Synthesis and Characterization of New Sr3(BO3)2 Crystal for Stimulated Raman Scattering Applications
Abstract
:1. Introduction
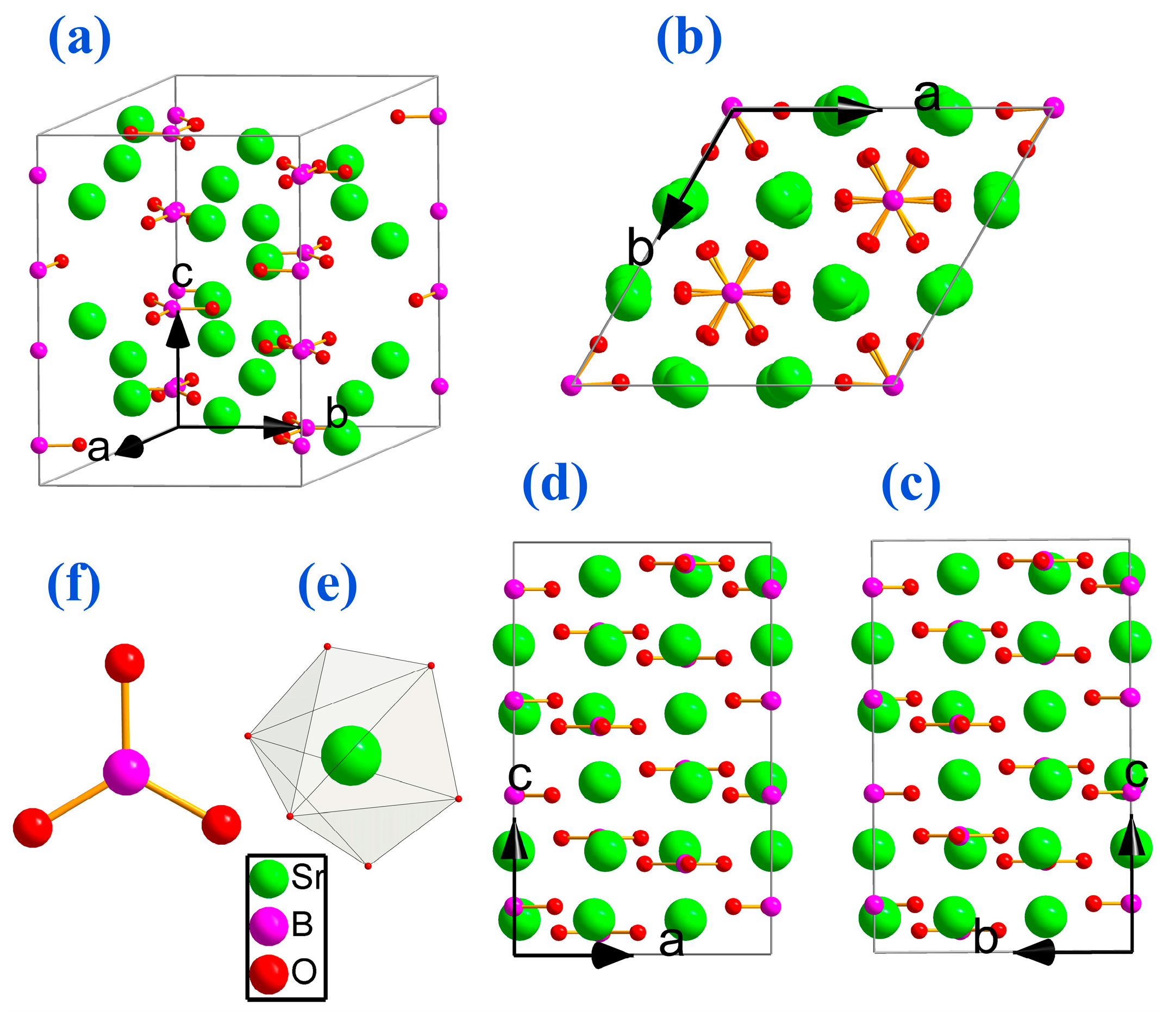
2. Structure Analysis and New Crystal Design
3. Experimental
4. Results and Discussion
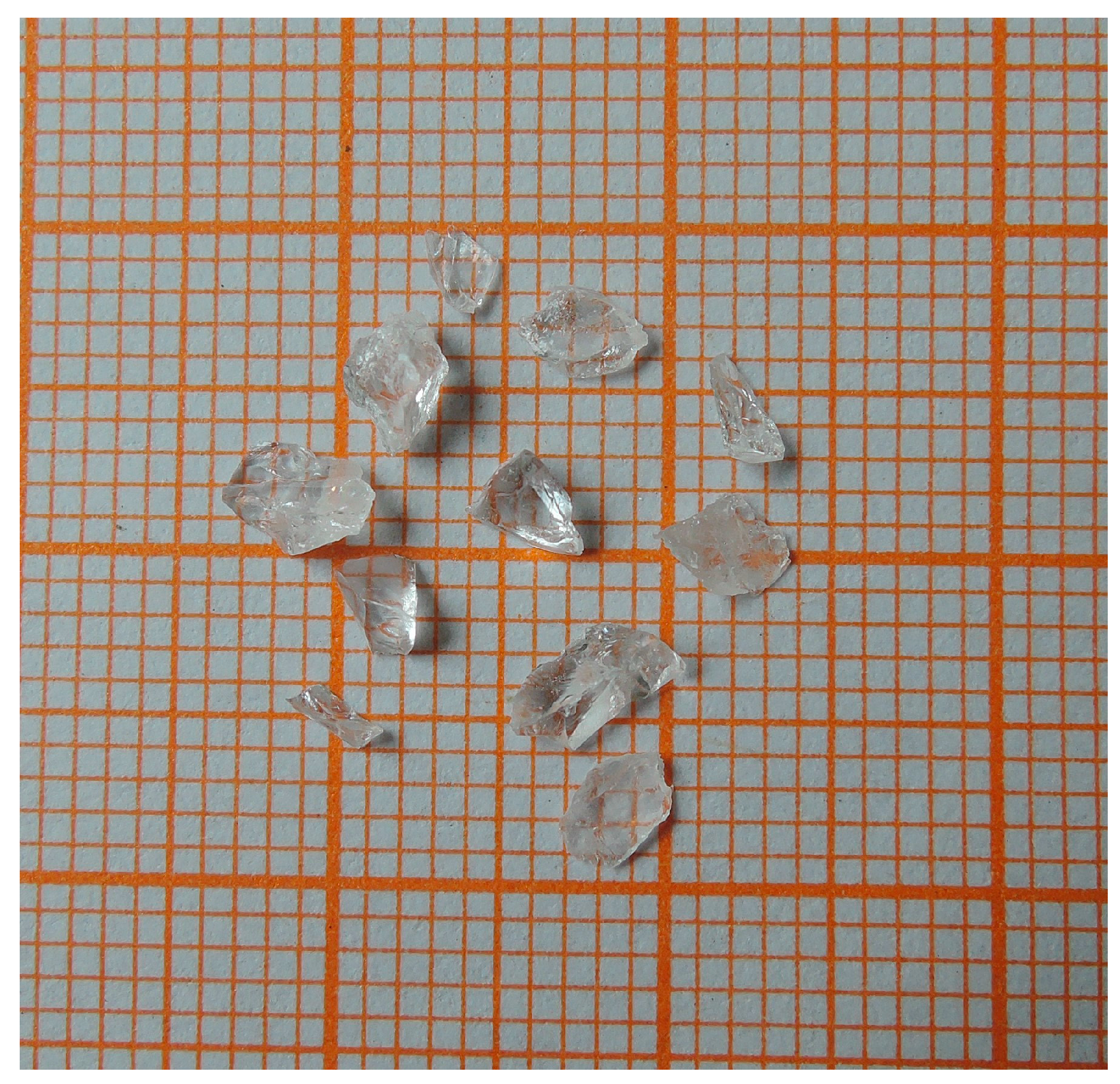
4.1. Single Crystal Growth
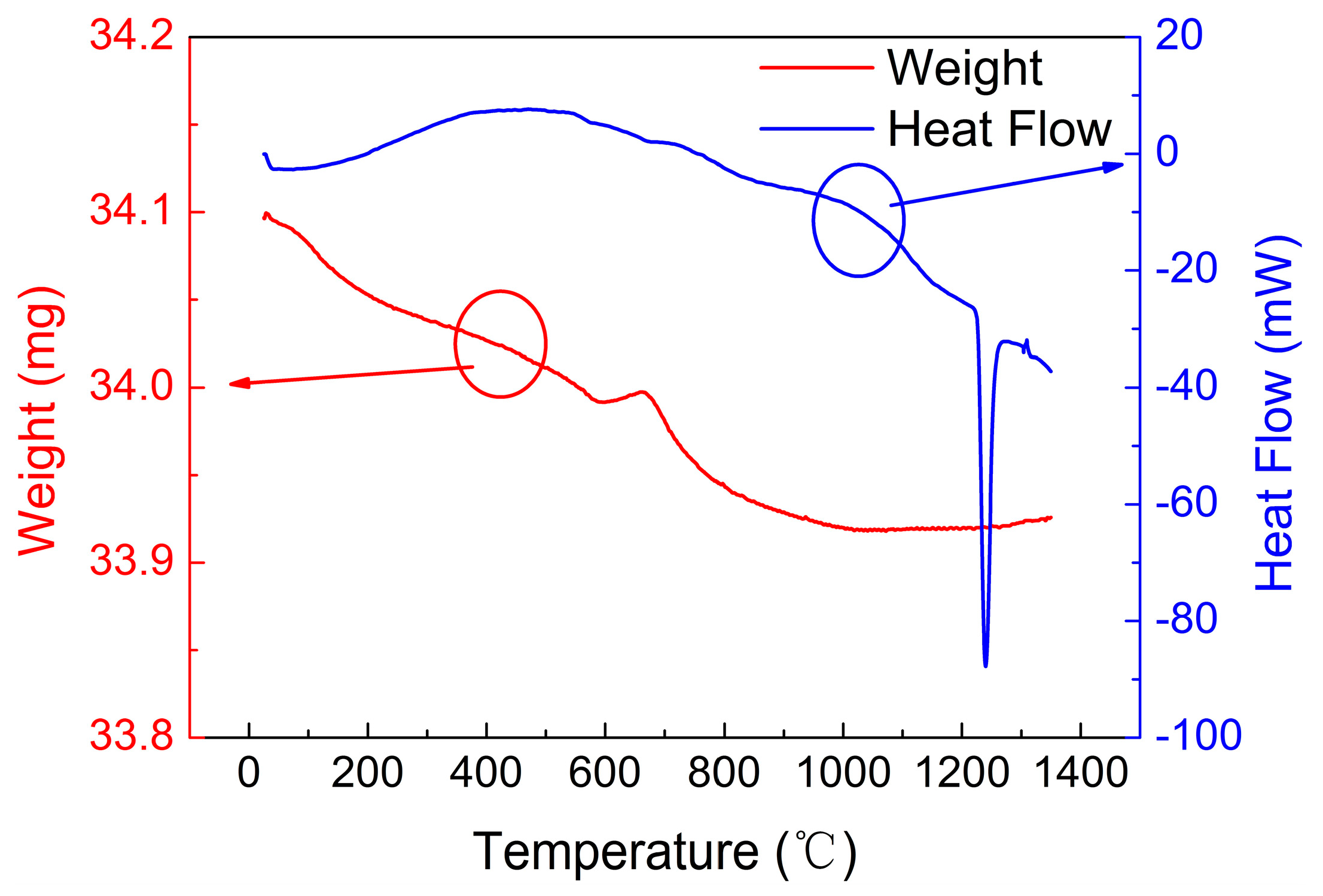
4.2. Thermogravimetric and Differential Thermal Analysis
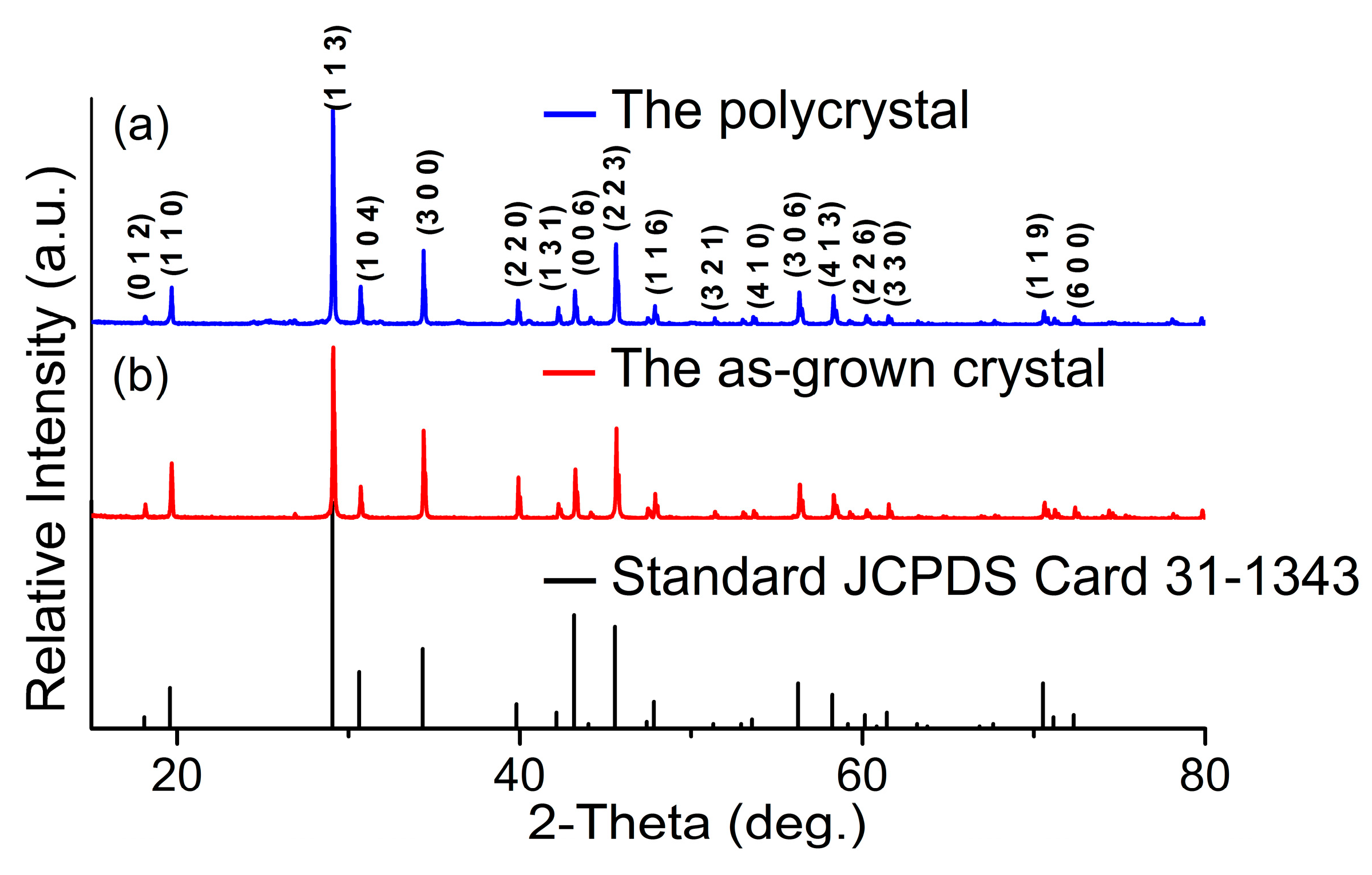
4.3. Structure and Phase Transition Analysis
4.4. Raman Spectra Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.L.; Ye, Y.; Fan, Y.D.; Zhou, J.; Jia, L.; Tang, B.; Wang, X.G. Silver nanoprism-loaded eggshell membrane: a facile platform for in situ sers monitoring of catalytic reactions. Crystals 2017, 7, 45. [Google Scholar] [CrossRef]
- Saar, B.G.; Freudiger, C.W.; Reichman, J.; Stanley, C.M.; Holtom, G.R.; Xie, X.S. Video-rate molecular imaging in vivo with stimulated raman scattering. Science 2010, 330, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Girlando, A.; Masino, M.; Brillante, A.; Toccoli, T.; Iannotta, S. Raman identification of polymorphs in pentacene films. Crystals 2016, 6, 41. [Google Scholar] [CrossRef]
- Du, Y.; Cai, Q.; Xue, J.D.; Zhang, Q. Raman and terahertz spectroscopic investigation of cocrystal formation involving antibiotic nitrofurantoin drug and coformer 4-aminobenzoic acid. Crystals 2016, 6, 164. [Google Scholar] [CrossRef]
- Lioe, D.X.; Mars, K.; Kawahito, S.; Yasutomi, K.; Kagawa, K.; Yamada, T.; Hashimoto, M. A stimulated raman scattering CMOS pixel using a high-speed charge modulator and lock-in amplifier. Sensors 2016, 16, 532. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Guo, M.; Lu, D.L.; Wang, W.M.; Fu, Z.Y. Effect of HNO3 concentration on the morphologies and properties of Bi2WO6 photocatalyst synthesized by a hydrothermal method. Crystals 2016, 6, 75. [Google Scholar] [CrossRef]
- Wu, D.; Huang, H.; Zhu, X.P.; He, Y.W.; Xie, Q.L.; Chen, X.L.; Zheng, X.M.; Duan, H.G.; Gao, Y.L. E’’ Raman Mode in Thermal Strain-Fractured CVD-MoS2. Crystals 2016, 6, 151. [Google Scholar] [CrossRef]
- Wu, Z.X.; Wang, Z.P.; Zhang, F.; Liu, Y.Q.; Lu, J.R.; Xu, X.G. Optical frequency fork based on stimulated Raman scattering. J. Alloys Compd. 2016, 682, 537–542. [Google Scholar] [CrossRef]
- Bergmann, V.; Meier, W.; Wolff, D.; Stricker, W. Application of spontaneous Raman and Rayleigh scattering and 2D LIF for the characterization of a turbulent CH4/H2/N2 jet diffusion flame. Appl. Phys. B 1998, 66, 489–502. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Sczancoski, J.C.; Lima, L.F., Jr.; Espinosa, J.W.M.; Pizani, P.S.; Varela, J.A.; Longo, E. Synthesis, characterization, anisotropic growth and photoluminescence of BaWO4. Cryst. Growth Des. 2009, 9, 1002–1012. [Google Scholar] [CrossRef]
- Ivleva, L.I.; Voronina, I.S.; Lykov, P.A.; Berezovskaya, L.Y.; Osiko, V.V. Growth of optically homogeneous BaWO4 single crystals for Raman lasers. J. Cryst. Growth 2007, 304, 108–113. [Google Scholar] [CrossRef]
- Zvereva, P.G.; Basieva, T.T.; Osikoa, V.V.; Kulkovb, A.M.; Voitsekhovskiib, V.N.; Yakobsonb, V.E. Physical, chemical and optical properties of barium nitrate Raman crystal. Opt. Mater. 1999, 11, 315–334. [Google Scholar] [CrossRef]
- Li, X.L.; Lee, A.J.; Huo, Y.J.; Zhang, H.J.; Wang, J.Y.; Piper, J.A.; Pask, H.M.; Spence, D.J. Managing SRS competition in a miniature visible Nd:YVO4/BaWO4 Raman laser. Opt. Express 2012, 20, 19305–19312. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Ueda, K.; Eichler, H.J.; Kuwano, Y.; Kouta, H.; Bagaev, S.N.; Chyba, T.H.; Barnes, J.C.; Gad, G.M.A.; Murai, T.; et al. Tetragonal vanadates YVO4 and GdVO4—New efficient χ(3)—Materials for Raman lasers. Opt. Commun. 2001, 194, 201–206. [Google Scholar] [CrossRef]
- Chen, Y.F. Compact efficient all-solid-state eye-safe laser with self-frequency Raman conversion in a Nd:YVO4 crystal. Opt. Lett. 2004, 29, 2172–2174. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.B.; Orley, M.J.; Roberts, K.J.; Jackson, R.A.; Quayle, M.J. An examination of the influence of divalent cationic dopants on the bulk and surface properties of Ba(NO3)2 associated with crystallization. Cryst. Growth Des. 2009, 9, 2588–2594. [Google Scholar] [CrossRef]
- Yu, H.H.; Hu, D.W.; Zhang, H.J.; Wang, Z.P.; Ge, W.W.; Xu, X.G.; Wang, J.Y.; Shao, Z.S.; Jiang, M.H. Picosecond stimulated Raman scattering of BaWO4 crystal. Opt. Laser Technol. 2007, 39, 1239–1242. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhang, F.; Wang, Z.P.; Yu, F.P.; Wei, L.; Xu, X.G.; Zhao, X. Ca3(BO3)2, a first wide waveband borate Raman laser crystal with strong Raman effects and outstanding anti-optical damage ability. J. Mater. Chem. C 2015, 3, 10687–10694. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Scott, J.F. Raman spectra of CaWO4, SrWO4 CaMoO4 and SrMoO4. Phys. Rev. 1967, 157, 716–719. [Google Scholar] [CrossRef]
- Černýa, P.; Zverevb, P.G.; Jelı́nkováa, H.; Basievb, T.T. Efficient Raman shifting of picosecond pulses using BaWO4 crystal. Opt. Comm. 2000, 177, 397–404. [Google Scholar] [CrossRef]
- Basiev, T.T.; Sobol, A.A.; Voronko, Y.K.; Zverev, P.G. Spontaneous Raman spectroscopy of tungstate and molybdate crystals for Raman lasers. Opt. Mater. 2000, 15, 205–216. [Google Scholar] [CrossRef]
- Goldschmidt, V. M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jia, G.H.; Wang, Y.; You, Z.Y.; Li, J.F.; Zhu, Z.J.; Yang, F.G.; Wei, Y.P.; Tu, C.Y. Crystal growth and spectral properties of pure and Co2+-doped Mg3B2O6 crystal. Opt. Mater. 2007, 29, 1635–1639. [Google Scholar] [CrossRef]
- Richter, L.; Muller, F. Zur Struktur von Sr3B2O6. Z. Anorg. Allg. Chem. 1980, 467, 123–125. [Google Scholar] [CrossRef]
- International Centre of Diffraction Data (ICDD). PCPDF 31–1343. Available online: http://www.icdd.com (accessed on 10 March 2017).
- International Centre of Diffraction Data (ICDD). PCPDF 38–1475. Available online: http://www.icdd.com (accessed on 10 March 2017).
- Segall, M.; Lindan, P.; Probert, M.; Pickard, C.; Hasnip, P.; Clark, S.; Payne, M. First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Fletcher, J.G.; Glasser, F.P. Phase relations in the system CaO-B2O3-SiO2. J. Mater. Sci. 1993, 28, 2677–2686. [Google Scholar] [CrossRef]
- Barseghyan, A.H.; Hovhannisyan, R.M.; Petrosyan, B.V.; Aleksanyan, H.A.; Toroyan, V.P. Glass formation and crystalline phases in the ternary systems CaO-Bi2O3-B2O3 and SrO-Bi2O3-B2O3. Phys. Chem. Glasses 2013, 54, 199–205. [Google Scholar]
- Liang, G.; Tang, Z.; Fang, H.; Katz, D.; Salama, K. Synthesis and X-ray diffraction pattern for MgCu2. J. Alloys Comp. 2006, 422, 73–77. [Google Scholar] [CrossRef]
- Piper, J.A.; Pask, H.M. Crystalline Raman Lasers. J. Sel. Top. Quant. 2007, 13, 692–704. [Google Scholar] [CrossRef]
- Pask, H.M. The design and operation of solid-state Raman lasers. Prog. Quant. Electron. 2003, 27, 3–56. [Google Scholar] [CrossRef]

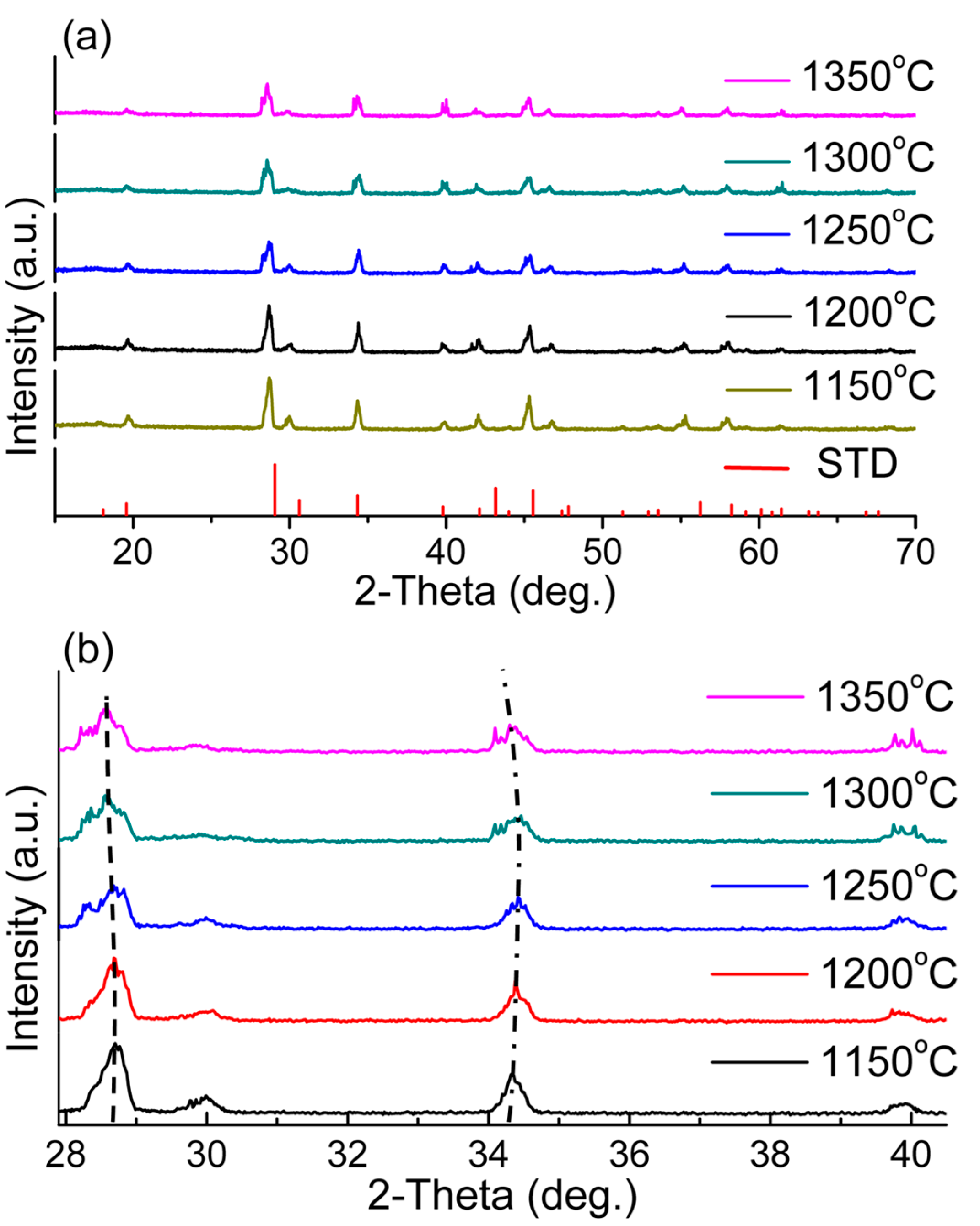
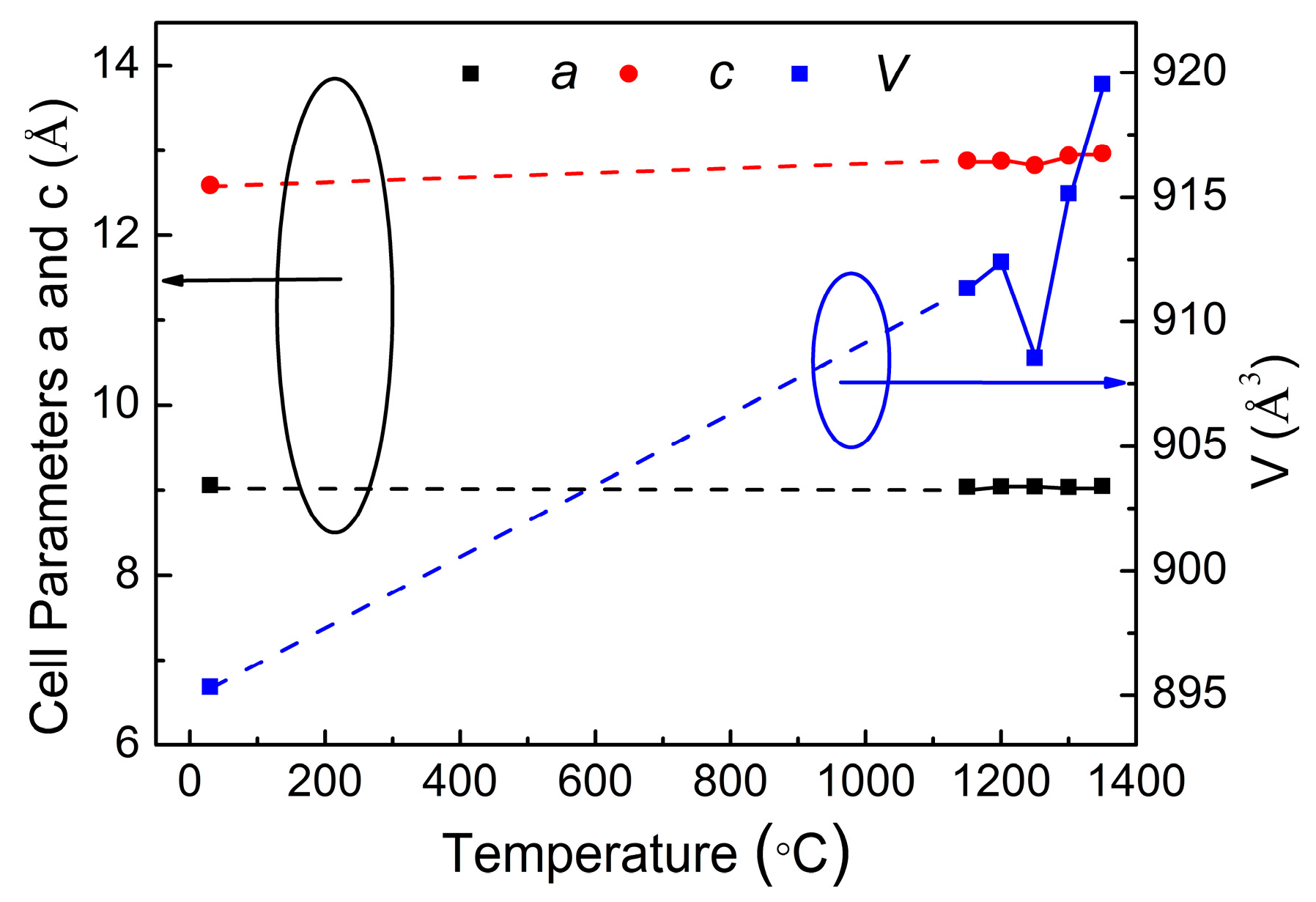

| T/°C | 1150 | 1200 | 1250 | 1300 | 1350 |
|---|---|---|---|---|---|
| a/Å | 9.03988 | 9.04522 | 9.04469 | 9.0376 | 9.04985 |
| b/Å | 9.03988 | 9.04522 | 9.04469 | 9.0376 | 9.04985 |
| c/Å | 12.87744 | 12.87707 | 12.82442 | 12.93778 | 12.96449 |
| V/Å3 | 911.35 | 912.4 | 908.56 | 915.16 | 919.54 |
| d113/Å | 3.36251 | 3.36241 | 3.34865 | 3.37827 | 3.38526 |
| d300/Å | 3.71739 | 3.71728 | 3.70207 | 3.73482 | 3.74254 |
| d220/Å | 3.94289 | 3.94279 | 3.92664 | 3.96137 | 3.96956 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Qi, H.; Li, Y.; Yu, F.; Wang, H.; Chen, F.; Liu, Y.; Wang, Z.; Xu, X.; Zhao, X. Synthesis and Characterization of New Sr3(BO3)2 Crystal for Stimulated Raman Scattering Applications. Crystals 2017, 7, 125. https://doi.org/10.3390/cryst7050125
Wang X, Qi H, Li Y, Yu F, Wang H, Chen F, Liu Y, Wang Z, Xu X, Zhao X. Synthesis and Characterization of New Sr3(BO3)2 Crystal for Stimulated Raman Scattering Applications. Crystals. 2017; 7(5):125. https://doi.org/10.3390/cryst7050125
Chicago/Turabian StyleWang, Xinle, Hongwei Qi, Yanlu Li, Fapeng Yu, Hewei Wang, Feifei Chen, Yanqing Liu, Zhengping Wang, Xinguang Xu, and Xian Zhao. 2017. "Synthesis and Characterization of New Sr3(BO3)2 Crystal for Stimulated Raman Scattering Applications" Crystals 7, no. 5: 125. https://doi.org/10.3390/cryst7050125








