Tri- and Mono-Nuclear Zinc(II) Complexes Based on Half- and Mono-Salamo Chelating Ligands
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
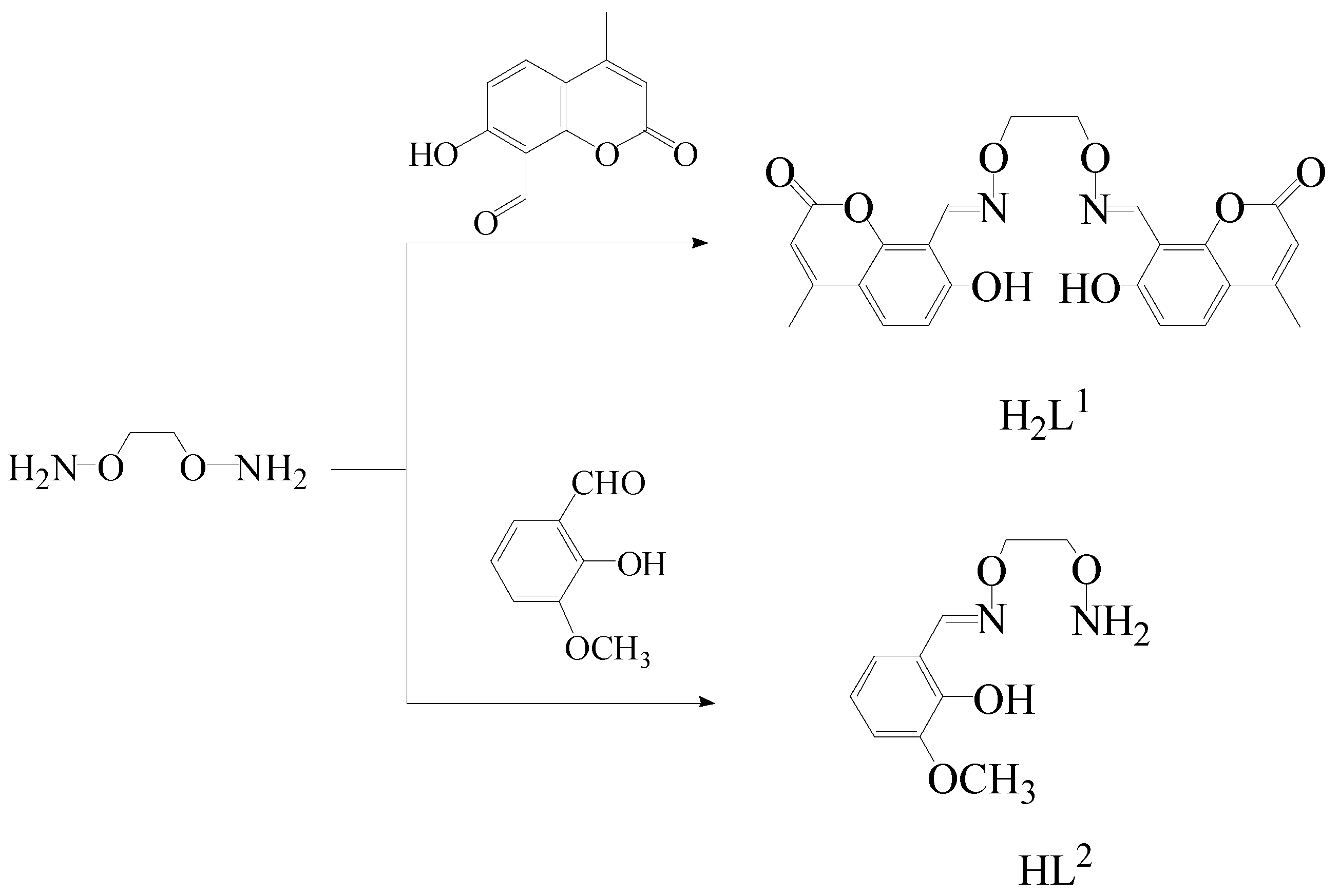
2.2. Synthesis of H2L1
2.3. Synthesis of HL2
2.4. Synthesis of Complex 1
2.5. Synthesis of Complex 2
2.6. Crystal Structure Determinations of Complexes 1 and 2
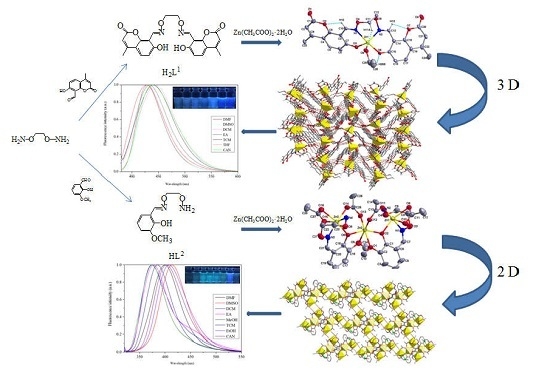
3. Results and Discussion
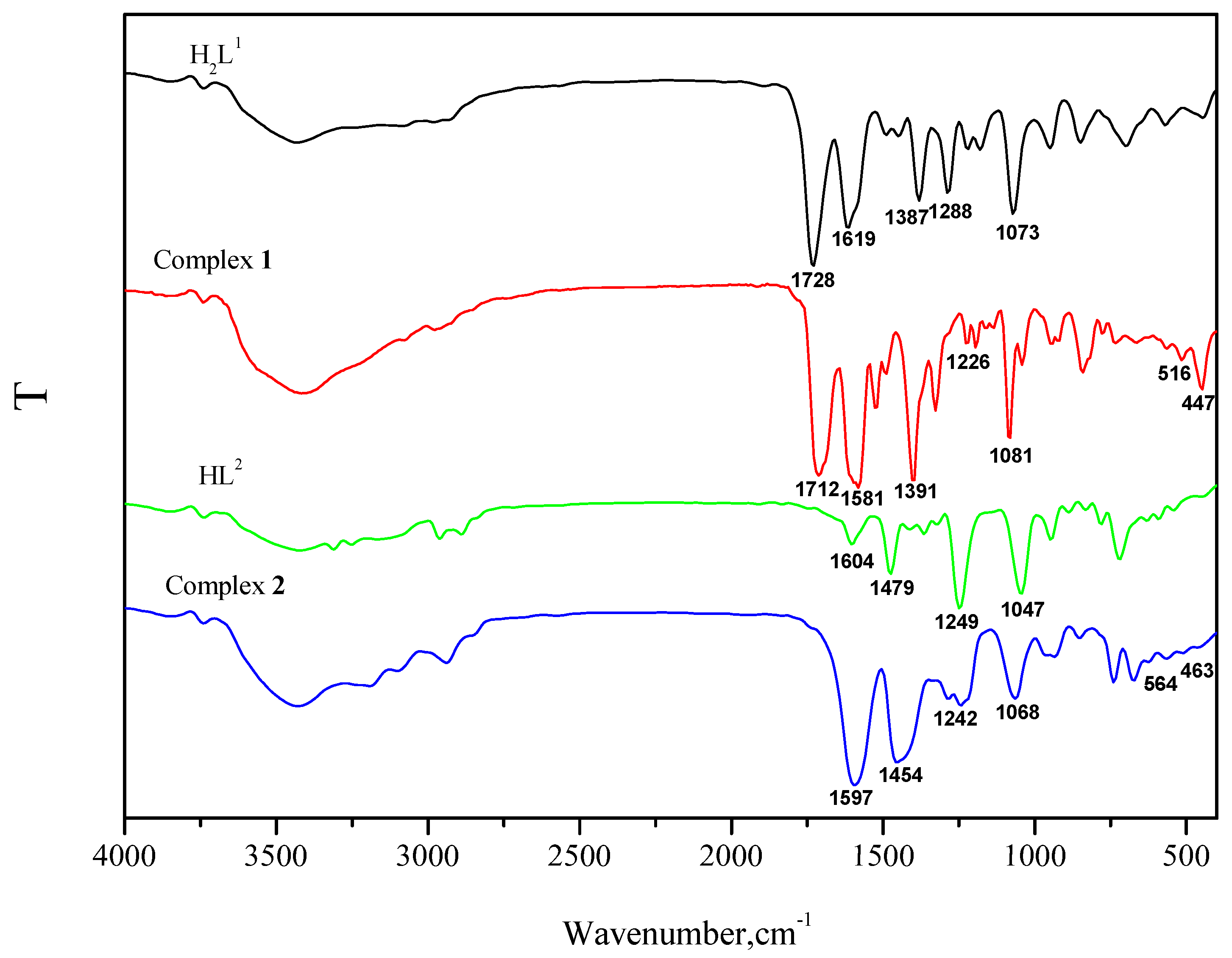
3.1. IR Spectra
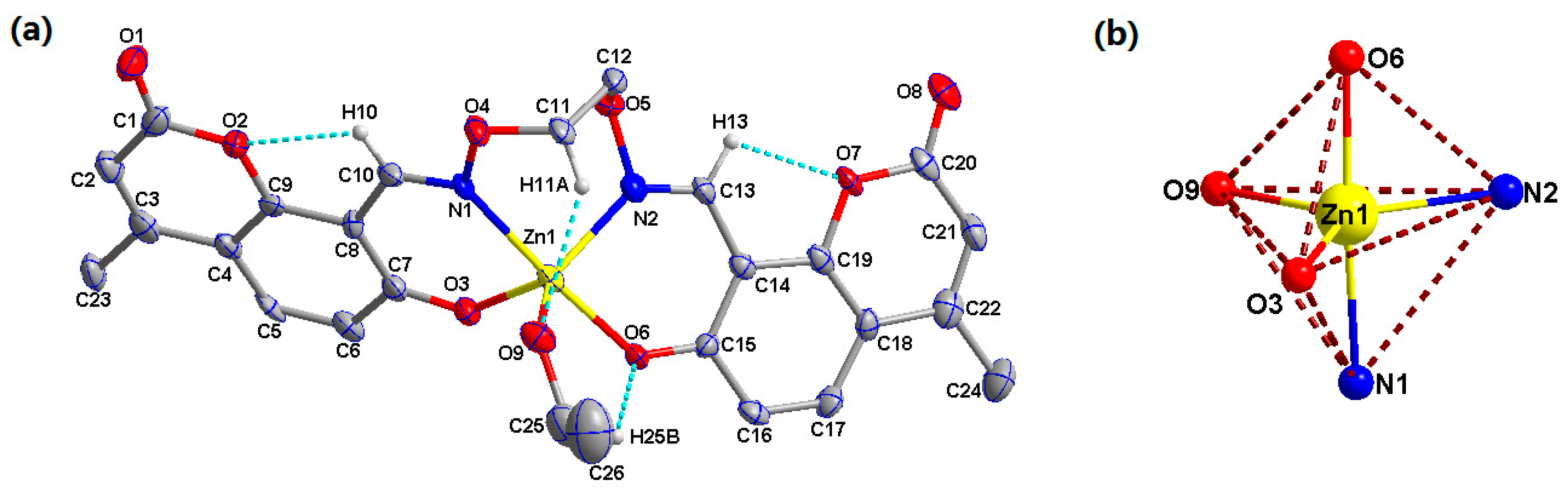
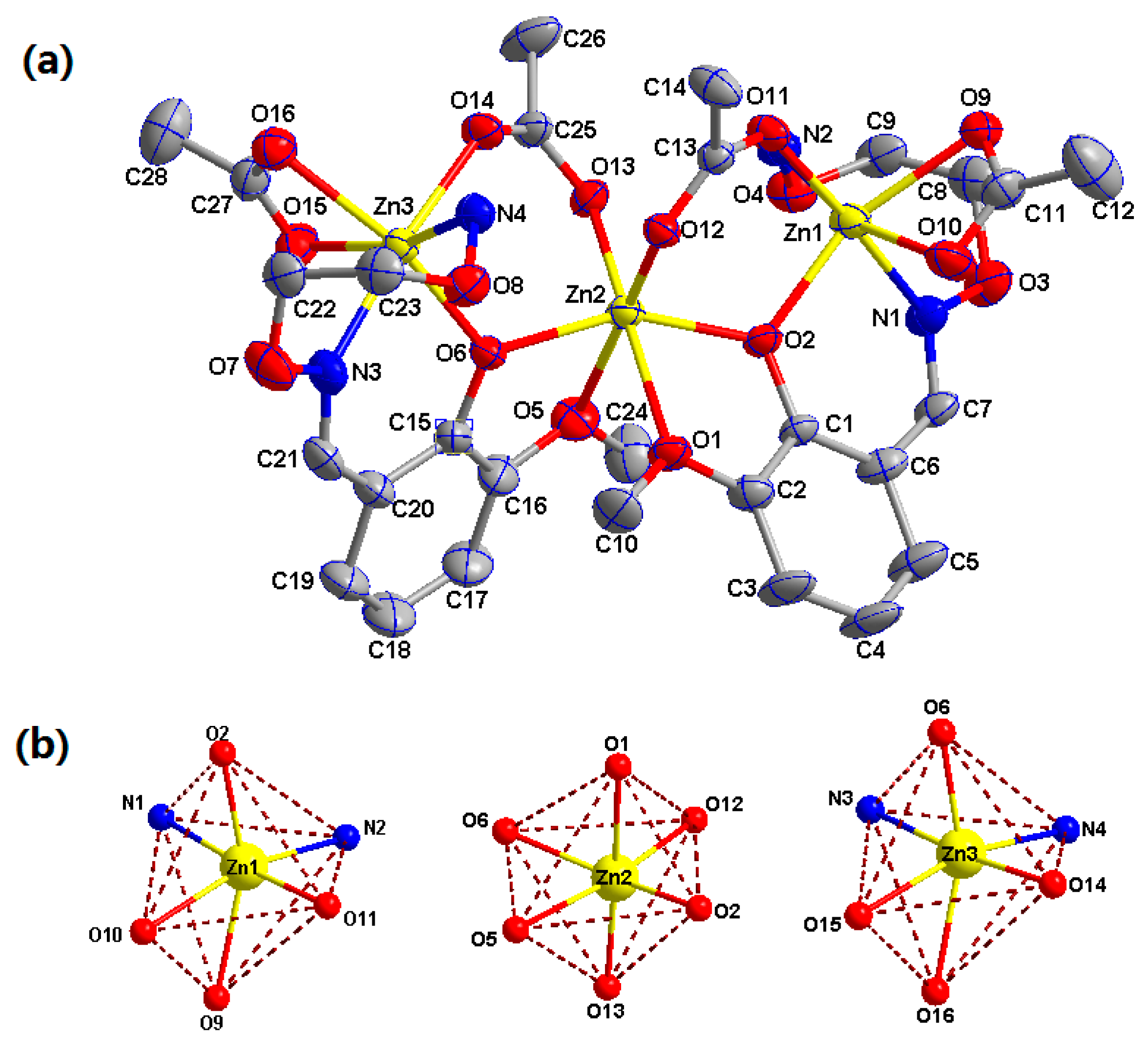
3.2. Crystal Structure of Complex 1
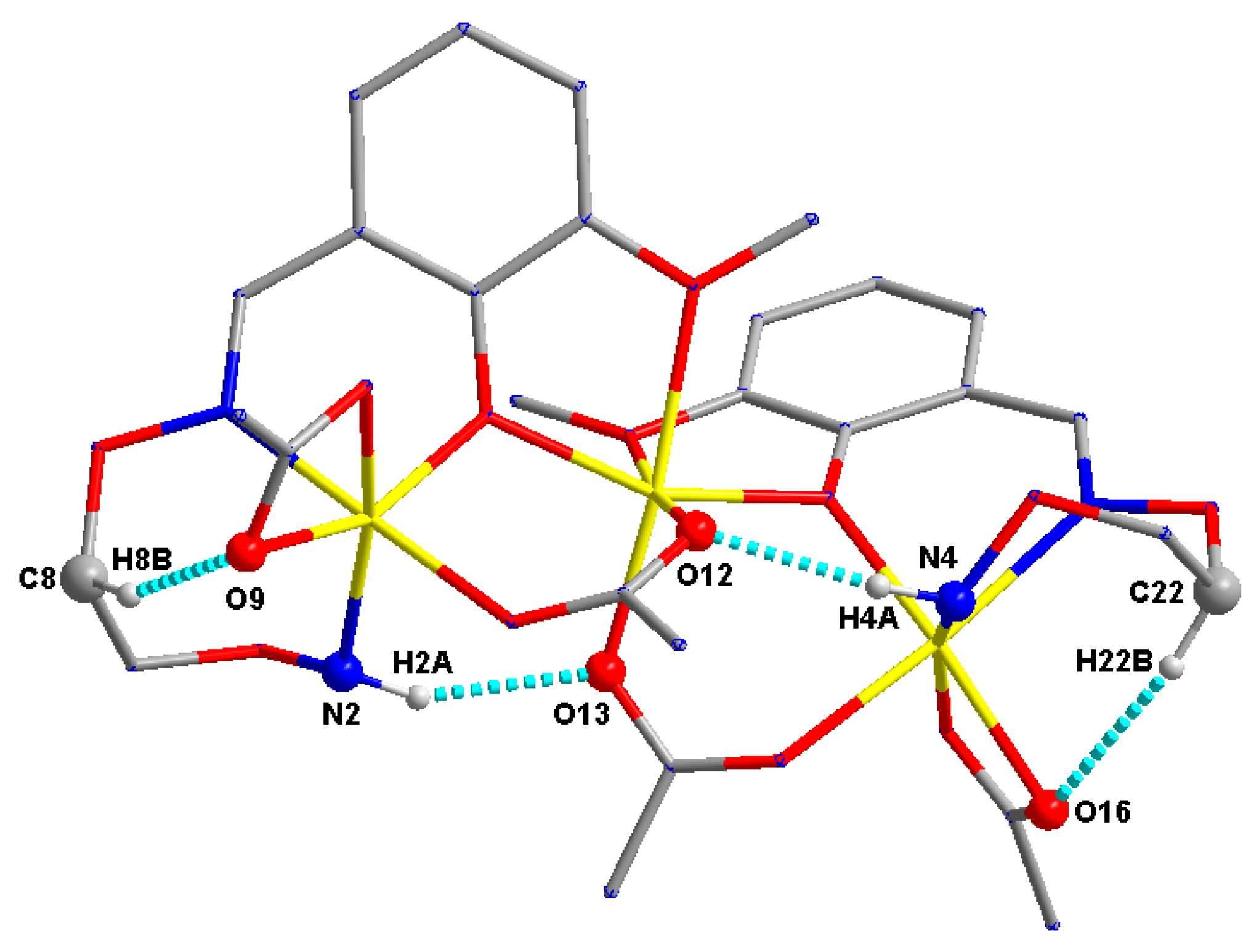
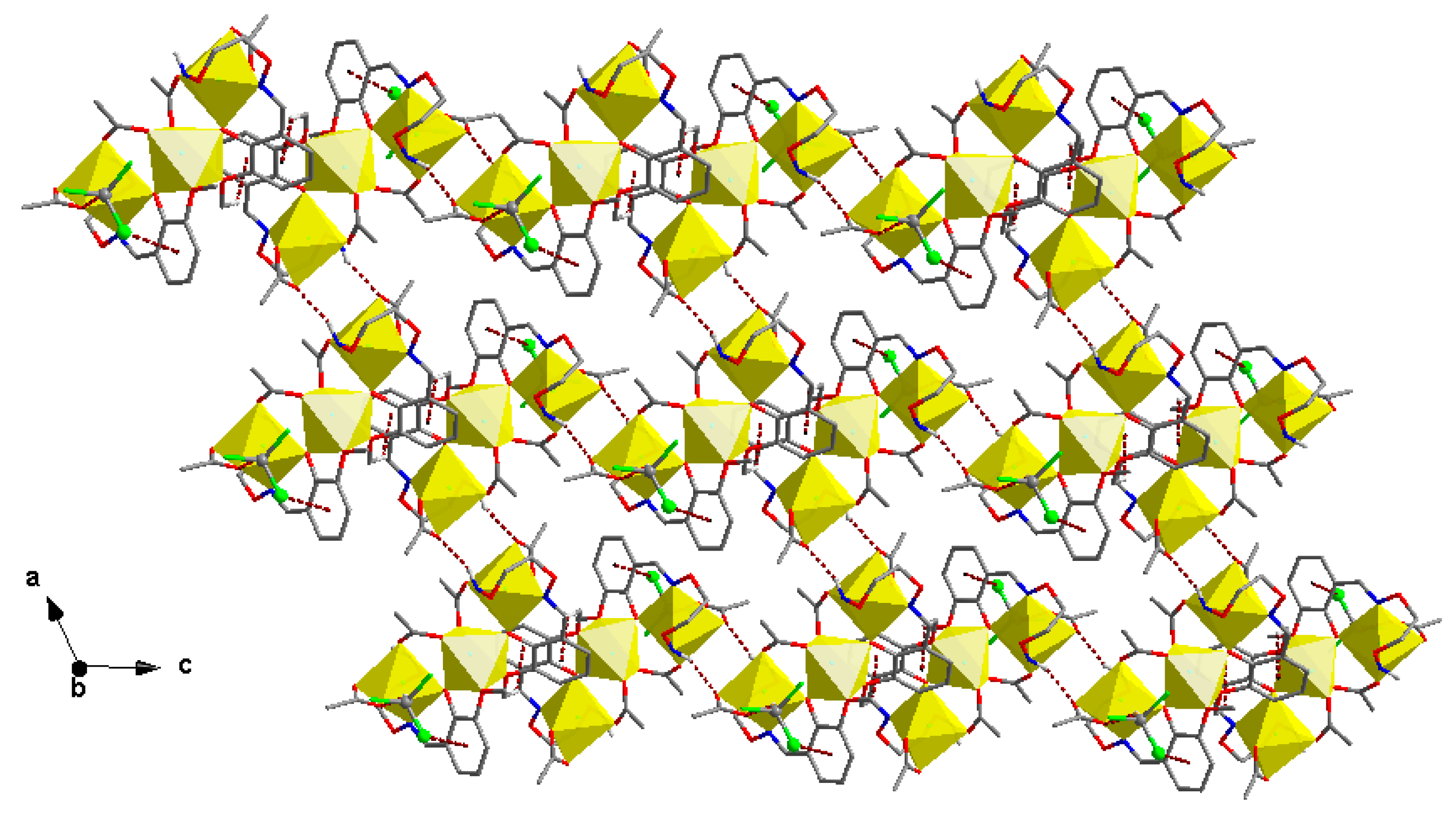
3.3. Crystal Structure of Complex 2
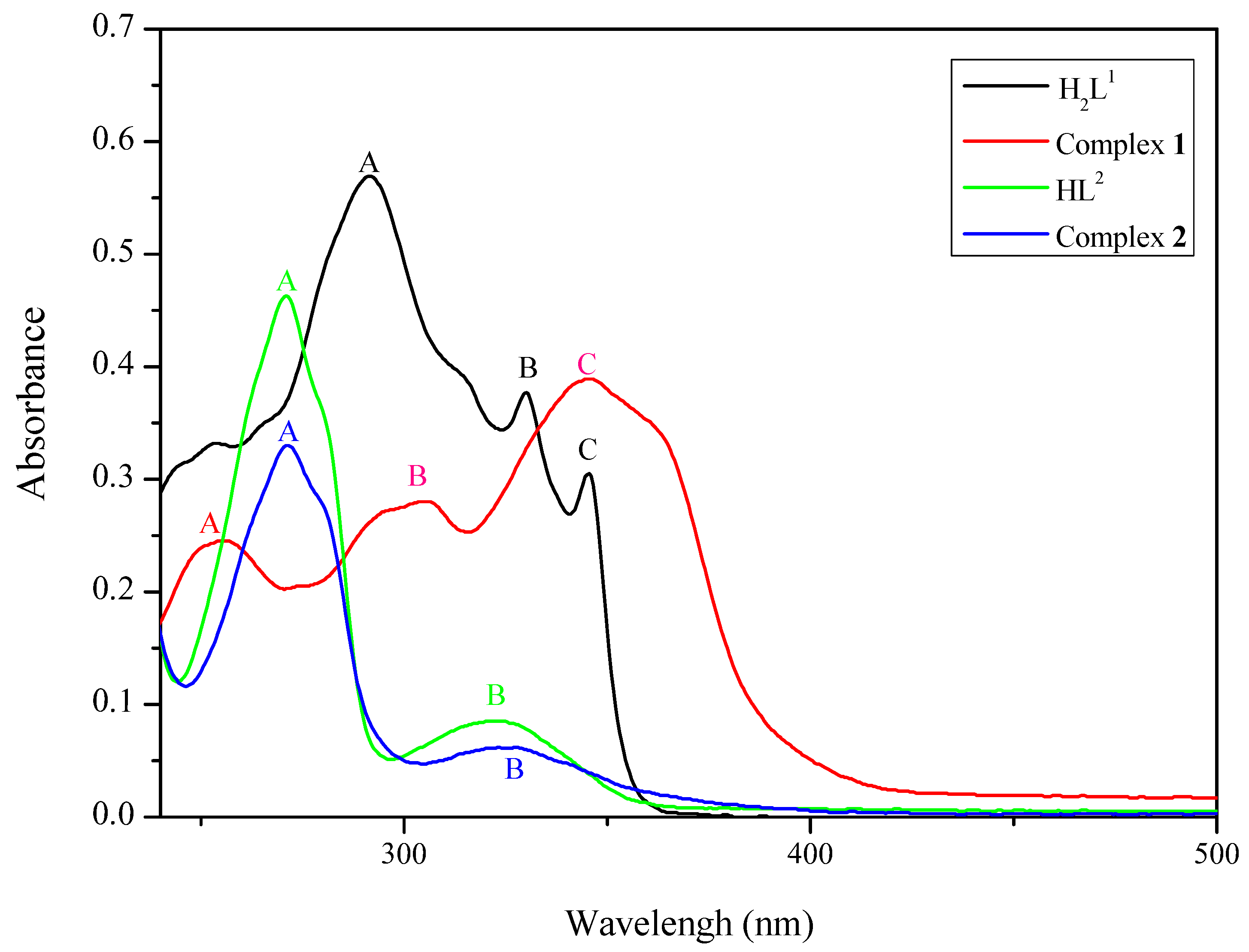
3.4. UV-VIS Spectra
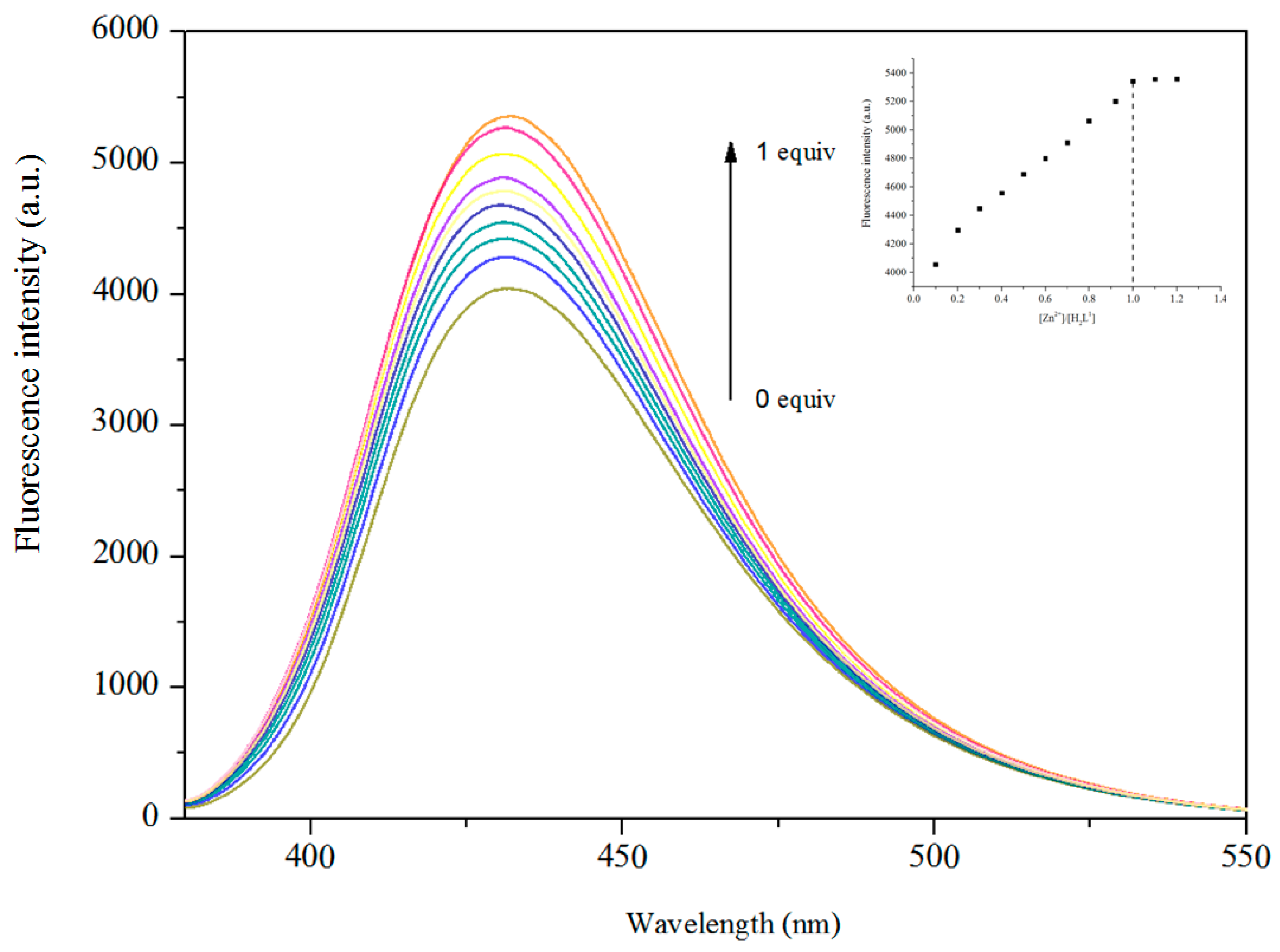
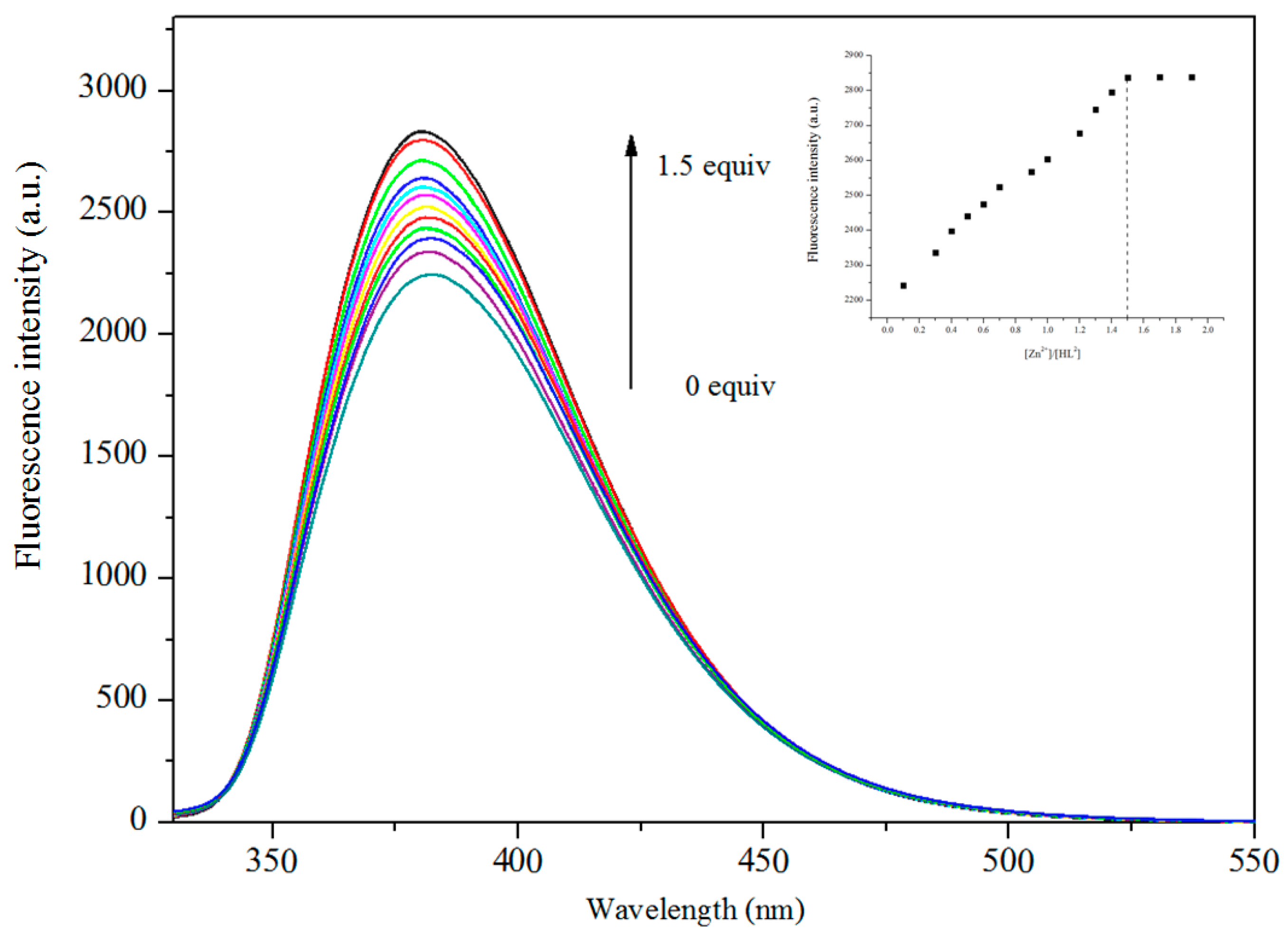
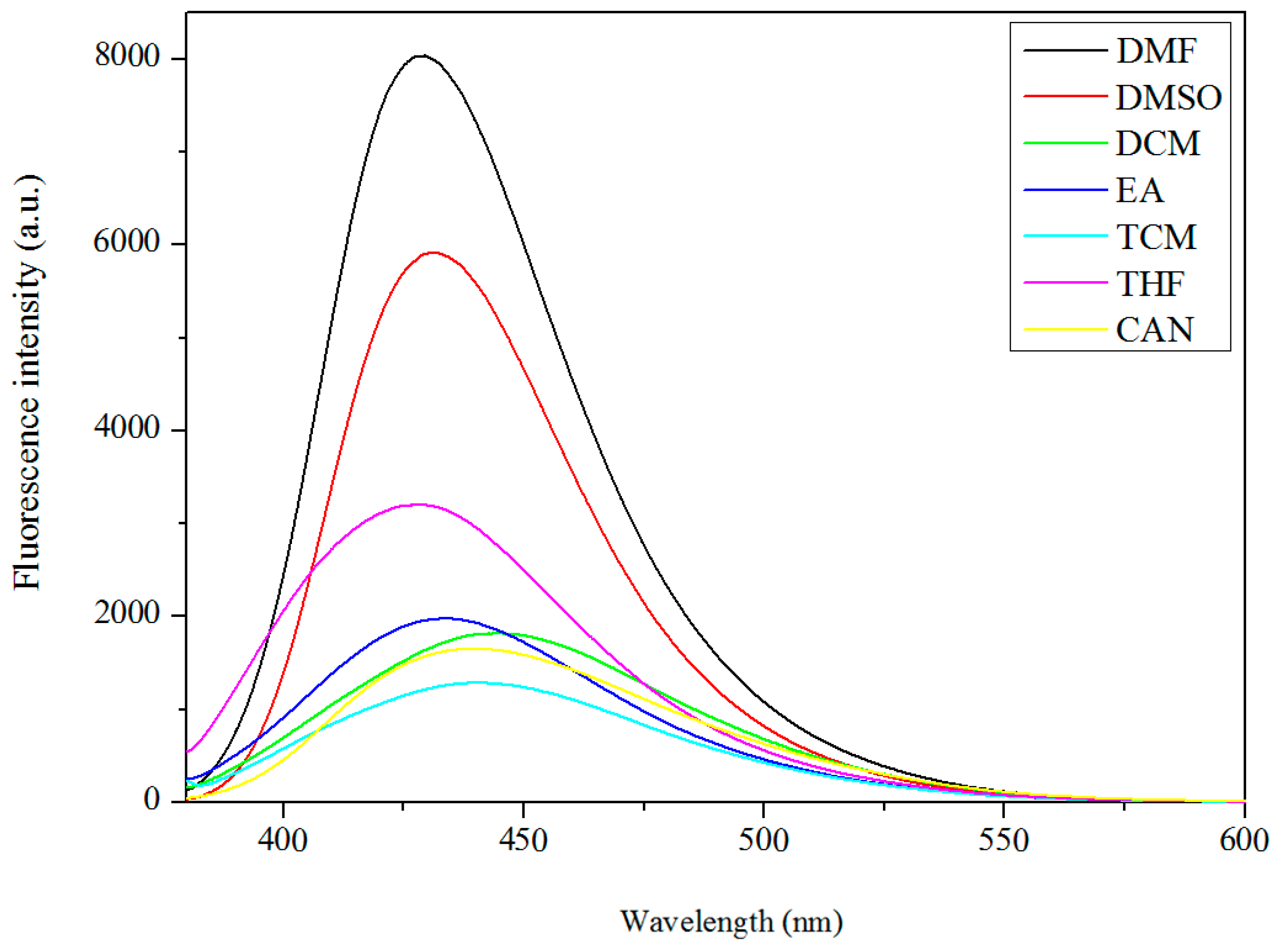
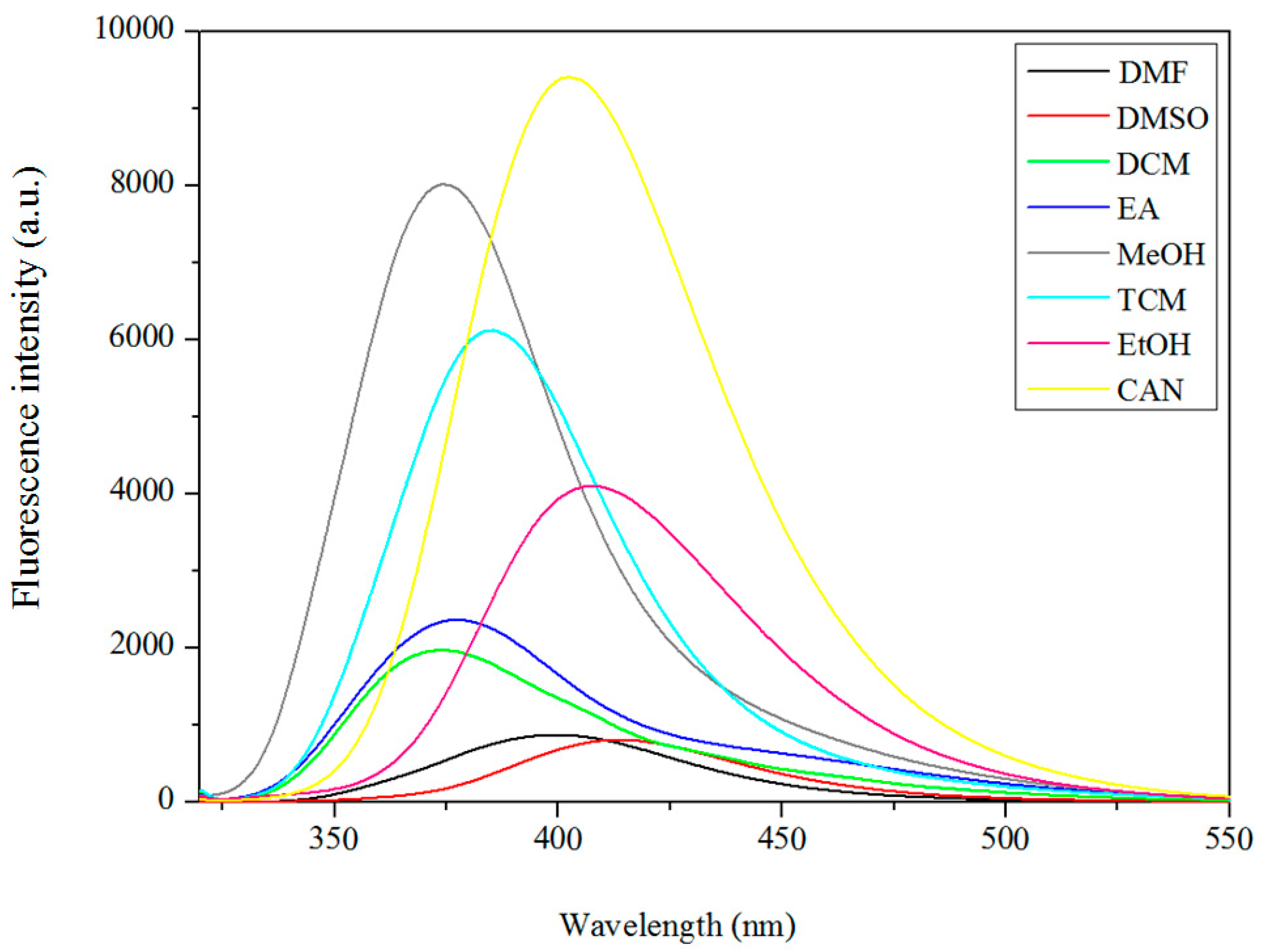
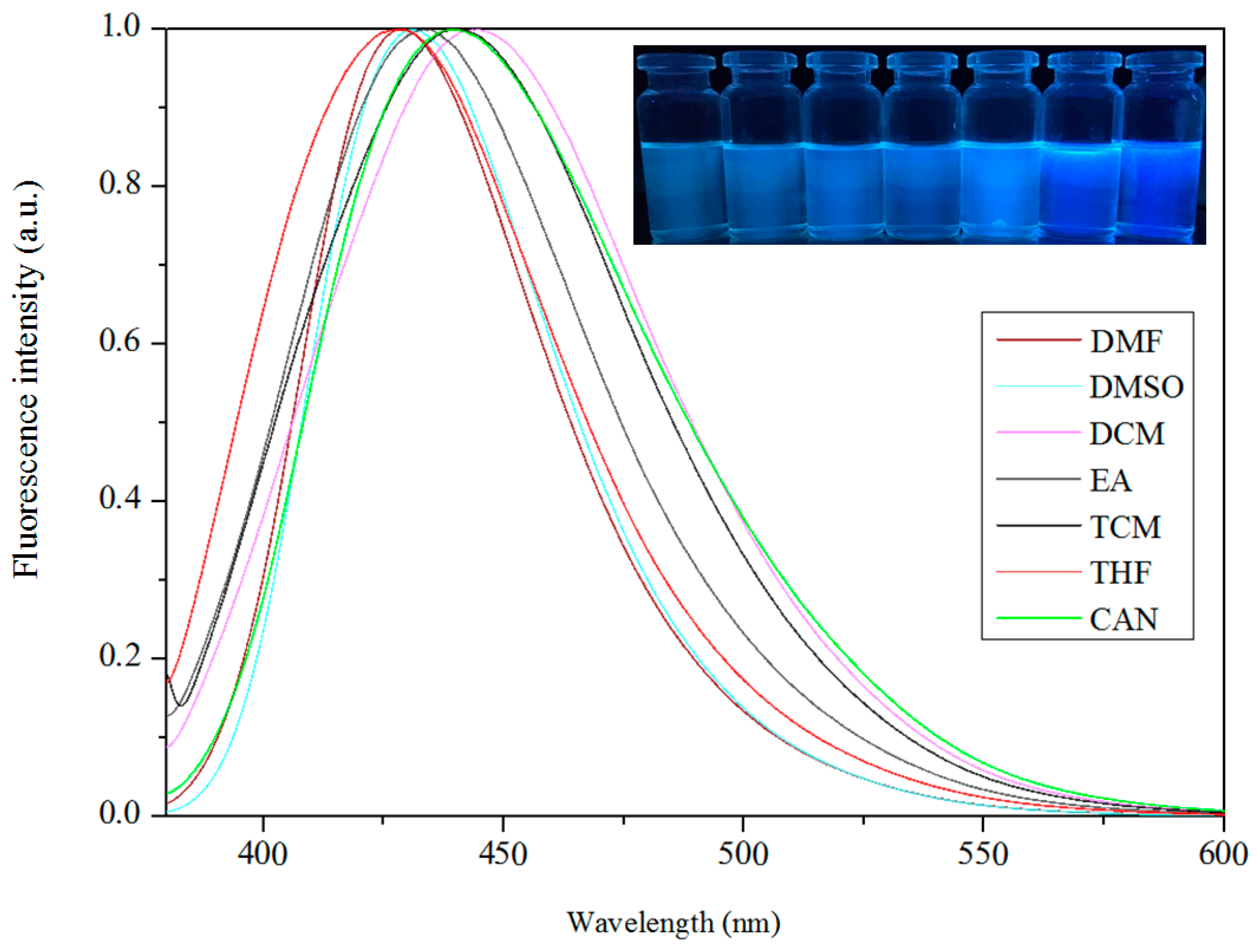
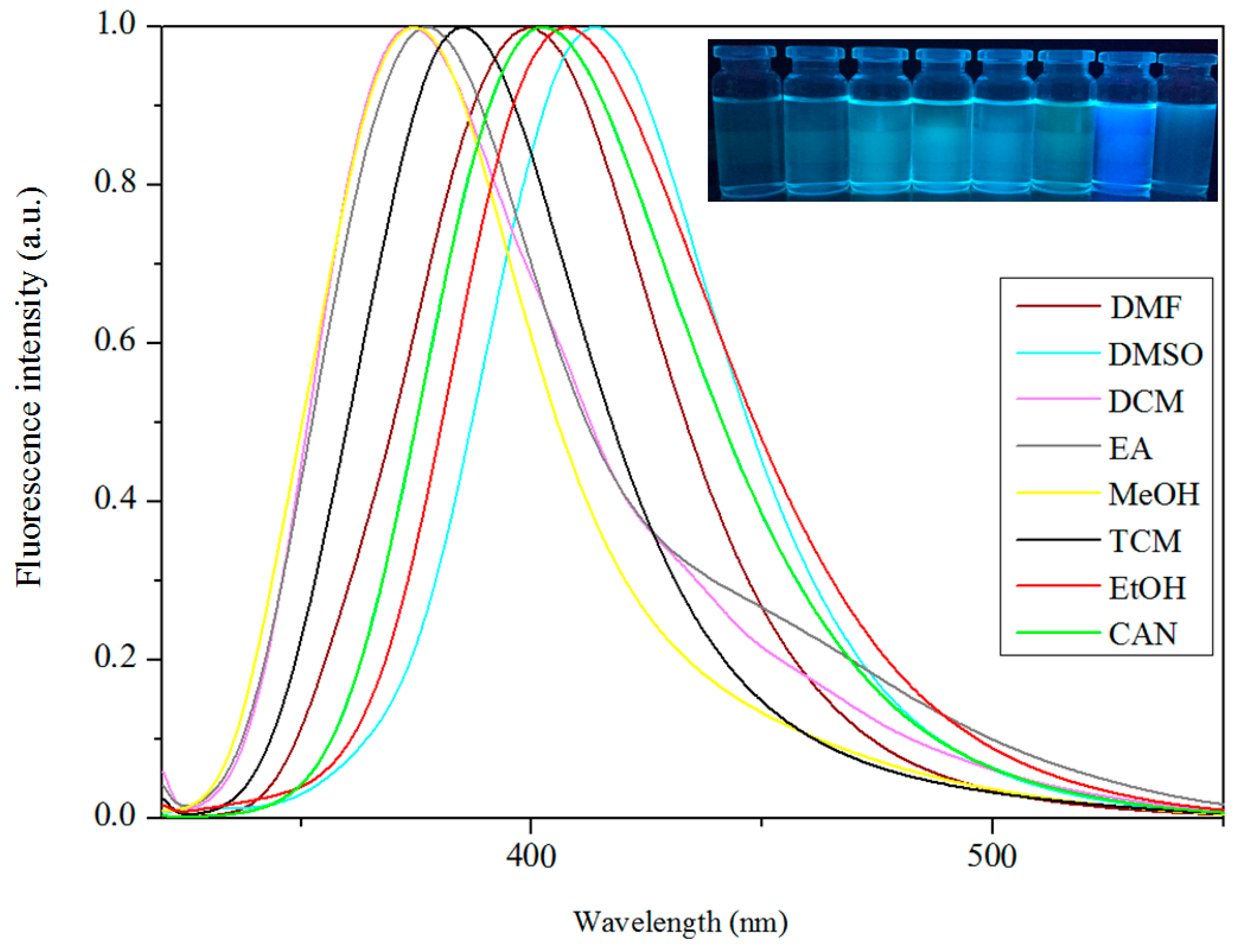
3.5. Fluorescence Properties
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
Author Contributions
Conflicts of Interest
References
- Dong, W.K.; Lan, P.F.; Zhou, W.M.; Zhang, Y. Salamo-type trinuclear and tetranuclear cobalt(II) complexes based on a new asymmetry salamo-type ligand: Syntheses, crystal structures and fluorescence properties. J. Coord. Chem. 2016, 65, 1272–1283. [Google Scholar] [CrossRef]
- Dong, X.Y.; Akogun, S.F.; Zhou, W.M.; Dong, W.K. Tetranuclear Zn(II) complex based on an asymmetrical Salamo-type chelating ligand: Synthesis, structural characterization, and fluorescence property. J. Chin. Chem. Soc. 2017, 64, 412–419. [Google Scholar] [CrossRef]
- Tao, C.H.; Ma, J.C.; Zhu, L.C.; Zhang, Y.; Dong, W.K. Heterobimetallic 3d–4f Zn(II)–Ln(III) (Ln = Sm, Eu, Tb and Dy) complexes with a N2O4 bisoxime chelate ligand and a simple auxiliary ligand Py: Syntheses, structures and luminescence properties. Polyhedron 2017, 128, 38–45. [Google Scholar] [CrossRef]
- Dong, Y.J.; Dong, X.Y.; Dong, W.K.; Zhang, Y.; Zhang, L.S. Three asymmetric Salamo-type copper(II) and cobalt(II) complexes: Syntheses, structures, fluorescent properties. Polyhedron 2017, 123, 305–315. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Dong, Y.J.; Zhao, L.; Zhu, L.C.; Sun, Y.X.; Zhang, Y. Two hetero-trinuclear Zn(II)-M(II) (M = Sr, Ba) complexes based on metallohost of mononuclear Zn(II) complex: Syntheses, structures and fluorescence properties. J. Coord. Chem. 2016, 69, 3231–3241. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, C.P.; Wang, F.; Peng, H.P.; Zhang, H.; Bai, Y.C. A new manganese(III) complex from bis(5-methylsalicylaldehyde)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidant activity and luminescence. J. Chin. Chem. Soc. 2015, 62, 1028–1034. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Li, Z.; Wu, M.C.; Chen, C.Y.; Zhang, J.W. Synthesis, crystal structure, antioxidation and DNA-binding properties of a dinuclear copper(II) complex with bis(N-salicylidene)-3-oxapentane-1, 5-diamine. J. Coord. Chem. 2014, 67, 3054–3066. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.; Yuan, J.K.; Wang, H.; Pan, G.L.; Fan, X.Y.; Kong, J. A zinc(II) complex with tris(2-(N-methyl)benzimidazlylmethyl)amine and salicylate: Synthesis, crystal structure, and DNA-binding. J. Coord. Chem. 2012, 65, 2839–2851. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Wang, H.; Wang, X.L.; Bai, Y.C.; Zhang, Y.H. Study on synthesis, crystal structure, antioxidant and DNA-binding of mono-, di- and poly-nuclear lanthanides complexes with bis(N-salicylidene)-3-oxapentane-1,5-diamine. J. Photochem. Photobiol. B Biol. 2014, 135, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Pan, G.L.; Kong, J.; Shi, F.; Wang, X.L. Two lanthanide(III) complexes based on the schiff base N,N-Bis(salicylidene)-1,5-diamino-3-oxapentane: Synthesis, characterization, DNA-binding properties, and antioxidation. Z. Anorg. Allg. Chem. 2014, 640, 2062–2071. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J.; Shi, F.; Zhang, Y.H.; Wang, X.L. Preparation, structure, DNA-binding properties, and antioxidant activities of a homodinuclear erbium(III) complex with a pentadentate Schiff base ligand. J. Chem. Res. 2014, 38, 211–217. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J. Synthesis, structure, antioxidation, and DNA-bindingstudies of a binuclear ytterbium(III) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. Res. Chem. Intermed. 2015, 41, 3375–3388. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.W.; Zhang, Y.H.; Yang, Z.H.; Wu, H.L. Gadolinium(III) and dysprosium(III) complexes with a Schiff base bis(N-salicylidene)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidation, and DNA-binding studies. J. Coord. Chem. 2015, 68, 1054–1071. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y.; Li, X.L. Four new nickel(II) complexes based on an asymmetric Salamo-type ligand: Synthesis, structure, solvent effect and electrochemical property. Inorg. Chim. Acta 2016, 445, 140–148. [Google Scholar] [CrossRef]
- Chai, L.Q.; Zhang, K.Y.; Tang, L.J.; Zhang, J.Y.; Zhang, H.S. Two mono- and dinuclear ni(II) complexes constructed from quinazoline-type ligands: Synthesis, x-ray structures, spectroscopic, electrochemical, thermal, and antimicrobial studies. Polyhedron 2017, 130, 100–107. [Google Scholar] [CrossRef]
- Yu, T.Z.; Zhang, K.; Zhao, Y.L.; Yang, C.H.; Zhang, H.; Qian, L.; Fan, D.W.; Dong, W.K.; Chen, L.L.; Qiu, Y.Q. Synthesis, crystal structure and photoluminescent properties of an aromatic bridged Schiff base ligand and its zinc complex. Inorg. Chim. Acta 2008, 361, 233–240. [Google Scholar] [CrossRef]
- Dong, Y.J.; Ma, J.C.; Zhu, L.C.; Dong, W.K.; Zhang, Y. Four 3d–4f heteromultinuclear zinc(II)–lanthanide(III) complexes constructed from a distinct hexadentate N2O2-type ligand: Syntheses, structures and photophysical properties. J. Coord. Chem. 2017, 70, 103–115. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.C.; Dong, W.K.; Zhu, L.C.; Zhang, Y. A novel Self–assembled nickel(II)–cerium(III) heterotetranuclear dimer constructed from N2O2-type bisoxime and terephthalic acid: Synthesis, structure and photophysical properties. Z. Anorg. Allg. Chem. 2016, 642, 834–839. [Google Scholar] [CrossRef]
- Dong, W.K.; Chen, X.; Sun, Y.X.; Yang, Y.H.; Zhao, L.; Xu, L.; Yu, T.Z. Synthesis, structure and spectroscopic properties of two new trinuclear nickel(II) clusters possessing solvent effect. Spectrochim. Acta Part A 2009, 74, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.K.; Li, G.; Wang, Z.K.; Dong, X.Y. A novel trinuclear cobalt(II) complex derived from an asymmetric Salamo-type N2O3 bisoxime chelate ligand: Synthesis, structure and optical properties. Spectrochimica Acta Part A 2014, 133, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.A.; Wang, C.Y.; Zhang, M.; Song, X.Q. Structures and magnetic properties of cyclic heterometallic tetranuclear clusters. Polyhedron 2017, 127, 278–286. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Dong, Y.J.; Zhu, L.C.; Zhang, Y. Di-and tetranuclear heterometallic 3d-4f cobalt(II)-lanthanide(III) complexes derived from a hexadentate bisoxime: Syntheses, structures and magnetic properties. Polyhedron 2016, 115, 228–235. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Xiao, Z.R.; Li, X.; Liu, Y.A. Four polynuclear complexes based on a versatile salicylamide salen-like ligand: Synthesis, structural variations and magnetic properties. Inorg. Chim. Acta 2015, 438, 232–244. [Google Scholar] [CrossRef]
- Liu, P.P.; Wang, C.Y.; Zhang, M.; Song, X.Q. Pentanuclear sandwich-type ZnII-LnIII clusters based on a new Salen-like salicylamide ligand: Structure, near-infrared emission and magnetic properties. Polyhedron 2017, 129, 133–140. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Nine self–assembled nickel(II)–lanthanide(III) heterometallic complexes constructed from a Salamo–type bisoxime and bearing N- or O-donor auxiliary ligand: Syntheses, structures and magnetic properties. New J. Chem. 2016, 40, 6998–7010. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Self-assembled zinc(II)-lanthanide(III) heteromultinuclear complexes constructed from 3-MeOsalamo ligand: Syntheses, structures and luminescent properties. Cryst. Growth Des. 2016, 16, 6903–6914. [Google Scholar] [CrossRef]
- Chen, L.; Dong, W.K.; Zhang, H.; Zhang, Y.; Sun, Y.X. Structural variation and luminescence properties of tri- and dinuclear CuII and ZnII complexes constructed from a naphthalenediol-based bis(Salamo)-type ligand. Cryst. Growth Des. 2017, 17, 3636–3648. [Google Scholar] [CrossRef]
- Song, X.Q.; Peng, Y.J.; Chen, G.Q.; Wang, X.R.; Liu, P.P.; Xu, W.Y. Substituted group-directed assembly of Zn(II) coordination complexes based on two new structural related pyrazolone based Salen ligands: Syntheses, structures and fluorescence properties. Inorg. Chim. Acta. 2015, 427, 13–21. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, J.; Zhang, Y.; Li, N. Novel multinuclear transition metal(II) complexes based on an asymmetric Salamo-type ligand: Syntheses, structure characterizations and fluorescent properties. Inorg. Chim. Acta. 2016, 444, 95–102. [Google Scholar] [CrossRef]
- Dong, W.K.; Akogun, S.F.; Zhang, Y.; Dong, X.Y. A reversible “turn-on” fluorescent sensor for selective detection of Zn2+. Sensors Actuators B Chem. 2017, 238, 723–734. [Google Scholar] [CrossRef]
- Dong, Y.J.; Li, X.L.; Zhang, Y.; Dong, W.K. A highly selective visual and fluorescent sensor for Pb2+ and Zn2+ and crystal structure of Cu2+ complex based-on a novel single-armed Salamo-type bisoxime. Supramol. Chem. 2017, 29, 518–527. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, X.L.; Wang, L.; Zhang, Y.; Ding, Y.J. A new application of Salamo-type bisoximes: as a relay-sensor for Zn2+/Cu2+ and its novel complexes for successive sensing of H+/OH‒. Sens. Actuators B Chem. 2016, 229, 370–378. [Google Scholar] [CrossRef]
- Zhao, L.; Dang, X.T.; Chen, Q.; Zhao, J.X.; Wang, L. Synthesis, crystal structure and spectral properties of a 2D supramolecular copper(II) complex with 1-(4-{[(E)-3-ethoxyl-2-hydroxybenzylidene]amino}phenyl)ethanone oxime. Synth. React. Inorg. Met. -Org. Nano-Met. Chem. 2013, 43, 1241–1246. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, L.; Zhao, T.H.; Liu, S.H.; Liu, G.H.; Dong, X.T. Synthesis and crystal structure of a 3D supramolecular copper(II) complex with 1-(3-{[(E)-3-bromo-5-chloro-2-hydroxybenzylidene]amino}phenyl) ethanone oxime. Synth. React. Inorg. Met. -Org. Nano-Met. Chem. 2013, 43, 509–513. [Google Scholar] [CrossRef]
- Sun, Y.X.; Dong, W.K.; Wang, L.; Zhao, L.; Yang, Y.H. Synthesis and crystal structure of nickel(II) cluster with salen-type bisoxime ligand. Chinese J. Inorg. Chem. 2009, 25, 1478–1482. [Google Scholar]
- Sun, Y.X.; Zhang, S.T.; Ren, Z.L.; Dong, X.Y.; Wang, L. Synthesis, characterization, and crystal structure of a new supramolecular CdII complex with halogen-substituted salen-type bisoxime. Synth. React. Inorg. Met.-Org. Nano-Met Chem. 2013, 43, 995–1000. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, X.Y.; Zhao, M.M.; Li, G.; Dong, X.Y. Syntheses and crystal structures of 5-Methoxy-6′-hydroxy-2,2′-[ethylenedioxybis(nitrilomethylidyne)]diphenol and its tetranuclear zinc(II) complex. Chin. J. Inorg. Chem. 2014, 30, 710–716. [Google Scholar]
- Zhou, J.A.; Tang, X.L.; Cheng, J.; Ju, Z.H.; Yang, L.Z.; Liu, W.S.; Chen, C.Y.; Bai, D.C. An 1 3,4-oxadiazole-based off-on fluorescent chemosensor for Zn2+ in aqueous solution and imaging application in living cells. Dalton Trans. 2012, 41, 10626–10632. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Taniguchi, T.; Dong, W.K.; Masubuchi, S.; Nabeshima, T. Oxime-Based Salen-Type Tetradentate Ligands with High Stability against Imine Metathesis Reaction. J. Org. Chem. 2005, 70, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Dong, W.K.; Nabeshima, T. Octanuclear zinc(II) and cobalt(II) clusters produced by cooperative tetrameric assembling of oxime chelate ligands. Inorg. Chem. 2006, 454, 677–4684. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Cheng, G.Q.; Liu, Y.A. Enhanced Tb(III) luminescence by d10 transition metal coordination. Inorg. Chim. Acta 2016, 450, 386–394. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Karroonnirun, O.; Wilson, S.J. Ring-Opening Polymerization of Cyclic Esters and Trimethylene Carbonate Catalyzed by Aluminum Half-Salen Complexes. Inorg. Chem. 2011, 50, 6775–6787. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Karroonnirun, O. Stereoselective Ring-Opening Polymerization of rac-Lactides Catalyzed by Chiral and Achiral Aluminum Half-Salen Complexes. Organometallics 2010, 29, 5627–5634. [Google Scholar] [CrossRef]
- Dong, Y.; Li, F.J.; Jiang, X.X.; Song, F.Y.; Cheng, Y.X.; Zhu, C.J. Na+ triggered fluorescence sensors for Mg2+ detection based on a coumarin salen moiety. Org. Lett. 2011, 9, 2252–2255. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.K.; Wang, Z.K.; Li, G.; Zhao, M.M.; Dong, X.Y.; Liu, S.H. Syntheses, crystal structures, and properties of a Salamo-type tetradentate chelating ligand and its pentacoordinated copper(II) complex. Z. Anorg. Allg. Chem. 2013, 639, 2263–2268. [Google Scholar] [CrossRef]
- Dong, W.K.; Feng, J.H.; Yang, X.Q. Synthesis and crystal structure of a five-coordinated cu(II) dimer with 4,4′-Dibromo-2,2′-[ethylenedioxybis(nitrilomethylidyne)]diphenol. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2007, 37, 189–192. [Google Scholar] [CrossRef]
- Ma, J.C.; Dong, X.Y.; Dong, W.K.; Zhang, Y.; Zhu, L.C.; Zhang, J.T. An unexpected dinuclear Cu(II) complex with a bis(Salamo) chelating ligand: synthesis, crystal structure, and photophysical properties. J. Coord. Chem. 2016, 69, 149–159. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97. In Program for the Solution and the Refinement of Crystal Structures; University of Gottingen: Germany, 1997. [Google Scholar]
- Dong, W.K.; Zhang, F.; Li, N.; Xu, L.; Zhang, Y.; Zhang, J.; Zhu, L.C. Trinuclear cobalt(II) and zinc(II) salamo-type complexes: Syntheses, crystal structures, and fluorescent properties. Z. Anorg. Allg. Chem. 2016, 642, 532–538. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, L.S.; Sun, Y.X.; Zhao, M.M.; Li, G.; Dong, X.Y. Synthesis, crystal structure and spectroscopic properties of a supramolecular zinc(II) complex with N2O2 coordination sphere. Spectrochim. Acta Part A 2014, 121, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, L.H.; Zhang, J.T.; Akogun, S.F.; Wang, L.; Dong, W.K. Four homo- and hetero-bismetallic 3d/3d-2s complexes constructed from a naphthalenediol-based acyclic bis(salamo)-type tetraoxime ligand. Polyhedron 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Li, L.H.; Dong, W.K.; Zhang, Y.; Akogun, S.F.; Xu, L. Syntheses, structures and catecholase activities of homo-and hetero-trinuclear cobalt(II) complexes constructed from an acyclic naphthalenediol-based bis(salamo)-type ligand. Appl. Organomet. Chem. [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Konno, T.; Tokuda, K.; Sakurai, J.; Okamoto, K.I. Five-Coordinate Geometry of Cadmium(II) with Octahedral Bidentate-S,S Complex-Ligand cis(S)-[Co(aet)2(en)]+ (aet = 2-aminoethanethiolate): Synthesis, Crystal Structures and Interconversion of S-Bridged CoIIICdII Polynuclear Complexes. Bull. Chem. Soc. Jpn. 2000, 73, 2767–2773. [Google Scholar] [CrossRef]
- Chai, L.Q.; Tang, L.J.; Chen, L.C.; Huang, J.J. Structural, spectral, electrochemical and DFT studies of two mononuclear manganese(II) and zinc(II) complexes. Polyhedron 2017, 122, 228–240. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.C.; Ma, J.C.; Zhang, Y.; Zhang, J.; Dong, W.K. Syntheses, structures and spectral properties of mononuclear CuII and dimeric ZnII complexes based on an asymmetric Salamo-type N2O2 ligand. Z. Anorg. Allg. Chem. 2015, 641, 2520–2524. [Google Scholar] [CrossRef]
- Boggs, J.M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim. Biophys. Acta 1987, 906, 353–404. [Google Scholar] [CrossRef]
- Karas, L.J.; Batista, P.R.; Viesser, R.V.; Tormena, C.F.; Rittner, R.; de Oliveira, P.R. Trends of intramolecular hydrogen bonding in substituted alcohols: a deeper investigation. Phys. Chem. Chem.Phys. 2017, 19, 16904–16913. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.P.; Simanek, E.E.; Whitesides, G.M. Self-Assembly through Hydrogen Bonding: Peripheral Crowding-A new strategy for the preparation of stable supramolecular aggregates based on parallel, connected CA3.cntdot.M3 rosettes. J. Am. Chem. Soc. 1994, 116, 4326–4340. [Google Scholar]
- Yabuuchi, K.; Marfo-Owusu, E.; Kato, T. A new urea gelator: incorporation of intra- and intermolecular hydrogen bonding for stable 1D self-assembly. Org. Biomol. Chem. 2003, 1, 3464–3469. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Q.; Huang, J.J.; Zhang, J.Y.; Li, Y.X. Two 1-D and 2-D cobalt(II) complexes: Synthesis, crystal structures, spectroscopic and electrochemical properties. J. Coord. Chem. 2015, 68, 1224–1237. [Google Scholar] [CrossRef]
- Sun, Y.X.; Wang, L.; Dong, X.Y.; Ren, Z.L.; Meng, W.S. Synthesis, characterization, and crystal structure of a supramolecular CoII complex containing Salen-type bisoxime. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 599–603. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L. Synthesis and crystal structure of supramolecular copper(II) complex based on N2O2 coordination Sphere. Asian J. Chem. 2015, 4, 1424–1426. [Google Scholar] [CrossRef]
- Liu, P.P.; Sheng, L.; Song, X.Q.; Xu, W.Y.; Liu, Y.A. Synthesis, structure and magnetic properties of a new one dimensional manganese coordination polymer constructed by a new asymmetrical ligand. Inorg. Chim. Acta 2015, 434, 252–257. [Google Scholar] [CrossRef]
- Wang, B.J.; Dong, W.K.; Zhang, Y.; Akogun, S.F. A novel relay-sensor for highly sensitive and selective detection of Zn2+/Pic− and fluorescence on/off switch response of H+/OH‒. Sens. Actuators B Chem. 2017, 247, 254–264. [Google Scholar] [CrossRef]
- Akine, S.; Morita, Y.; Utsuno, F.; Nabeshima, T. Multiple folding structures mediated by metal coordination of acyclic multidentate ligand. Inorg. Chem. 2009, 48, 10670–10678. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Liu, P.P.; Liu, Y.A.; Zhou, J.J.; Wang, X.L. Two dodecanuclear heterometallic [Zn6Ln6] clusters constructed by a multidentate salicylamide salen-like ligand: Synthesis, structure, luminescence and magnetic properties. Dalton Trans. 2016, 45, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Wang, Y.Y.; Xu, K.Z.; Zhu, H.L.; Liu, P.; Shi, Q.Z. Crystal structures, bioactivities and fluorescent properties of four diverse complexes with a new symmetric benzimidazolic ligand. Polyhedron 2008, 27, 3529–3536. [Google Scholar] [CrossRef]
- Che, G.B.; Liu, C.B.; Liu, B.; Wang, Q.W.; Xu, Z.L. Syntheses, structures and photoluminescence of a series of metal-organic complexes with 1,3,5-benzenetricarboxylate and pyrazino[2,3-f][1,10]-phenanthroline ligands. Cryst. Eng. Commun. 2008, 10, 184–191. [Google Scholar] [CrossRef]

| Complex | 1 | 2 |
|---|---|---|
| Formula | C26H24N2O9Zn | C29H39Cl3N4O16Zn3 |
| Formula weight | 573.84 | 1002.16 |
| Temperature (K) | 173.00(10) | 292.38(10) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Triclinic |
| Space group | P21/n | P–1 |
| Unit cell dimensions | ||
| a (Å) | 12.811(3) | 13.1694(13) |
| b (Å) | 13.4725(9) | 13.3568(13) |
| c (Å) | 15.393(3) | 16.1452(14) |
| α (°) | 90 | 94.664(8) |
| β (°) | 107.665(19) | 113.074(10) |
| γ (°) | 90 | 112.722(9) |
| V (Å3) | 2531.6(8) | 2317.6(4) |
| Z | 4 | 2 |
| Dc (g cm‒3) | 1.506 | 1.430 |
| μ (mm‒1) | 1.028 | 1.775 |
| F (000) | 1184 | 1012 |
| Crystal size (mm) | 0.24 × 0.19 × 0.17 | 0.22 × 0.18 × 0.14 |
| θ Range (°) | 3.338–25.008 | 3.34–26.02 |
| Index ranges | −13 ≤ h ≤ 15, | −10 ≤ h ≤ 16, |
| −16 ≤ k ≤ 14, | −16 ≤ k ≤ 16, | |
| −14 ≤ l ≤ 18 | −19 ≤ l ≤ 15 | |
| Reflections collected | 8334 | 16,912 |
| Independent reflections | 4446 | 9102 |
| Rint | 0.0996 | 0.0522 |
| Completeness | 99.6% | 99.79% |
| Data/restraints/parameters | 4446/16/349 | 9102/1/502 |
| GOF | 1.001 | 0.963 |
| Final R1, wR2 indices | 0.0581/0.0934 | 0.0633/0.1473 |
| R1, wR2 indices (all data) | 0.0721/0.1598 | 0.1236/0.1774 |
| Largest differences | 0.926/‒0.772 | 0.873/‒0.587 |
| peak and hole (e Å‒3) | ||
| Bond | |||
|---|---|---|---|
| Zn1-O3 | 1.940(5) | Zn1-N1 | 2.187(7) |
| Zn1-O6 | 1.994(5) | Zn1-N2 | 2.036(7) |
| Zn1-O9 | 2.049(6) | ||
| Angles | |||
| O3-Zn1-O6 | 97.1(2) | O3-Zn1-O9 | 111.5(2) |
| O3-Zn1-N1 | 86.7(2) | O3-Zn1-N2 | 125.1(3) |
| O6-Zn1-O9 | 91.7(2) | O6-Zn1-N1 | 175.8(2) |
| O6-Zn1-N2 | 87.0(2) | N1-Zn1-O9 | 88.5(3) |
| N2-Zn1-O9 | 123.2(3) | N2-Zn1-N1 | 89.4(3) |
| D-H⋅⋅⋅A | D-H | H⋅⋅⋅A | D⋅⋅⋅A | D-H⋅⋅⋅A |
|---|---|---|---|---|
| C10-H10 ⋅⋅⋅O2 | 0.93 | 2.30 | 2.670(11) | 103 |
| C11-H11A⋅⋅⋅O9 | 0.97 | 2.59 | 3.421(10) | 143 |
| C13-H13⋅⋅⋅O7 | 0.93 | 2.25 | 2.636(10) | 104 |
| C25-H25B ⋅⋅⋅O6 | 0.97 | 2.46 | 3.055(14) | 119 |
| C2-H2 ⋅⋅⋅O4 | 0.93 | 2.58 | 3.454(12) | 158 |
| C11-H11B ⋅⋅⋅O8 | 0.97 | 2.49 | 3.281(9) | 139 |
| C12-H12A⋅⋅⋅O1 | 0.97 | 2.51 | 3.424(12) | 157 |
| C12-H12B⋅⋅⋅O3 | 0.97 | 2.56 | 3.494(11) | 161 |
| C21-H21⋅⋅⋅O3 | 0.93 | 2.54 | 3.277(11) | 137 |
| O9-H9⋅⋅⋅O8 | 0.86 | 1.99 | 2.725(10) | 144 |
| C26-H26B⋅⋅⋅Cg3 | 0.96 | 2.99 | 3.398(17) | 107 |
| Bond | |||
|---|---|---|---|
| Zn1-O2 | 2.017(4) | Zn1-O9 | 2.203(4) |
| Zn1-O10 | 2.191(5) | Zn1-O11 | 2.112(4) |
| Zn1-N1 | 2.218(5) | Zn1-N2 | 2.077(5) |
| Zn2-O1 | 2.269(4) | Zn2-O2 | 2.006(4) |
| Zn2-O5 | 2.336(4) | Zn2-O6 | 2.001(4) |
| Zn2-O12 | 2.032(4) | Zn2-O13 | 2.022(4) |
| Zn3-O6 | 2.006(4) | Zn3-O14 | 2.147(4) |
| Zn3-O15 | 2.209(5) | Zn3-O16 | 2.160(5) |
| Zn3-N3 | 2.206(5) | Zn3-N4 | 2.085(5) |
| Angles | |||
| O2-Zn1-O9 | 155.36(16) | O2-Zn1-O10 | 96.75(16) |
| O2-Zn1-O11 | 95.76(15) | O2-Zn1-N1 | 81.53(17) |
| O2-Zn1-N2 | 99.03(17) | O9-Zn1-N1 | 94.48(18) |
| O10-Zn1-O9 | 59.04(16) | O10-Zn1-N1 | 93.23(19) |
| O11-Zn1-O9 | 89.09(16) | O11-Zn1-O10 | 89.72(18) |
| O11-Zn1-N1 | 176.21(18) | N2-Zn1-O9 | 105.28(18) |
| N2-Zn1-O10 | 164.21(18) | N2-Zn1-O11 | 88.08(17) |
| N2-Zn1-N1 | 89.72(18) | O1-Zn2-O5 | 84.12(15) |
| O2-Zn2-O1 | 74.99(15) | O2-Zn2-O5 | 85.59(16) |
| O2-Zn2-O12 | 97.76(16) | O2-Zn2-O13 | 100.51(15) |
| O6-Zn2-O1 | 84.90(16) | O6-Zn2-O2 | 152.58(15) |
| O6-Zn2-O5 | 73.79(16) | O6-Zn2-O12 | 101.12(15) |
| O6-Zn2-O13 | 96.80(16) | O12-Zn2-O1 | 91.08(15) |
| O12-Zn2-O5 | 173.26(15) | O13-Zn2-O1 | 171.40(15) |
| O13-Zn2-O5 | 88.25(16) | O13-Zn2-O12 | 96.84(15) |
| O6-Zn3-O14 | 92.82(15) | O6-Zn3-O15 | 95.64(16) |
| O6-Zn3-O16 | 155.32(16) | O6-Zn3-N3 | 82.60(17) |
| O6-Zn3-N4 | 99.71(18) | O14-Zn3-O15 | 88.68(17) |
| O14-Zn3-O16 | 88.30(16) | O14-Zn3-N3 | 174.96(19) |
| O16-Zn3-O15 | 59.72(16) | O16-Zn3-N3 | 96.74(19) |
| N3-Zn3-O15 | 93.87(19) | N4-Zn3-O14 | 88.59(17) |
| N4-Zn3-O15 | 164.52(19) | N4-Zn3-O16 | 104.96(18) |
| N4-Zn3-N3 | 90.08(19) |
| D-H⋅⋅⋅A | D-H | H⋅⋅⋅A | D⋅⋅⋅A | D-H⋅⋅⋅A |
|---|---|---|---|---|
| N2-H2A ⋅⋅⋅O13 | 0.90 | 2.06 | 2.890(7) | 153 |
| N4-H4A⋅⋅⋅O12 | 0.90 | 2.10 | 2.945(8) | 155 |
| C8-H8B⋅⋅⋅O9 | 0.90 | 2.03 | 2.860(7) | 154 |
| C22-H22B ⋅⋅⋅O16 | 0.90 | 2.13 | 2.965(8) | 153 |
| N2-H2B ⋅⋅⋅O9 | 0.97 | 2.33 | 3.253(9) | 158 |
| N4-H4B ⋅⋅⋅O16 | 0.97 | 2.38 | 3.300(8) | 157 |
| C29-H29⋅⋅⋅O15 | 0.98 | 2.35 | 3.19(2) | 145 |
| N2-H2A ⋅⋅⋅O13 | 0.90 | 2.06 | 2.890(7) | 153 |
| C29-Cl2⋅⋅⋅Cg7 | 1.687 | 3.979 | 4.652(18) | 102.7 |
| C10-H10B ⋅⋅⋅Cg6 | 0.96 | 2.69 | 3.481(9) | 140 |
| Compound | c | A(ε) | B(ε) | C(ε) |
|---|---|---|---|---|
| H2L1 | 1.0 × 10‒5 | 291 (5.7 × 10‒4) | 329 (3.7 × 10‒4) | 345 (3.0 × 10‒4) |
| Complex 1 | 1.0 × 10‒5 | 252 (2.4 × 10‒4) | 304 (2.8 × 10‒4) | 344 (3.9 × 10‒4) |
| HL2 | 1.0 × 10‒5 | 270 (4.6 × 10‒4) | 322 (0.8 × 10‒4) | |
| Complex 2 | 1.0 × 10‒5 | 271 (3.3 × 10‒4) | 325 (0.6 × 10‒4) |
| Compound | DMF | DMSO | DCM | EA | TCM | CAN | THF | MeOH | EtOH |
|---|---|---|---|---|---|---|---|---|---|
| Complex 1 | 429 | 431 | 440 | 433 | 439 | 439 | 428 | ||
| Complex 2 | 399 | 414 | 372 | 377 | 384 | 402 | 373 | 407 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.-Y.; Gao, L.; Wang, F.; Zhang, Y.; Dong, W.-K. Tri- and Mono-Nuclear Zinc(II) Complexes Based on Half- and Mono-Salamo Chelating Ligands. Crystals 2017, 7, 267. https://doi.org/10.3390/cryst7090267
Dong X-Y, Gao L, Wang F, Zhang Y, Dong W-K. Tri- and Mono-Nuclear Zinc(II) Complexes Based on Half- and Mono-Salamo Chelating Ligands. Crystals. 2017; 7(9):267. https://doi.org/10.3390/cryst7090267
Chicago/Turabian StyleDong, Xiu-Yan, Lei Gao, Fei Wang, Yang Zhang, and Wen-Kui Dong. 2017. "Tri- and Mono-Nuclear Zinc(II) Complexes Based on Half- and Mono-Salamo Chelating Ligands" Crystals 7, no. 9: 267. https://doi.org/10.3390/cryst7090267