Synthesis, Crystal Structure and Nonlinear Optical Property of RbHgI3
Abstract
:1. Introduction
2. Results and Discussion
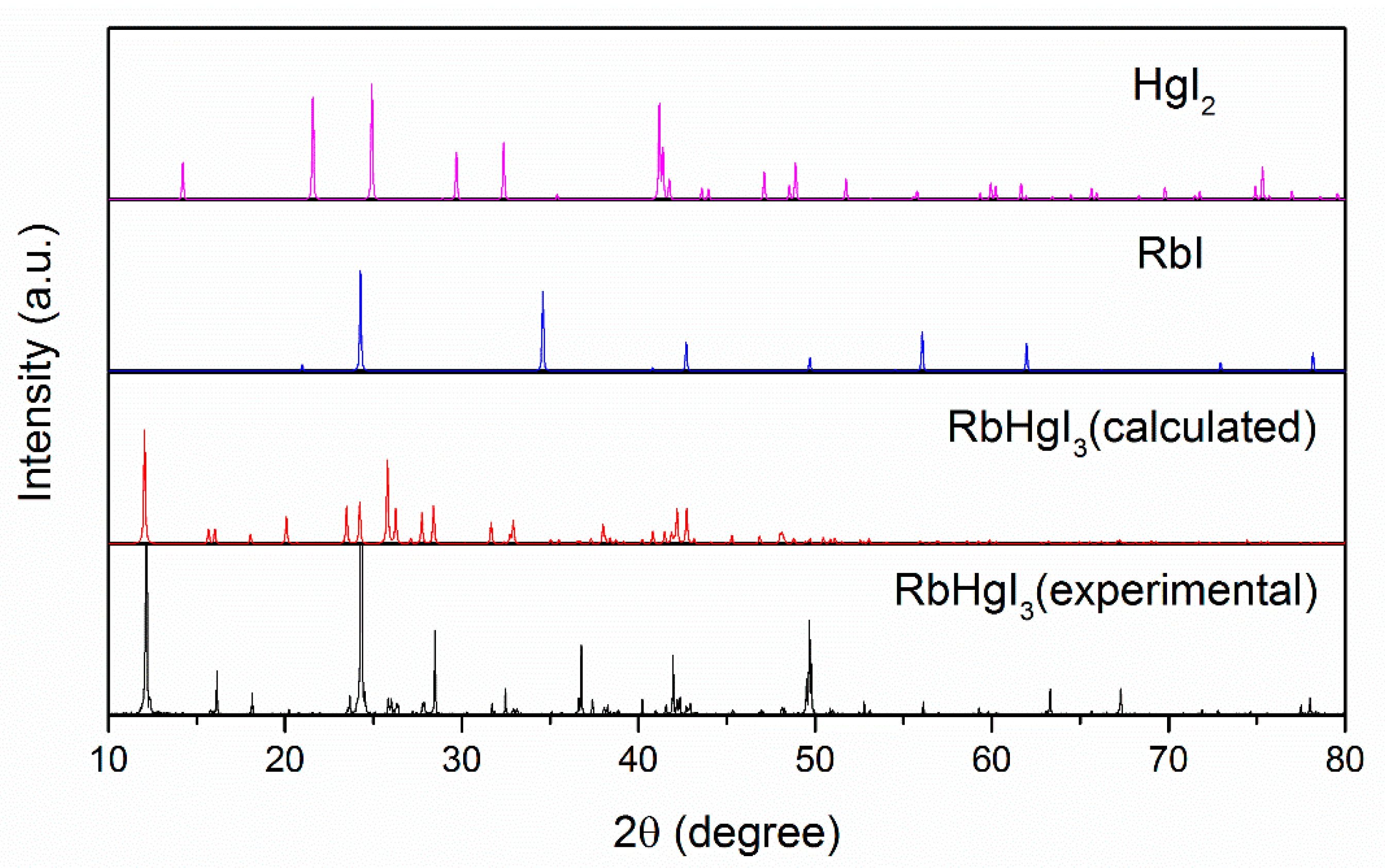
2.1. Synthesis and Analysis
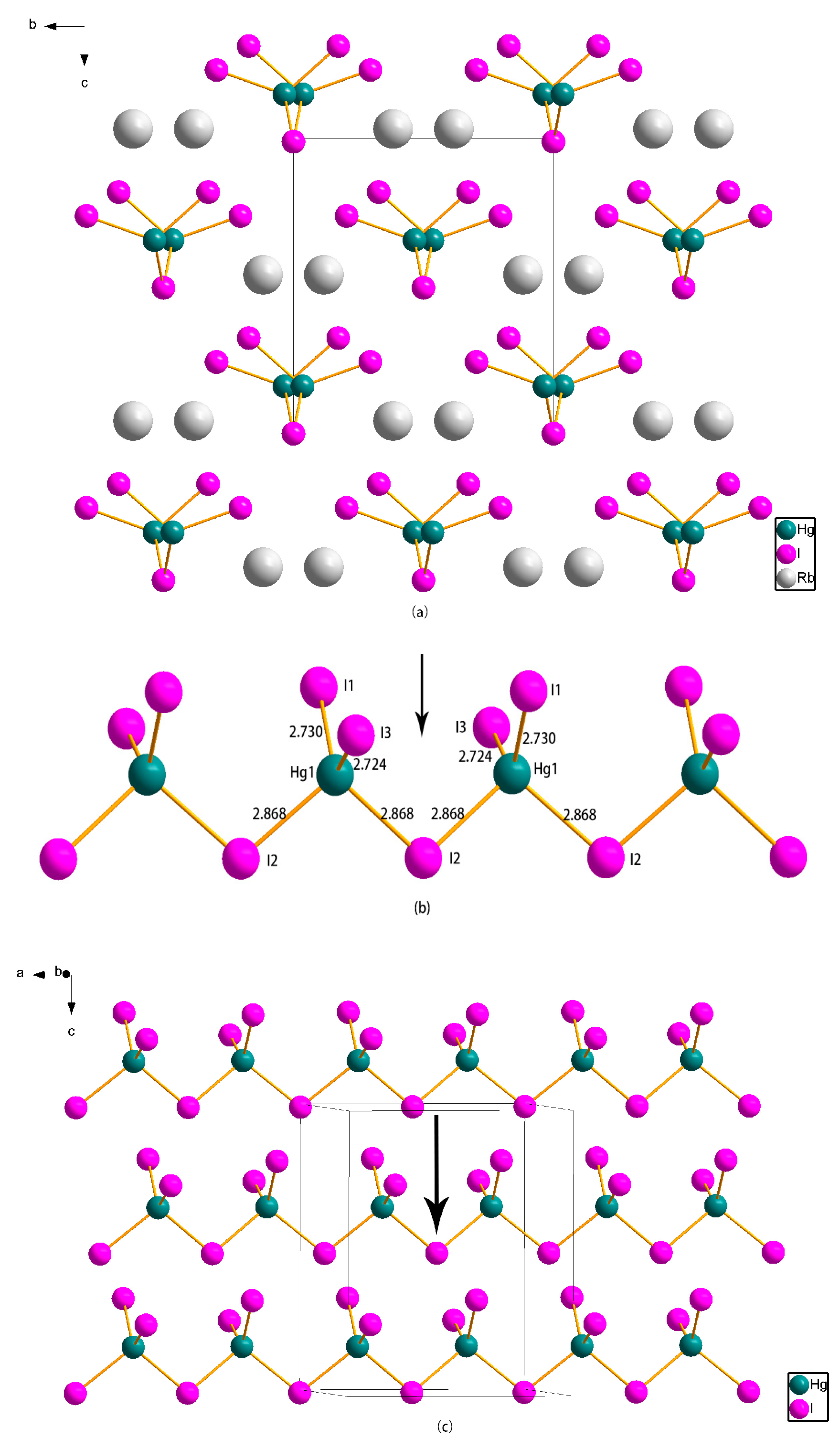
2.2. Crystal Structure
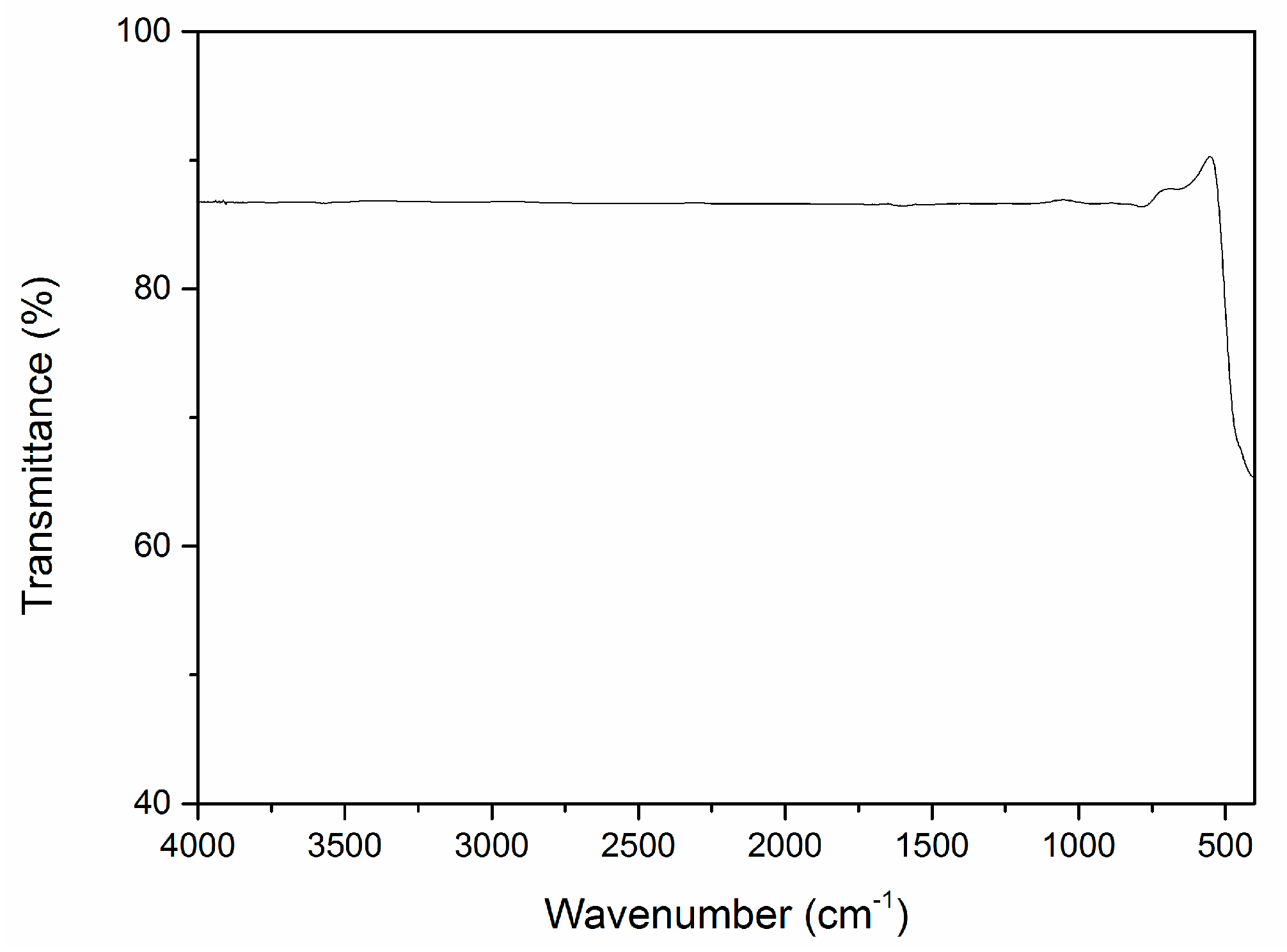
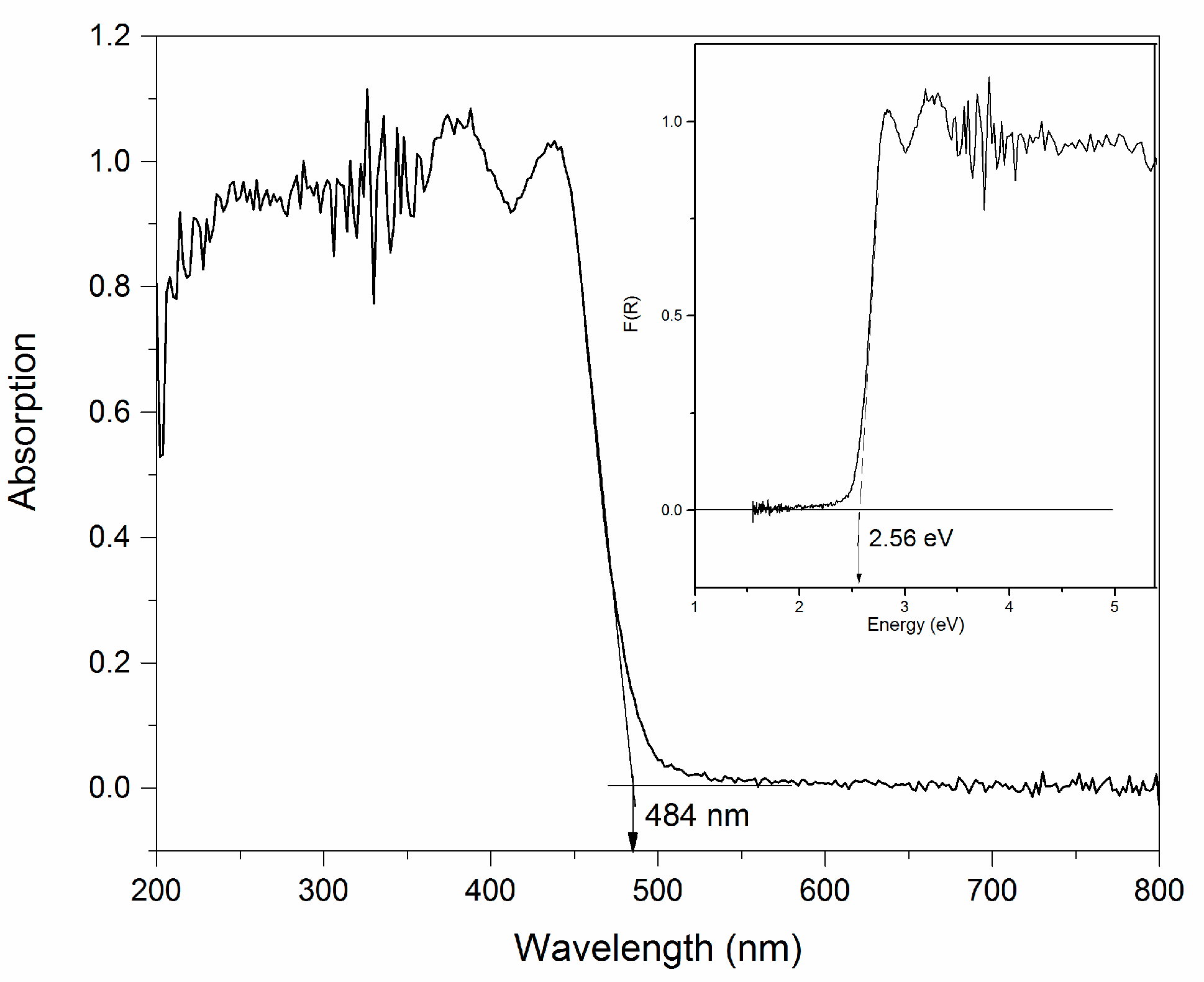
2.3. Infrared Spectrum and UV-Vis Diffuse Reflectance Spectrum
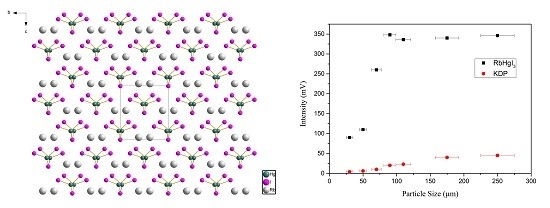
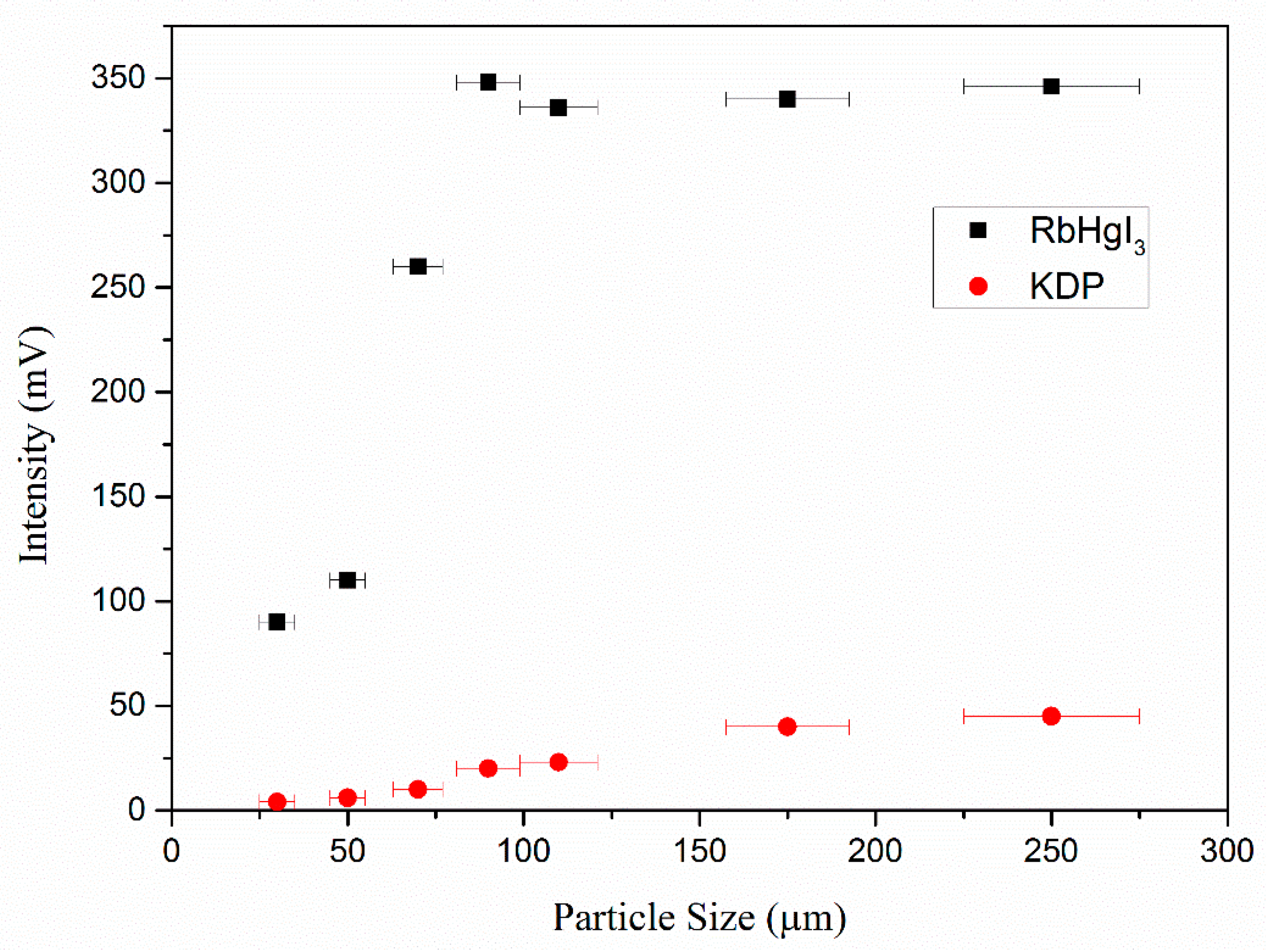
2.4. NLO Property and LDT Measurement
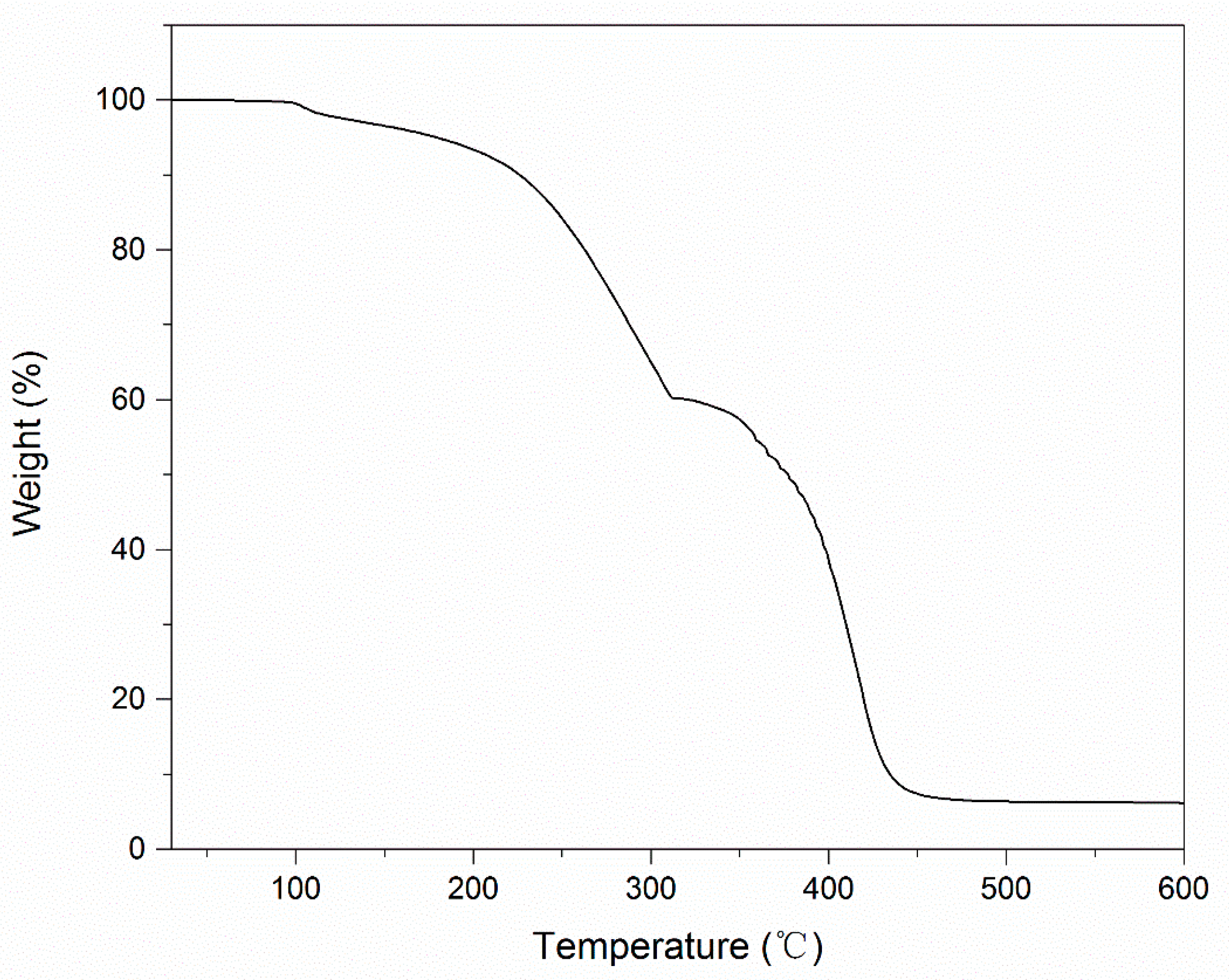
2.5. Thermogravimetric Analysis
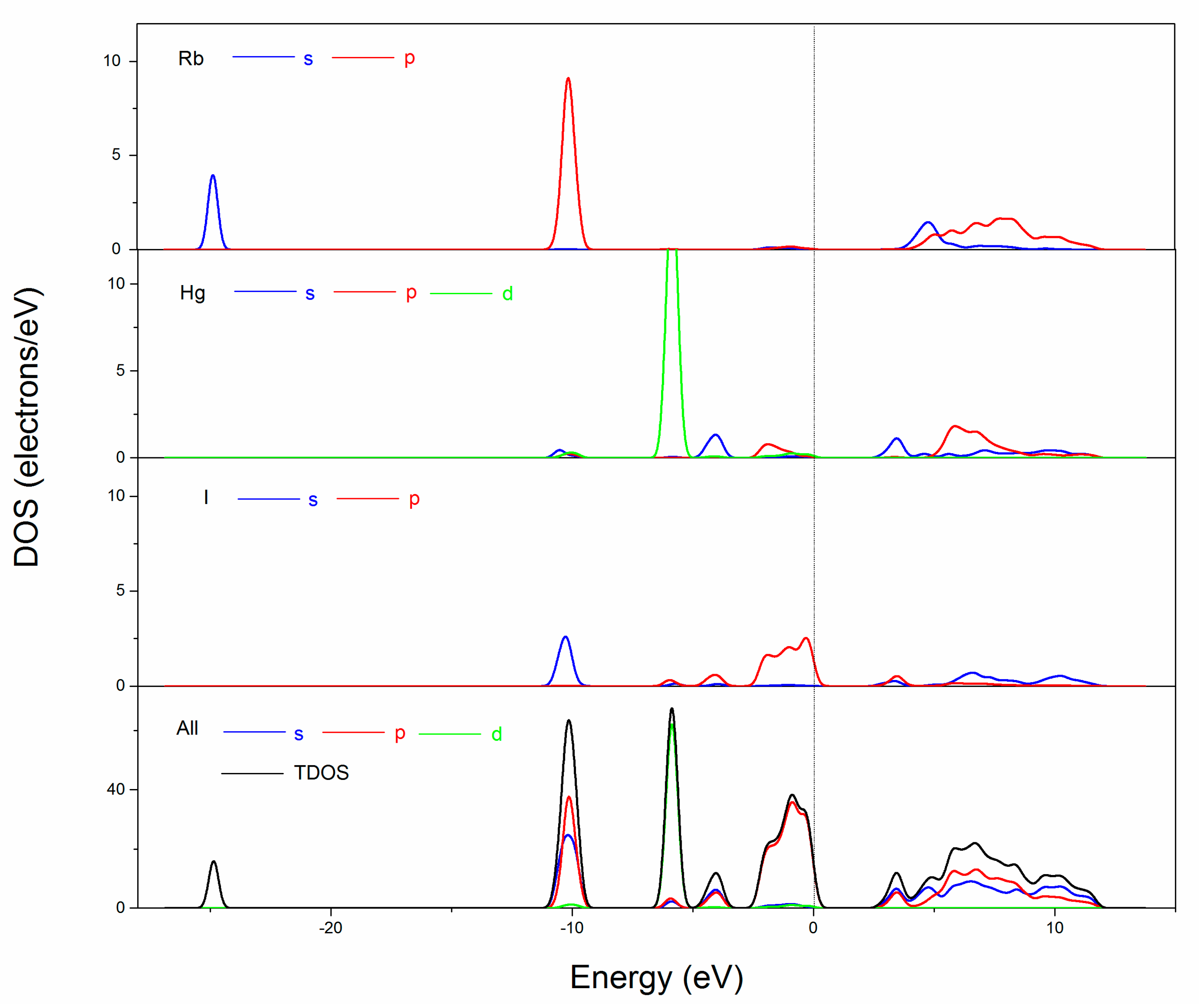
2.6. Electronic Structure and Optical Properties Calculation
3. Materials and Methods
3.1. Synthesis and Crystal Growth
3.2. Structure Determination
3.3. Powder XRD Measurement
3.4. Optical Spectroscopy
3.5. Second-Harmonic Generation (SHG) and Laser Damage Threshold (LDT) Measurement
3.6. Thermogravimetric Analysis
3.7. Theoretical Calculation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burland, D.M.; Miller, R.D.; Walsh, A.C. Second-order nonlinearity in poled-polymer systems. Chem. Rev. 1994, 94, 31–75. [Google Scholar] [CrossRef]
- Chai, B.H.T. CRC Handbook of Laser Science and Technology Supplement 2: Optical Materials; Weber, M.J., Ed.; CRC: Boca Raton, FL, USA, 1995. [Google Scholar]
- Chen, C.T.; Wu, B.C.; Jiang, A.D.; You, M.G. A new-type ultraviolet SHG crystal—β-BaB2O4. Sci. Sin. Ser. B 1985, 28, 235–243. [Google Scholar]
- Chen, C.T.; Wu, Y.C.; Jiang, A.D.; Wu, B.C.; You, G.M.; Li, R.K.; Lin, S.J. New nonlinear-optical crystal: LiB3O5. J. Opt. Soc. Am. B 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Chen, C.T.; Xu, Z.Y.; Deng, D.Q.; Zhang, J.; Wrong, G.K.L. The vacuum ultraviolet phase-matching characteristics of nonlinear optical KBe2BO3F2 crystal. Appl. Phys. Lett. 1996, 28, 2930–2932. [Google Scholar] [CrossRef]
- Chen, C.T.; Ge, N.; Lin, J.; Jiang, J.; Zeng, W.R.; Wu, B.C. Computer-Assisted Search for Nonlinear Optical Crystals. Adv. Mater. 1999, 11, 1071–1078. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, G.L.; Wang, X.Y.; Xu, Z.Y. Deep-UV nonlinear optical crystal KBe2BO3F2 —discovery, growth, optical properties and applications. Appl. Phys. B 2009, 97, 9–25. [Google Scholar] [CrossRef]
- Smith, W.L. KDP and ADP transmission in the vacuum ultraviolet. Appl. Opt. 1977, 16, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Kato, K. Parametric oscillation at 3.2 µm in KTP pumped at 1.064 µm. IEEE J. Quantum Electron. 1991, 27, 1137–1140. [Google Scholar] [CrossRef]
- Chemla, D.S.; Kupecek, P.J.; Robertson, D.S.; Smith, R.C. Silver thiogallate, a new material with potential for infrared devices. Opt. Commun. 1971, 3, 29–31. [Google Scholar] [CrossRef]
- Boyd, G.D.; Kasper, E.H.M.; Mcfee, J.H.; Storz, F.G. Linear and nonlinear optical properties of AgGaS2, CuGaS2, and CuInS2, and theory of the wedge technique for the measurement of nonlinear coefficients. IEEE J. Quantum Electron. 1972, 8, 563–573. [Google Scholar]
- Boyd, G.D.; Buehler, E.; Storz, F.G. Linear and nonlinear optical properties of ZnGeP2 and CdSe. Appl. Phys. Lett. 1971, 18, 301–304. [Google Scholar] [CrossRef]
- Liang, F.; Lin, L.K.Z.; Wu, Y.; Chen, C. Analysis and prediction of mid-IR nonlinear optical metal sulfides with diamond-like structures. Coordin. Chem. Rev. 2017, 333, 57. [Google Scholar] [CrossRef]
- Guo, S.; Chi, Y.; Guo, G. Recent achievements on middle and far-infrared second-order nonlinear optical materials. Coord. Chem. Rev. 2017, 335, 44. [Google Scholar] [CrossRef]
- Jackson, A.G.; Ohmer, M.C.; LeClair, S.R. Relationship of the second order nonlinear optical coefficient to energy gap in inorganic non-centrosymmetric crystals. Infrared Phys. Technol. 1997, 38, 233–244. [Google Scholar] [CrossRef]
- Chen, C.T.; Liu, G.Z. Recent Advances in Nonlinear Optical and Electro-Optical Materials. Annu. Rev. Mater. Sci. 1986, 16, 203–243. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Jiang, K.; Zeng, H.; Liu, T.; Chen, X.; Qin, J.; Lin, Z.; Fu, P.; Wu, Y.; et al. A New Mixed Halide, Cs2HgI2Cl2: Molecular Engineering for a New Nonlinear Optical Material in the Infrared Region. J. Am. Chem. Soc. 2012, 134, 14818–14822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, J.; Liu, T.; Fu, P.; Wu, Y.; Chen, C. Synthesis, Characterization, and Crystal Growth of Cs2Hg3I8: A New Second-Order Nonlinear Optical Material. Cryst. Growth Des. 2008, 8, 2946–2949. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Chen, H.; Jiang, K.; Li, H.; Zhong, C.; Chen, X.; Qin, J. HgBrI: A promising nonlinear optical material in IR region. Inorg. Chem. Commun. 2013, 34, 1–3. [Google Scholar] [CrossRef]
- Liu, T.; Qin, J.; Zhang, G.; Zhu, T.; Niu, F.; Wu, Y.; Chen, C. Mercury Bromide (HgBr2): A promising nonlinear optical material in IR region with a high laser damage threshold. Appl. Phys. Lett. 2008, 93, 091102. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Zhu, T.; Meng, X.; Zhong, C.; Chen, X.; Qin, J. Synthesis, crystal structure and properties of a new candidate for nonlinear optical material in the IR region: Hg2BrI3. Dalton Trans. 2012, 41, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.W.; Wu, Q.; Meng, X.; Kang, L.; Zhong, C.; Lin, Z.; Hu, Z.; Chen, X.; Qin, J. A promising new nonlinear optical crystal with high laser damage threshold for application in the IR region: Synthesis, crystal structure and properties of noncentrosymmetric CsHgBr3. J. Mater. Chem. C. 2014, 2, 6796–6801. [Google Scholar] [CrossRef]
- Dang, Y.; Meng, X.; Jiang, K.; Zhong, C.; Chen, X.; Qin, J. A promising nonlinear optical material in the Mid-IR region: New results on synthesis, crystal structure and properties of noncentrosymmetric β-HgBrCl. Dalton Trans. 2013, 42, 9893–9897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, J.; Liu, T.; Li, Y.; Wu, Y.; Chen, C. NaSb3F10: A new second-order nonlinear optical crystal to be used in the IR region with very high laser damage threshold. Appl. Phys. Lett. 2009, 95, 1. [Google Scholar] [CrossRef]
- Wu, Q.; Meng, X.; Zhong, C.; Chen, X.; Qin, J. Rb2CdBr2I2: A new IR nonlinear optical material with a large laser damage threshold. J. Am. Chem. Soc. 2014, 136, 5683–5686. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Harvill, M.L.; Bogan, L.D. A survey of ABX3 halides for optical second harmonic generation. Mat. Res. Bull. 1975, 10, 753–759. [Google Scholar] [CrossRef]
- George, A.J.; Marcus, V. Crystal structures of the red, yellow, and orange forms of mercuric iodide. Inorg. Chem. 1967, 6, 396–399. [Google Scholar]
- Cortona, P. Direct determination of self-consistent total energies and charge densities of solids: A study of the cohesive properties of the alkali halides. Phys. Rev. B 1992, 46, 2008. [Google Scholar] [CrossRef]
- Avdienko, K.I.; Badikov, D.V.; Badikov, V.V.; Chizhikov, V.I.; Panyutin, V.L.; Shevyrdyaeva, G.S.; Scherbakov, S.I.; Scherbakova, E.S. Optical properties of thallium mercury iodide. Opt. Mater. 2003, 23, 569–573. [Google Scholar] [CrossRef]
- Sathiskumar, S.; Kathiravan, P.; Balakrishnan, T. Cationic Coordination Compound Cs2Hg3I8 for IR NLO Material: Synthesis, Crystal Growth and Characterizations. In Proceedings of the AIP conference Proceedings 1665, Tamilnadu, India, 16–20 December 2014; p. 2814. [Google Scholar]
- Kurtz, S.K.; Perry, T.T. A Powder Technique for the Evaluation of Nonlinear Optical Materials. J. Appl. Phys. 1968, 39, 3798–3813. [Google Scholar] [CrossRef]
- Nikogosyan, D.N. Nonlinear Optical Crystals: A Complete Survey; Springer: New York, NY, USA, 2005; pp. 102–103. [Google Scholar]
- Godby, R.W.; Schlüter, M.; Sham, L.J. Trends in self-energy operators and their corresponding exchange-correlation potentials. Phys. Rev. B 1987, 36, 6497. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL, Version 6.14; Bruker Analytical x-ray Instruments, Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXS-97—A Program for Automatic Solution of Crystal Structures; University of Goettingen: Goettingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97—A Program for Crystal Structure Refinement; University of Goettingen: Goettingen, Germany, 1997. [Google Scholar]
- Wendlandt, W.H.; Hecht, H.G. Reflectance Spectroscopy; Interscience: New York, NY, USA, 1966; pp. 62–65. [Google Scholar]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Lin, J.S.; Qtseish, A.; Payne, M.C.; Heine, V. Optimized and transferable nonlocal separable ab initio pseudopotentials. Phys. Rev. B 1993, 47, 4174. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lee, M.; Lin, Z.; Chen, C.; Pickard, C. Mechanism for linear and nonlinear optical effects in beta-BaB2O4 crystals. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 60, 13380. [Google Scholar] [CrossRef]
| Wavelength | nx | ny | nz | Δn (ny − nx) |
|---|---|---|---|---|
| 1000 nm | 2.01 | 2.09 | 2.07 | 0.08 |
| 1064 nm | 2.00 | 2.08 | 2.06 | 0.08 |
| 2000 nm | 1.97 | 2.05 | 2.03 | 0.08 |
| ~∞ | 1.96 | 2.04 | 2.02 | 0.08 |
| Empirical Formula | RbHgI3 |
|---|---|
| Formula weight | 666.76 |
| Temperature | 296(2) |
| Wavelength | 0.71073 |
| Crystal color | Yellow |
| Crystal system | Orthorhombic |
| Space group | Ama2 (No.40) |
| Crystal size (mm3) | 0.10 × 0.10 × 0.08 |
| Unit cell dimensions (Å) | a = 8.838(3), b = 9.824(3), c = 11.050(3) |
| Z | 4 |
| V (Å3) | 959.4(5) |
| Absorption coefficient (mm−1) | 30.640 |
| Density (calculated) | 4.616 |
| Goodness-of-fit on F2 | 1.149 |
| Reflections collected | 1473 |
| Independent reflection | 1337 [R (int) = 0.0293] |
| R1, wR1 [I > 2σ (I)] | 0.0682/0.1845 |
| R2, wR2 (all data) | 0.0637/0.1818 |
| Min/max Δρ/e·Å−3 | −3.472/3.022 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ding, Y.; Li, Y.; Liu, H.; Meng, X.; Cong, Y.; Zhang, J.; Li, X.; Chen, X.; Qin, J. Synthesis, Crystal Structure and Nonlinear Optical Property of RbHgI3. Crystals 2017, 7, 148. https://doi.org/10.3390/cryst7050148
Li Y, Ding Y, Li Y, Liu H, Meng X, Cong Y, Zhang J, Li X, Chen X, Qin J. Synthesis, Crystal Structure and Nonlinear Optical Property of RbHgI3. Crystals. 2017; 7(5):148. https://doi.org/10.3390/cryst7050148
Chicago/Turabian StyleLi, Yanjun, Yuxun Ding, Yaming Li, Hongming Liu, Xianggao Meng, Ye Cong, Jiang Zhang, Xuanke Li, Xingguo Chen, and Jingui Qin. 2017. "Synthesis, Crystal Structure and Nonlinear Optical Property of RbHgI3" Crystals 7, no. 5: 148. https://doi.org/10.3390/cryst7050148