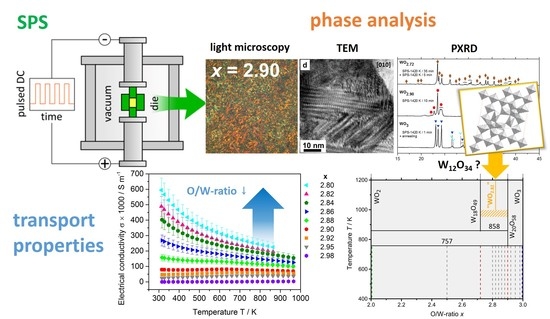
Spark Plasma Sintering of Tungsten Oxides WOx (2.50 ≤ x ≤ 3): Phase Analysis and Thermoelectric Properties
Abstract
:1. Introduction
2. Results & Discussion
2.1. Spark Plasma Preparation
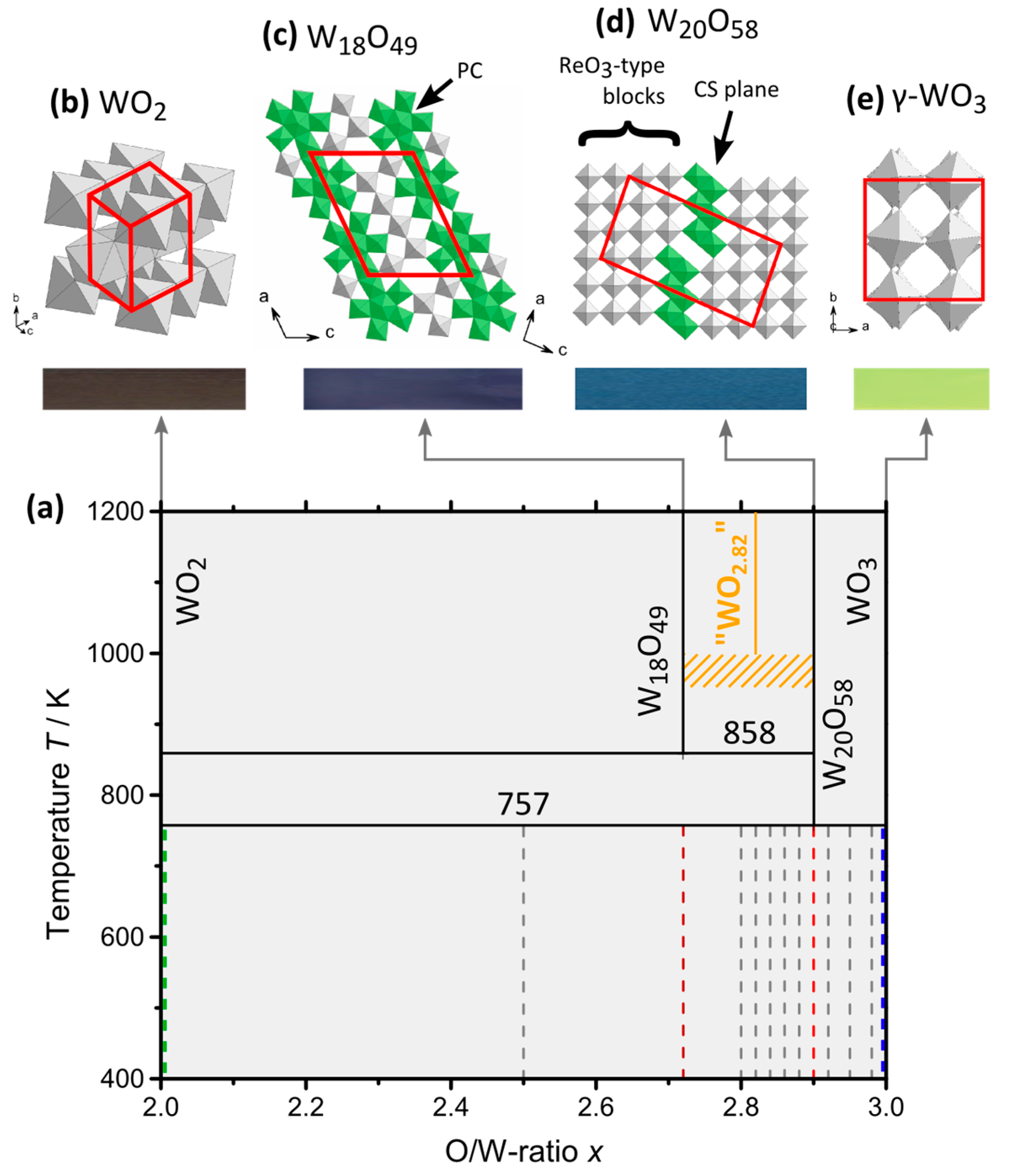
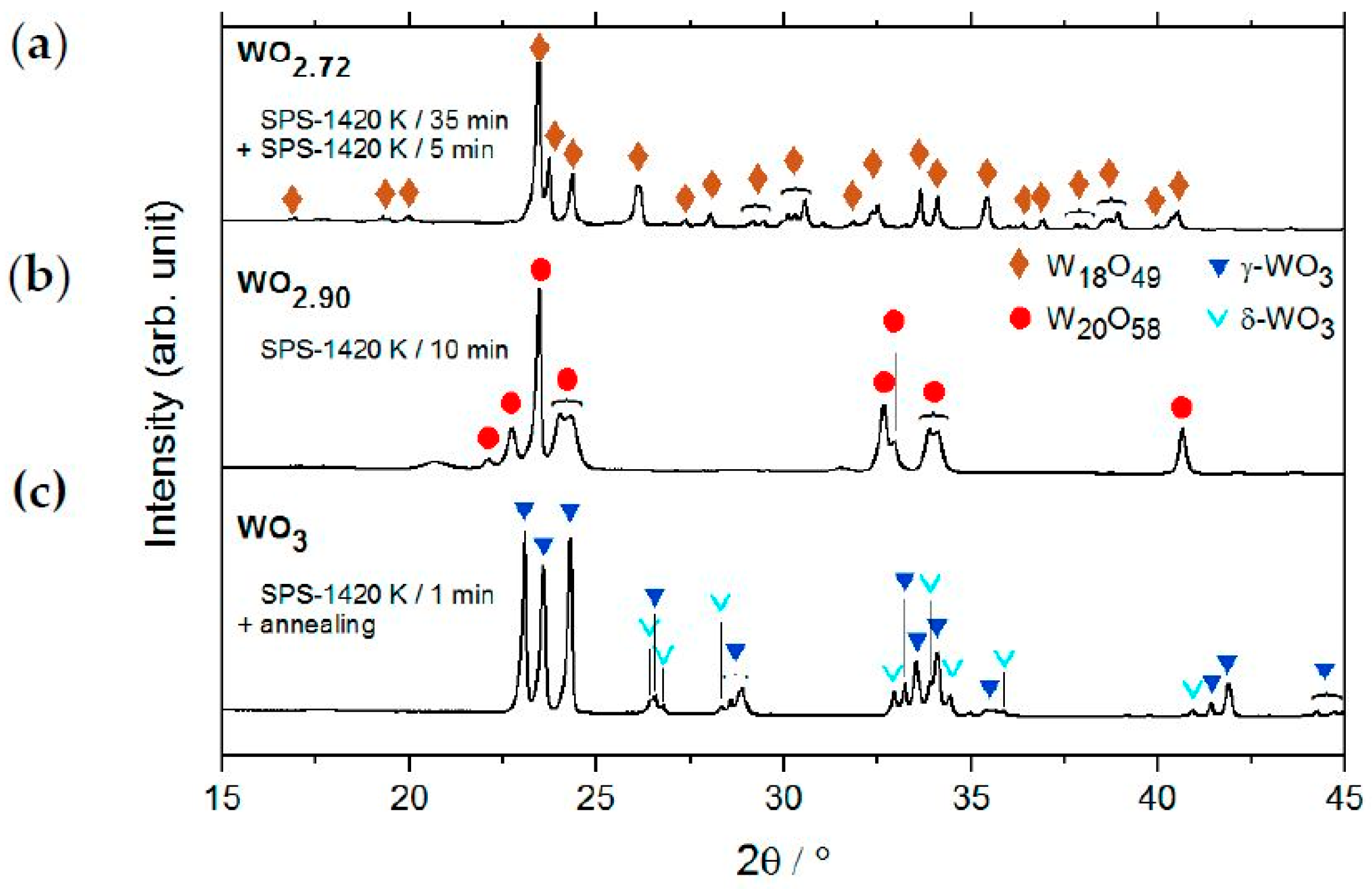
2.2. Single-Phase Materials WO2, WO2.72, WO2.90 and WO3
2.2.1. Crystal Structure on Atomic Resolution
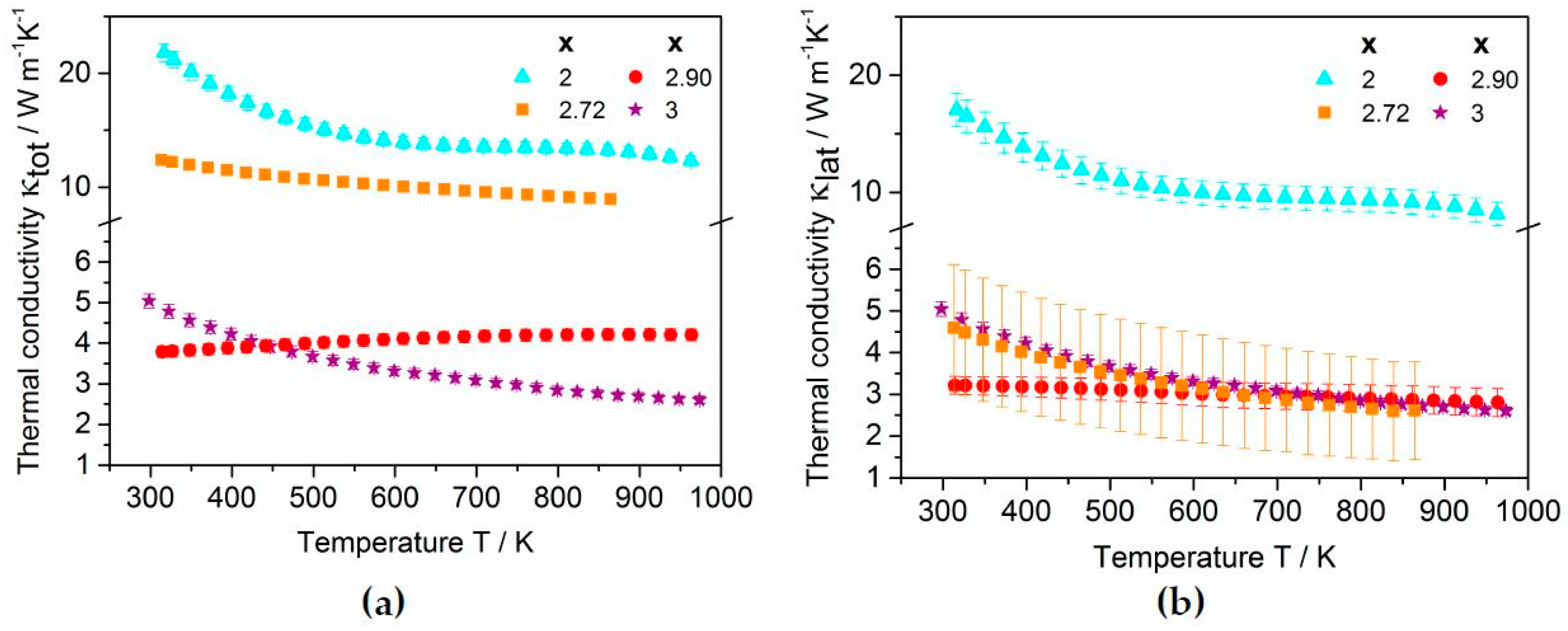
2.2.2. Thermoelectric Properties
2.3. Compositions WOx
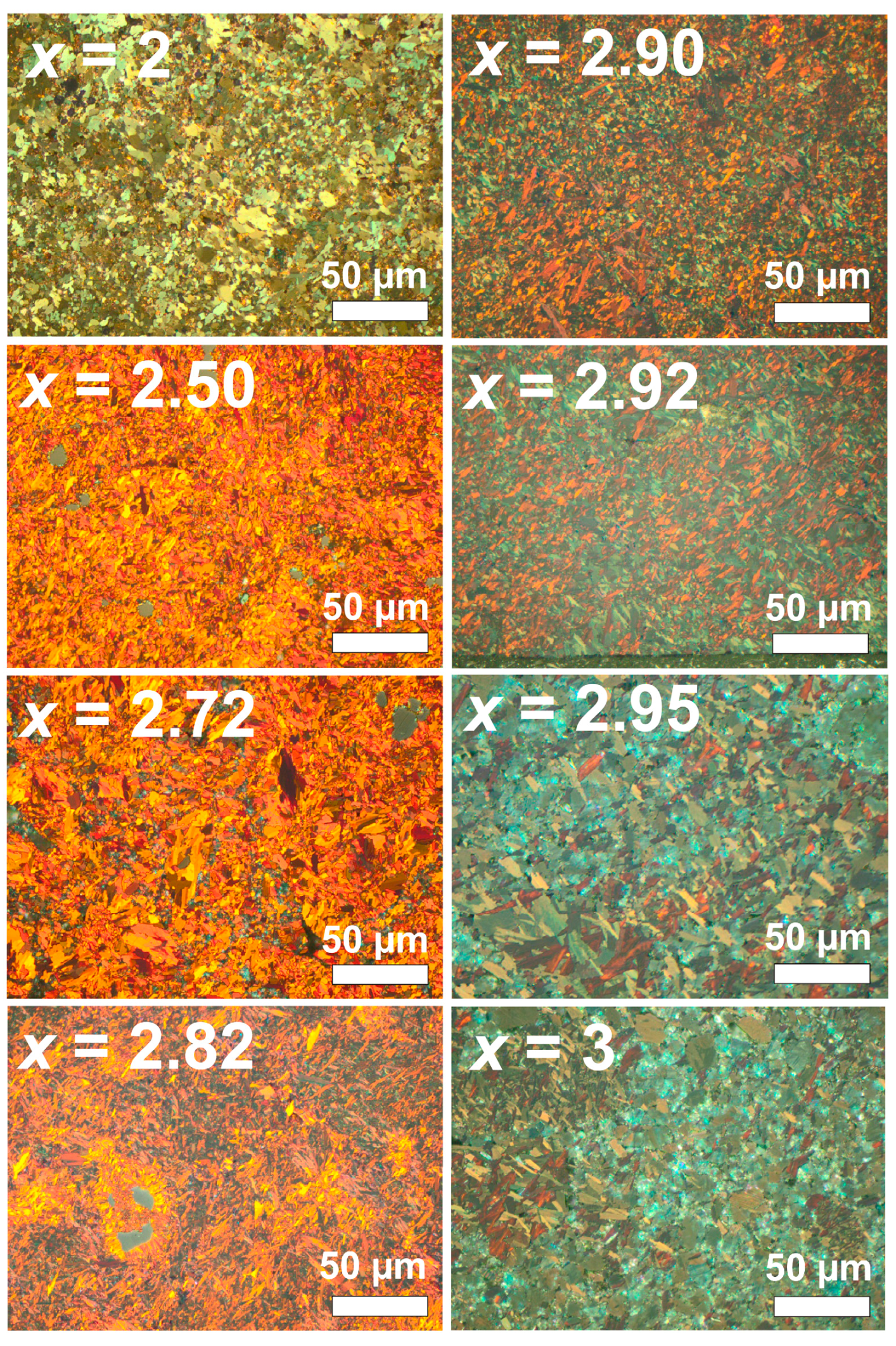
2.3.1. Microstructure
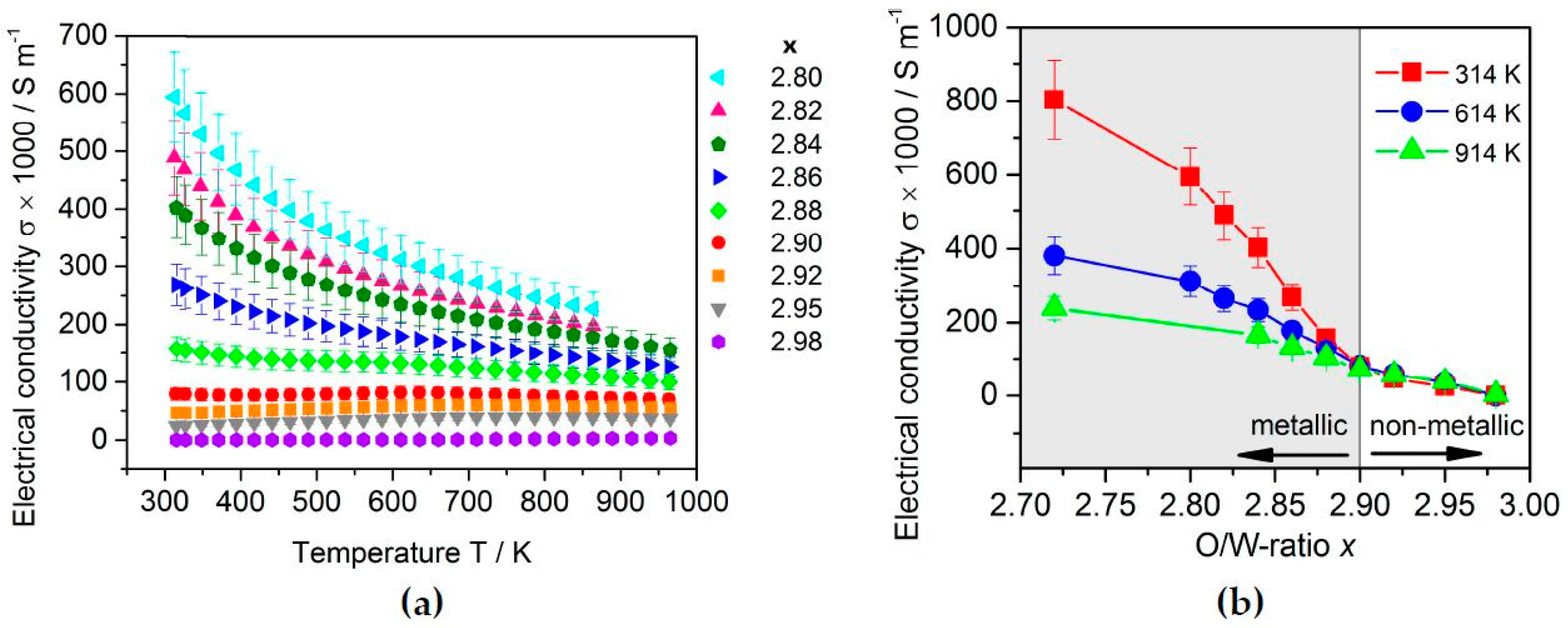
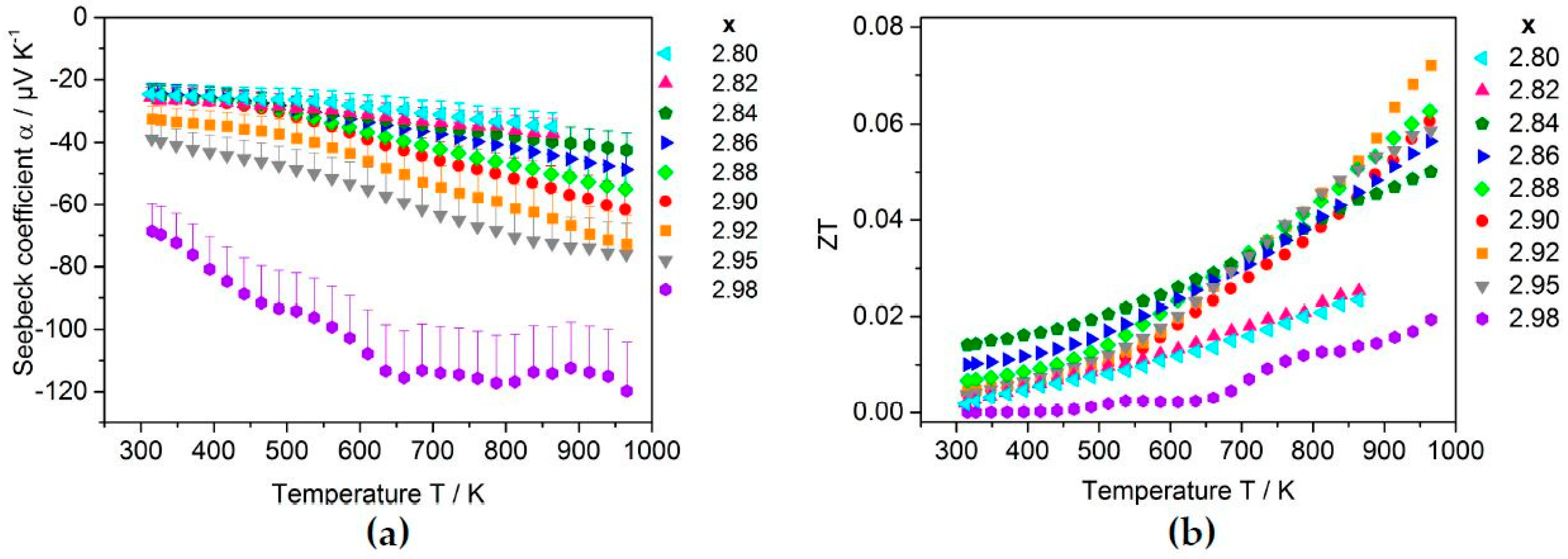
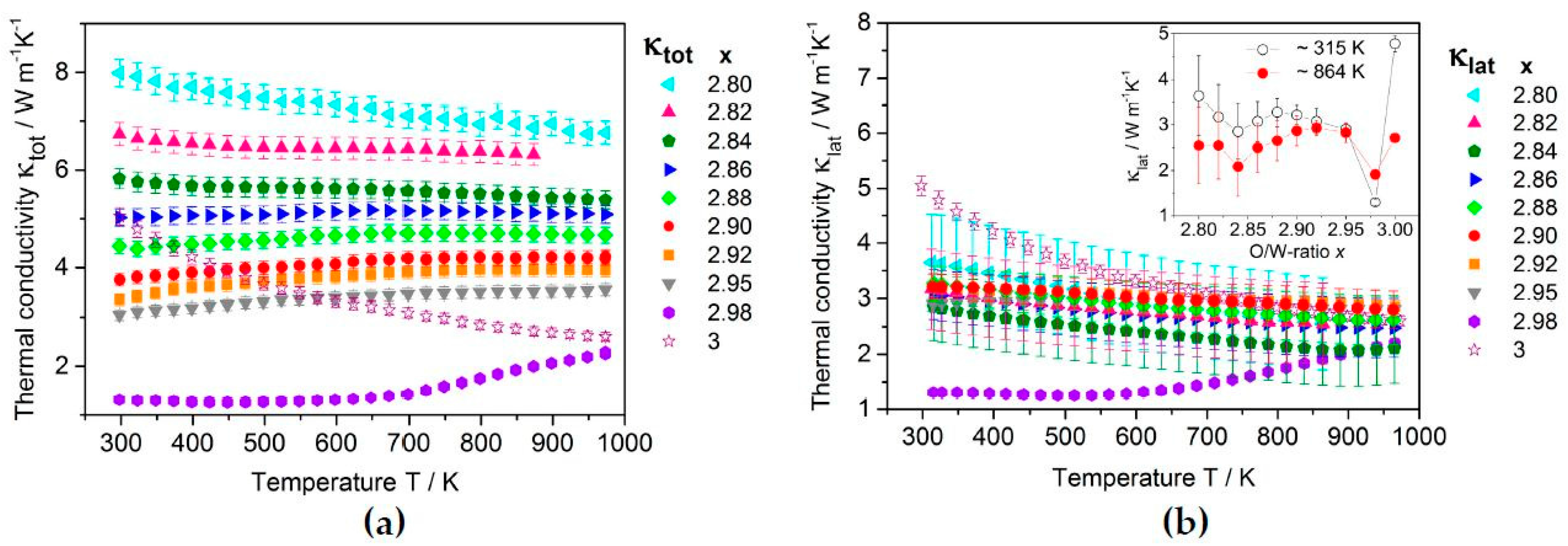
2.3.2. Thermoelectric Properties
3. Materials and Methods
3.1. Materials
3.2. Spark Plasma Sintering
3.3. Powder X-ray Diffraction
3.4. High-Resolution Transmission Electron Microscopy
3.5. Thermoelectric Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev. B 1997, 56, R12685–R12687. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Walia, S.; Balendhran, S.; Nili, H.; Zhuiykov, S.; Rosengarten, G. Transition metal oxides—Thermoelectric properties. Prog. Mater. Sci. 2013, 58, 1443–1489. [Google Scholar] [CrossRef]
- Kieslich, G.; Cerretti, G.; Veremchuk, I.; Hermann, R.P.; Panthöfer, M.; Grin, Y.; Tremel, W. A chemists view: Metal oxides with adaptive structures for thermoelectric applications. Phys. Status Solidi A 2016, 213, 1–16. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Lei, W.; Sinclair, D.C.; Reaney, I.M. High-Figure-of-Merit Thermoelectric La-Doped A-Site-Deficient SrTiO3 Ceramics. Chem. Mater. 2016, 28, 925–935. [Google Scholar] [CrossRef]
- Liang, X. Structure and Thermoelectric Properties of Zinc Based Materials. Doctoral Dissertation, Harvard University, Cambridge, MA, USA, 29 August 2013. [Google Scholar]
- Mikami, M.; Ozaki, K. Thermoelectric properties of nitrogen-doped TiO2−x compounds. J. Phys. Conf. Ser. 2012, 379, 012006. [Google Scholar] [CrossRef]
- Feng, B.; Martin, H.-P.; Börner, F.-D.; Lippmann, W.; Schreier, M.; Vogel, K.; Lenk, A.; Veremchuk, I.; Dannowski, M.; Richter, C.; et al. Manufacture and Testing of Thermoelectric Modules Consisting of BxC and TiOx Elements. Adv. Eng. Mater. 2014, 16, 1252–1263. [Google Scholar] [CrossRef]
- Martin, H.-P.; Pönicke, A.; Kluge, M.; Rost, A.; Conze, S.; Wätzig, K.; Schilm, J.; Michaelis, A. TiOx-Based Thermoelectric Modules: Manufacturing, Properties, and Operational Behavior. J. Electron. Mater. 2016, 45, 1570–1575. [Google Scholar] [CrossRef]
- Harada, S.; Tanaka, K.; Inui, H. Thermoelectric properties and crystallographic shear structures in titanium oxides of the Magnèli phases. J. Appl. Phys. 2010, 108, 083703. [Google Scholar] [CrossRef] [Green Version]
- Kieslich, G.; Veremchuk, I.; Antonyshyn, I.; Zeier, W.G.; Birkel, C.S.; Weldert, K.; Heinrich, C.P.; Visnow, E.; Panthöfer, M.; Burkhardt, U.; et al. Using crystallographic shear to reduce lattice thermal conductivity: High temperature thermoelectric characterization of the spark plasma sintered Magnéli phases WO2.90 and WO2.722. Phys. Chem. Chem. Phys. 2013, 15, 15399. [Google Scholar] [CrossRef] [PubMed]
- Kieslich, G.; Tremel, W. Magnéli oxides as promising n-type thermoelectrics. AIMS Mater. Sci. 2014, 1, 184–190. [Google Scholar] [CrossRef]
- Migas, D.; Shaposhnikov, V.; Borisenko, V. Tungsten oxides. II. The metallic nature of Magnéli phases. J. Appl. Phys. 2010, 108, 093714. [Google Scholar] [CrossRef]
- Sundberg, M. The crystal and defect structures of W25O73, a member of the homologous series WnO3n-2. Acta Cryst. B 1976, 32, 2144–2149. [Google Scholar] [CrossRef]
- Sundberg, M. Structure and “oxidation behavior” of W24O70, a new member of the {103} CS series of tungsten oxides. Solid State Chem. 1980, 35, 120–127. [Google Scholar] [CrossRef]
- Sundberg, M.; Zakharov, N.D.; Zibrov, I.P.; Barabanenkov, Y.A.; Filonenko, V.P.; Werner, P. Two high-pressure tungsten oxide structures of W3O8 stoichiometry deduced from high-resolution electron microscopy images. Acta Cryst. B 1993, 49, 951–958. [Google Scholar] [CrossRef]
- Barabanenkov, Y.A.; Zakharov, N.D.; Zibrov, I.P.; Filonenko, V.P.; Werner, P. High-pressure phases in the system W-O.I. Structure of WO1.09 by HRTEM. Acta Cryst. B 1992, 48, 572–577. [Google Scholar] [CrossRef]
- McColm, I.J.; Steadman, R.; Wilsoni, S.J. Iron-promoted phases in the tungsten-oxygen system. J. Solid State Chem. 1978, 23, 33–42. [Google Scholar] [CrossRef]
- Stoneham, A.M.; Durham, P.J. The ordering of crystallographic shear planes: Theory of regular arrays. Phys. Chem. Solids 1973, 34, 2127–2135. [Google Scholar] [CrossRef]
- Wriedt, H.A. O-W (Oxygen-Tungsten). In Binary Alloy Phase Diagrams, 2nd ed.; Massalski, T.B., Ed.; ASM International: Materials Park, OH, USA, 1990; Volume 3, pp. 2933–2935. [Google Scholar]
- Magneli, A. Structure of β-Tungsten Oxide. Nature 1950, 165, 356–357. [Google Scholar] [CrossRef]
- Gebert, E.; Ackermann, R.J. Substoichiometry of Tungsten Trioxide; the Crystal Systems of WO3.00, WO2.98, and WO2.96. Inorg. Chem. 1966, 5, 136–142. [Google Scholar] [CrossRef]
- Berak, J.M.; Sienko, M.J. Effect of oxygen-deficiency on electrical transport properties of tungsten trioxide crystals. J. Solid State Chem. 1970, 2, 109–133. [Google Scholar] [CrossRef]
- Kieslich, G.; Birkel, C.; Douglas, J.E.; Gaultois, M.; Veremchuk, I.; Seshadri, R.; Stucky, G.D.; Grin, Y.; Tremel, W. SPS-assisted preparation of the Magnéli phase WO2.90 for thermoelectric applications. J. Mater. Chem. A 2013, 1, 13050–13054. [Google Scholar] [CrossRef]
- Kieslich, G.; Burkhardt, U.; Birkel, C.; Veremchuk, I.; Douglas, J.E.; Gaultois, M.; Lieberwirth, I.; Seshadri, R.; Stucky, G.D.; Grin, Y.; et al. Enhanced thermoelectric properties of the n-type Magnéli phase WO2.90: Reduced thermal conductivity through microstructure engineering. J. Mater. Chem. A 2014, 2, 13492–13497. [Google Scholar] [CrossRef]
- Venables, D.; Brown, M. Reduction of tungsten oxides with carbon. Part 1: Thermal analyses. Thermochim. Acta 1996, 282, 251–264. [Google Scholar] [CrossRef]
- Woodward, P.M.; Sleight, A.W.; Vogt, T. Ferroelectric Tungsten Trioxide. J. Solid State Chem. 1997, 131, 9–17. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Andrew, K.F. Kinetics of the Oxidation of Pure Tungsten from 500 to 1300 C. J. Electrochem. Soc. 1960, 107, 619–628. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Arakawa, H. The Visible Light Induced Photocatalytic Activity of Tungsten Trioxide Powders. Appl. Catal. A Gen. 2001, 210, 181–191. [Google Scholar] [CrossRef]
- Bolzan, H.; Kennedy, B.; Howard, C. Neutron Powder Diffraction Study of Molybdenum and Tungsten Dioxides. Aust. J. Chem. 1995, 48, 1473–1477. [Google Scholar] [CrossRef]
- Magneli, A. Crystal structure studies on β–tungsten oxide. Ark. Kemi 1949, 1, 223–230. [Google Scholar]
- Viswanathan, K.; Brandt, K.; Salje, E. Crystal structure and charge carrier concentration of W18O49. J. Solid State Chem. 1981, 36, 45–51. [Google Scholar] [CrossRef]
- Lamire, M.; Labbe, P.; Goreaud, M.; Raveau, B. Refining and new analysis of W18O49 structure. Rev. Chim. Miner. 1987, 24, 369–381. [Google Scholar]
- Sundberg, M. Structure determination from HREM images: Application to a new binary tungsten oxide. Chem. Scr. 1979, 14, 161–166. [Google Scholar]
- Magnéli, A. Structures of the ReO3-type with recurrent dislocations of atoms: ‘homologous series’ of molybdenum and tungsten oxides. Acta Cryst. 1953, 6, 495–500. [Google Scholar] [CrossRef]
- Vogt, T.; Woodward, P.M.; Hunter, B.A. The High-Temperature Phases of WO3. J. Solid State Chem. 1999, 144, 209–215. [Google Scholar] [CrossRef]
- Molenda, J.; Kubik, A. Transport properties and reactivity of tungsten trioxide. Solid State Ion. 1999, 117, 57–64. [Google Scholar] [CrossRef]
- Akselrud, L.; Grin, Y. WinCSD: Software package for crystallographic calculations (Version 4). J. Appl. Cryst. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- NIST Chemical Kinetics Database: Tungsten Oxide (WO3). Available online: http://kinetics.nist.gov/janaf/html/O-065.html (accessed on 20 April 2017).
- NIST Chemical Kinetics Database: Tungsten Oxide (WO3). Available online: http://kinetics.nist.gov/janaf/html/O-047.html (accessed on 20 April 2017).
- Hyun-Sik, K.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Wadsley, H.D. Nonstoichiometric Metal Oxides—Order and Disorder. In Nonstoichiometric Compounds; Gould, R.D., Ed.; American Chemical Society: Washington, DC, USA, 1963; pp. 23–36. [Google Scholar] [CrossRef]
- Salje, E.K.; Rehmann, S.; Pobell, F.; Morris, D.; Knight, K.S.; Herrmannsdörfer, T.; Dovey, M.T. Crystal structure and paramagnetic behaviour of ε-WO3−x. J. Phys. Condens. Matter 1997, 9, 6563–6577. [Google Scholar] [CrossRef]
- Woodward, P.M.; Sleight, A.W.; Vogt, T. Structure refinement of triclinic tungsten trioxide. J. Phys. Chem. Solids 1995, 56, 1305–1315. [Google Scholar] [CrossRef]



| x | Phase | Source | Space Group | Lattice Parameters | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | β | V | |||||
| Å | Å | Å | deg | Å3 | |||||
| 2 | WO2 | n.a. | P21/c | 5.58 | 4.90 | 5.664 | 120.7 | 133.1 | [30] |
| commercial | 5.574(1) | 4.898(1) | 5.662(1) | 120.69(1) | 132.9(2) | this work | |||
| SPS | 5.575(1) | 4.900(1) | 5.663(1) | 120.70(1) | 133.0(1) | this work | |||
| 2.72 | W18O49 | n.a. | P2/m | 18.32 | 3.79 | 14.04 | 115.0 | 883.3 | [31] |
| iv | 18.33 | 3.79 | 14.04 | 115.2 | 882.1 | [32] | |||
| n.a. | 18.32 | 3.78 | 14.03 | 115.2 | 879.5 | [33] | |||
| SPS | 18.32 | 3.79 | 14.04 | n/a | --- | [11] | |||
| SPS | 18.329(1) | 3.784(1) | 14.037(1) | 115.20(1) | 880.9(4) | this work | |||
| 2.83 | W12O34 | n.a. | P2/m | 17.0 | 3.8 | 19.4 | 105.3 | [34] | |
| SPS a | 17.229(1) | 3.782(1) | 19.496(1) | 105.77(1) | 1223(3) | this work | |||
| 2.90 | W20O58 | iv | P2/m | 12.1 | 3.78 | 23.4 | 85 | 1066.2 | [21] |
| calculated | 12.05 | 3.77 | 23.59 | 85.3 | 1067.2 | [35] | |||
| SPS | 12.08 | 3.78 | 23.59 | n/a | --- | [11] | |||
| SPS | 12.00 | 3.78 | 23.51 | 84.8 | 1062.0 | [24] | |||
| SPS | 12.080(3) | 3.782(1) | 23.62(1) | 85.36(1) | 1075.6(7) | this work | |||
| 2.92 | W25O73 | iv | P2/c | 11.93 | 3.82 | 59.72 | 98.3 | 2693.1 | [14] |
| 3 | γ-WO3 | commercial | P21/n | 7.33 | 7.56 | 7.73 | 90.5 | 428.3 | [36] |
| commercial | 7.299(1) | 7.537(1) | 7.689(1) | 90.88(1) | 422.9(1) | this work | |||
| x | Phases | |||||
|---|---|---|---|---|---|---|
| WO2 | W18O49 | “WO2.82” a | W20O58 | W25O73 b | γ/δ-WO3 | |
| 2 | ● | □ | □ | □ | □ | □ |
| 2.50 | ● | ● | □ | □ | □ | □ |
| 2.72–2.80 | □ | ● | ● | □ | □ | □ |
| 2.82–2.88 | □ | ● | ● | ● | □ | □ |
| 2.90 | □ | □ | □ | ● | □ | □ |
| 2.92–3 | □ | □ | □ | ● | ● | ● |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiser, F.; Simon, P.; Burkhardt, U.; Kieback, B.; Grin, Y.; Veremchuk, I. Spark Plasma Sintering of Tungsten Oxides WOx (2.50 ≤ x ≤ 3): Phase Analysis and Thermoelectric Properties. Crystals 2017, 7, 271. https://doi.org/10.3390/cryst7090271
Kaiser F, Simon P, Burkhardt U, Kieback B, Grin Y, Veremchuk I. Spark Plasma Sintering of Tungsten Oxides WOx (2.50 ≤ x ≤ 3): Phase Analysis and Thermoelectric Properties. Crystals. 2017; 7(9):271. https://doi.org/10.3390/cryst7090271
Chicago/Turabian StyleKaiser, Felix, Paul Simon, Ulrich Burkhardt, Bernd Kieback, Yuri Grin, and Igor Veremchuk. 2017. "Spark Plasma Sintering of Tungsten Oxides WOx (2.50 ≤ x ≤ 3): Phase Analysis and Thermoelectric Properties" Crystals 7, no. 9: 271. https://doi.org/10.3390/cryst7090271