Yarrison-Rice, J.M.; Parkinson, P.; et al. III–V semiconductor nanowires for optoelectronic device applications. Prog. Quantum Electron. 2011, 35, 23–75. [Google Scholar] [CrossRef]
- Prince, A.; Raynor, G.V.; Evans, D.S. Ternary systems Au-Sn-Te. In Phase Diagram of Ternary Gold Alloys; The Institute of Metals: London, UK, 1990; pp. 456–466. [Google Scholar]
- Okamoto, H.; Massalski, T.B. (Eds.) Phase diagram of binary gold alloys. In Binary Alloy Phase Diagrams; ASM International: Metals Park, OH, USA, 1987; p. 343.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atherton, S.; Steele, B.; Sasaki, S. Unexpected Au Alloying in Tailoring In-Doped SnTe Nanostructures with Gold Nanoparticles. Crystals 2017, 7, 78. https://doi.org/10.3390/cryst7030078
Atherton S, Steele B, Sasaki S. Unexpected Au Alloying in Tailoring In-Doped SnTe Nanostructures with Gold Nanoparticles. Crystals. 2017; 7(3):78. https://doi.org/10.3390/cryst7030078
Chicago/Turabian StyleAtherton, Samuel, Benjamin Steele, and Satoshi Sasaki. 2017. "Unexpected Au Alloying in Tailoring In-Doped SnTe Nanostructures with Gold Nanoparticles" Crystals 7, no. 3: 78. https://doi.org/10.3390/cryst7030078




