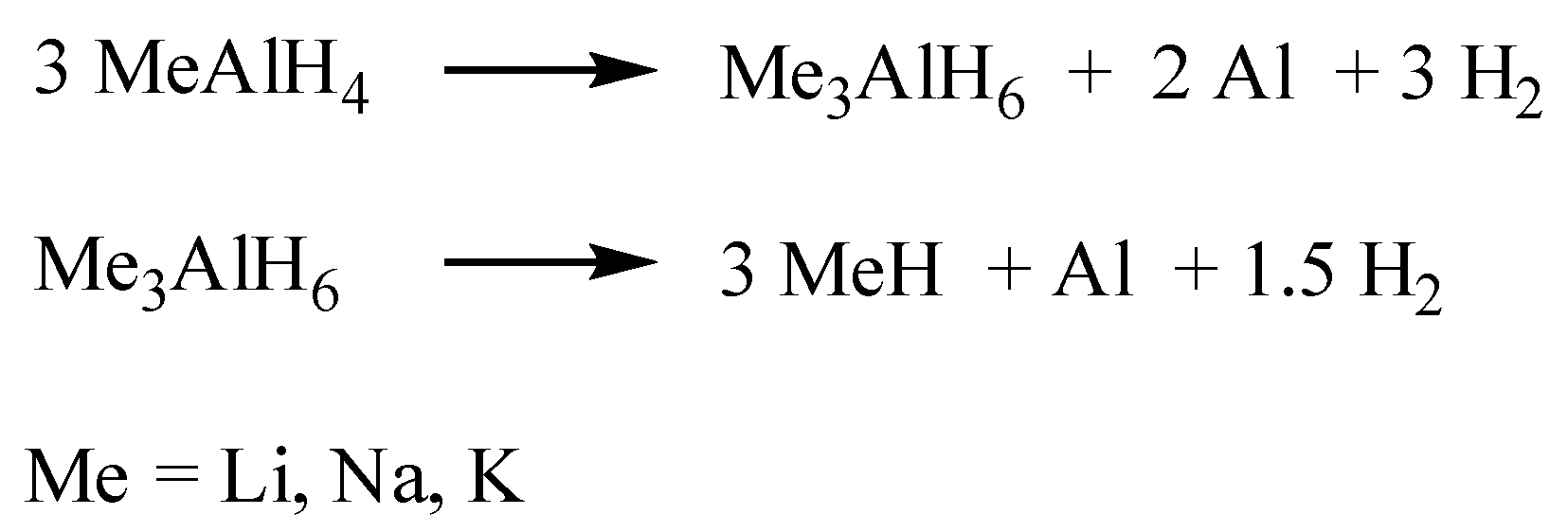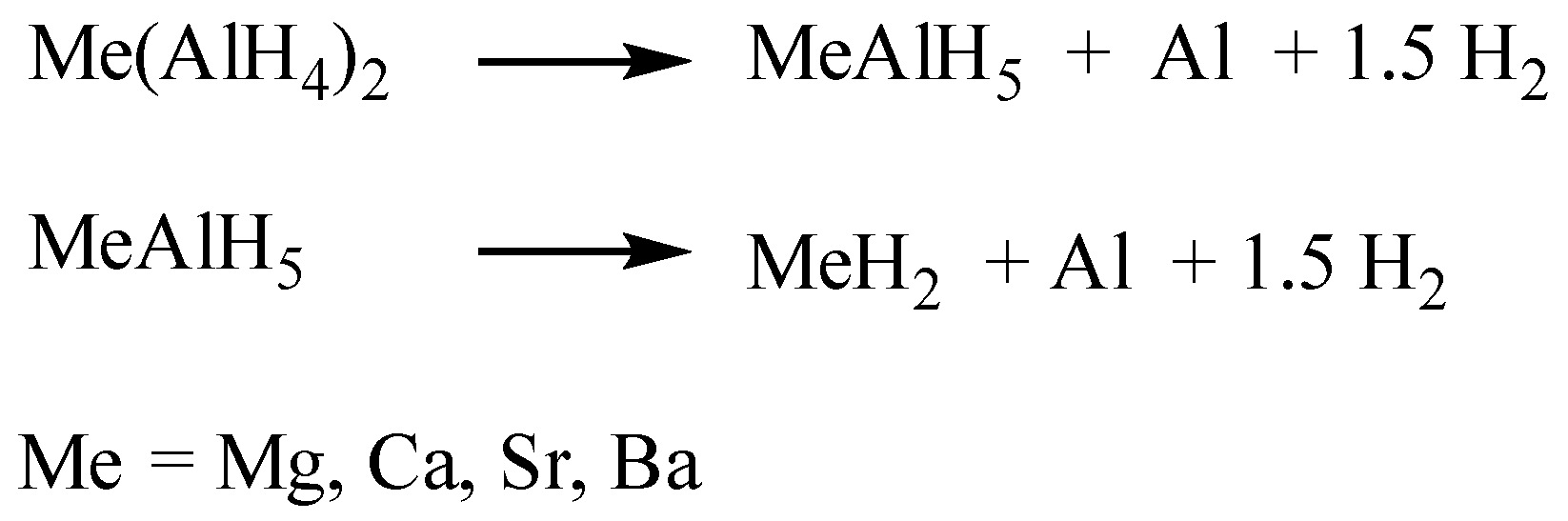1. Introduction
RbAlH
4 belongs to the family of complex metal aluminum hydrides, which have been studied intensively as potential candidates for reversible hydrogen storage in solid-state materials [
1,
2,
3]. For both groups, alkali and alkaline earth aluminum hydride crystalline structures are obtained after synthesis, which are built from isolated [AlH
4]
− anions coordinating the metal cations, Me
+/2+. So far, the known tetrahedral structures, MeAlH
4 or Me(AlH
4)
2 with
Me representing the alkali and alkaline earth metal cations, release hydrogen in defined dehydrogenation steps. For the light alkali metal aluminum hydrides, the subsequent decomposition steps are described in
Scheme 1. First, a part of the hydrogen is released, and intermediate hexahydride structures, Me
3AlH
6, are formed together with metallic aluminum. For the Li, K, and Na compounds, the intermediate structures are built of isolated [AlH
6]
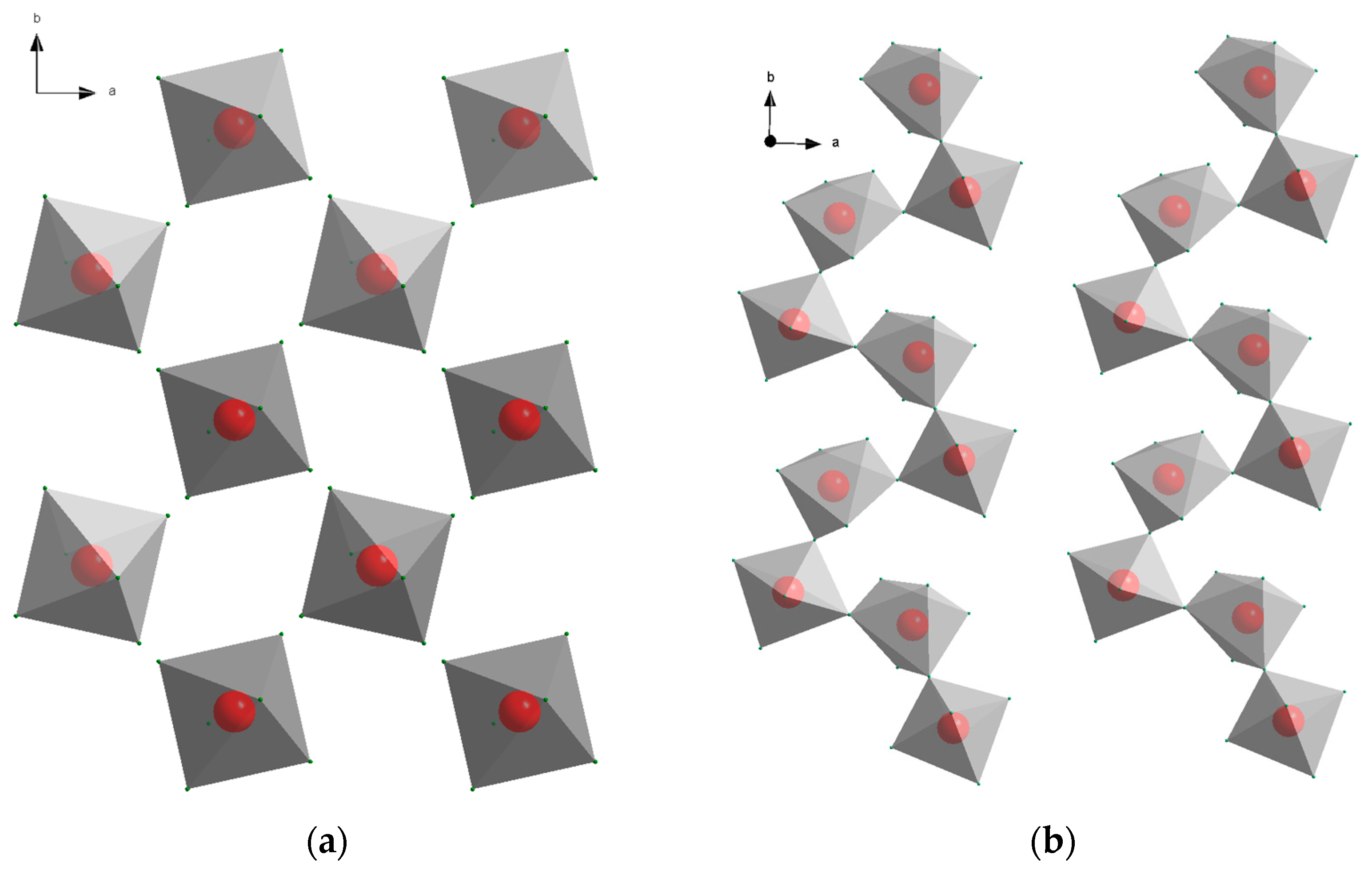
3− octahedra (
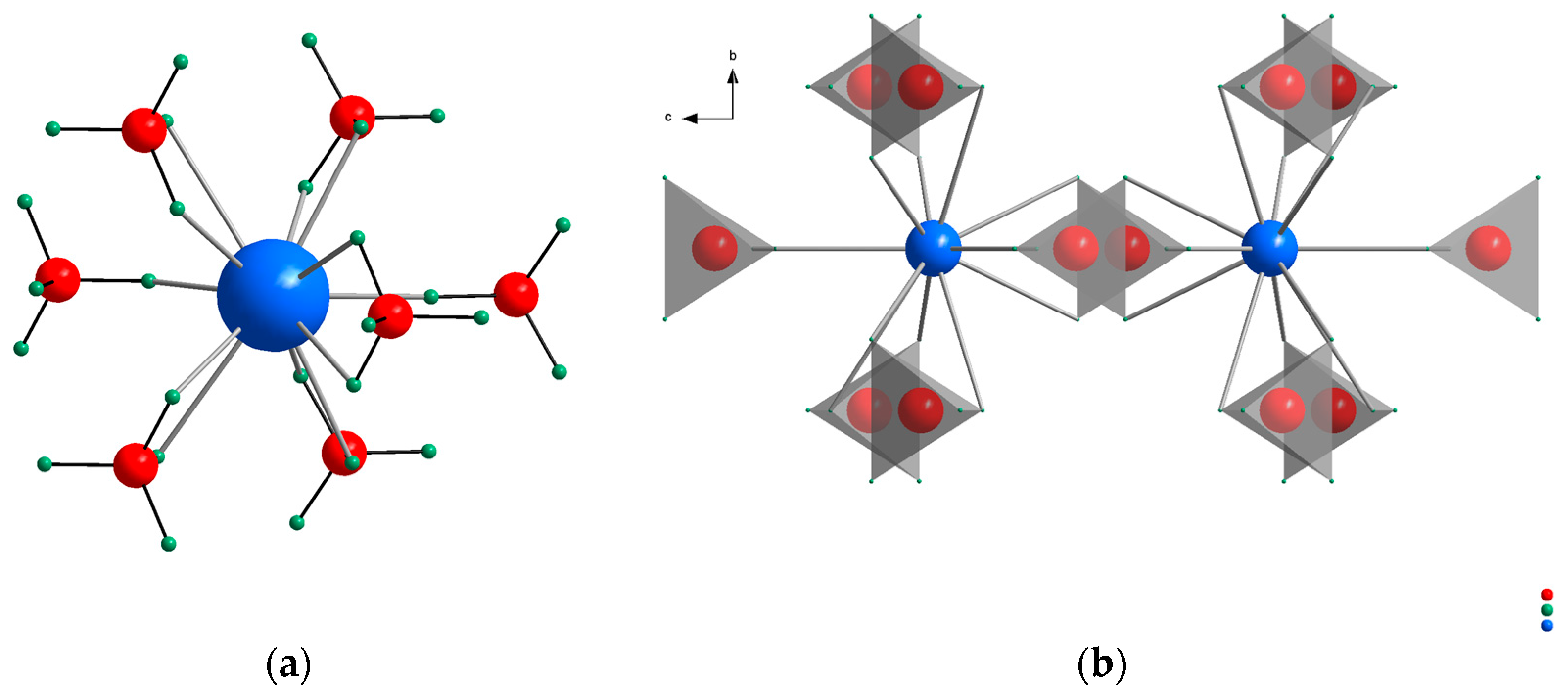
Figure 1a), which decompose in a second step, forming the simple hydrides MeH, Al, and H
2. Further heating leads to the complete decomposition of the hydrides to metals and hydrogen.
For the alkaline earth aluminum hydrides the decomposition and the intermediate structures are different. While for Mg(AlH
4)
2 so far only the direct decomposition to MgH
2 has been observed [
4,
5], intermediate structures have been reported for the Ca, Sr, and Ba compounds (
Scheme 2) [
6,
7,
8,
9]. The crystal structures of the alkaline earth aluminum intermediates consist of corner-sharing octahedra forming chains in different conformations. The octahedra can either form helical chains along the crystallographic
c-axis as determined for CaAlH
5 [
6] or a zig-zag like arrangement as observed for SrAlH
5 and BaAlH
5 (
Figure 1b) [
9].
The second hydrogen release step leads to the formation of simple hydrides, such as SrH
2 or CaH
2. The final decomposition step leads to the formation of intermetallic compounds of type Me
xAl
y. The crystal structure principles of the intermediates are shown in
Figure 1, with Na
3AlD
6 [
10] as representative for the alkali metal aluminum hydride and CaAlD
5 [
6] for the alkaline earth aluminum hydride.
Based on these results, the crystal structures and the decomposition pathways of aluminum hydrides containing heavier and larger Rb
+ and Cs
+ cations were expected to be similar to the known compounds. However, recently published results on CsAlH
4 showed that even though the crystal structure is related to the NaAlH
4 structure, the compound with the large cation has some different structural properties and decomposition behavior. Interestingly, CsAlH
4 crystallizes into two polymorphic structures, which undergo reversible phase transformations [
11,
12]. Contrary to the lightweight alkali aluminum hydrides, the decomposition does not follow the steps shown in
Scheme 1.
The synthesis of RbAlH
4 and the first spectroscopy data were described almost 50 years ago [
13]. The hydride was synthesized by an exchange reaction between LiAlH
4 and Rb metal. From the infrared data it was concluded that the structure of RbAlH
4 consists of [AlH
4]
− tetrahedra. Gavrilenko et al. report the synthesis of RbAlH
4 by a reaction between alkylated tetrahydroaluminates or tetraalkylonium cations with LiAlH
4 in ether [
14]. A wet-chemical synthesis route for the preparation of RbAlH
4 was published by Bastide et al. [
15]. The authors measured powder X-ray diffraction (PXD) data and analyzed the data by a least square refinement of the lattice parameters resulting in an orthorhombic unit cell (a = 9.253, b = 5.950, c = 7.599 Å). The structure was related to barite-type but no atomic positions were determined from the diffraction data. The complete crystal structure of RbAlH
4, including hydrogen positions, was first predicted by first-principle density-functional-calculations (DFT) [
16]. Calculations were based on the orthorhombic space group, Pnma with Rb and Al both on Wyckoff position 4c.
In this work, different mechanochemical synthesis procedures for the preparation of RbAlH4, crystal structure analysis based on powder X-ray and neutron diffraction data, and first results on the decomposition of RbAlH4 will be addressed.
2. Materials and Methods
2.1. Synthesis Procedure and Reagents
The synthesis of RbAlH
4 can be achieved by ball-milling using two different procedures. The first route is the synthesis of RbAlH
4 by ball-milling of RbCl or RbF and NaAlH
4 or LiAlH
4. For the deuterated forms, LiAlD
4 is used instead of LiAH
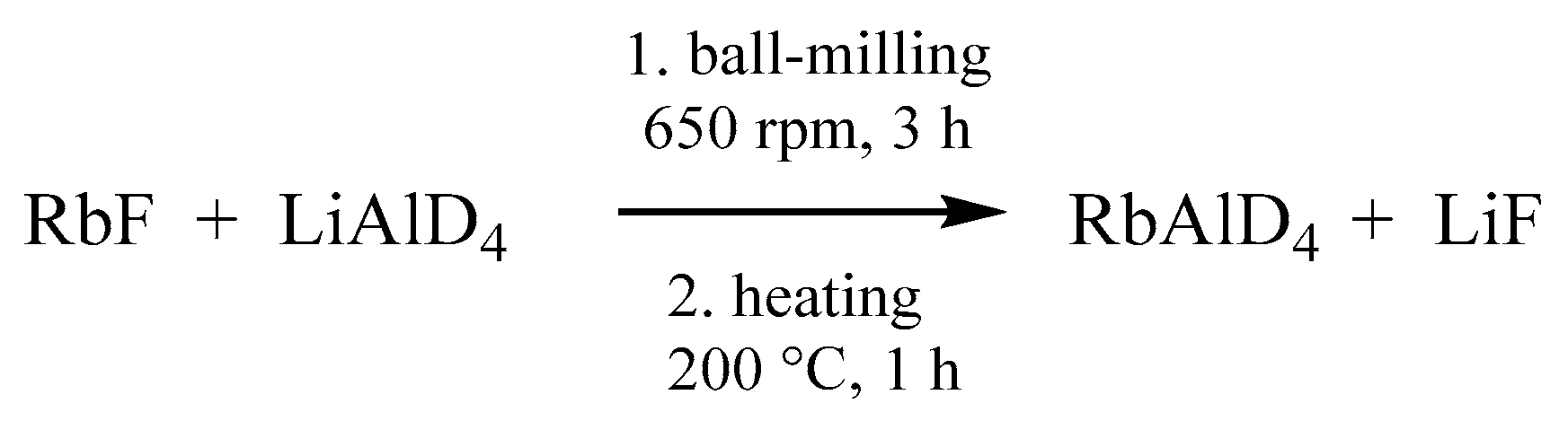
4. This step is followed by a heating treatment in an autoclave and a subsequent purification via dissolution in diglyme and precipitation in toluene (
Scheme 3) [
17].
Commercial LiAlD4 (>90%, 98% D, ABCR) and RbF (99%, Sigma Aldrich, St. Louis, MO, USA) were used without further purification. Used solvents were carefully dried, followed by distillation. Ball-milling experiments were carried out in a Pulverisette P7 (Fritsch, Idar-Oberstein, Germany) planetary ball-mill, using hardened steel balls and vessels.
RbAlD4 was prepared by ball-milling RbF (1.0 g, 9.6 mmol) and LiAlD4 (0.402 g, 9.6 mmol,) with 4 balls (5.0 g each, 10 mm diameter) at a rotational speed of 650 rpm. The milling time was set to three hours with a break of 10 min after each hour. Afterwards, the sample was heated in an autoclave to 200 °C for 1 h to complete the reaction. For purification, the sample was transferred into a Schlenk flask and suspended in 1-methoxy-2-(2-methoxyethoxy) ethane (diglyme) and stirred at room temperature for 1 h. The suspension was filtered through a P4 frit into toluene. The precipitated RbAlD4 was filtered off and carefully dried in vacuum.
Another option to produce RbAlH
4 is the direct synthesis starting from the metals, Rb and Al (
Scheme 4) [
18]. For the direct synthesis of RbAlH
4 commercial Rb-metal (99.9%, ABCR), Al-metal (99.5%, Sigma Aldrich) and TiCl
3 (99.995 Sigma Aldrich) were used. Ball-milling experiments were carried out in a Pulverisette P6 (Fritsch), using a high pressure milling vial constructed for hydrogen pressure p
max = 30 MPa.
Rb (1.158 g, 13.55 mmol), Al (0.366 g, 13.55 mmol) and TiCl3 (0.105 g, 0.68 mmol) were transferred into the high-pressure milling vial (10 balls hardened steel, 4 g each, diameter 9.8 mm) and pressurized to 20 MPa H2. The first hydrogenation (synthesis of RbH) was done at a rotational speed of 250 rpm for 1 h (10 min break) and the second reaction step (synthesis of RbAlH4) was done at a rotational speed of 450 rpm for 4 h. Purification process was similar to the synthesis of RbAlD4 as described above
Rubidium is a very soft and ductile material. Starting from the pure metals Rb and Al, direct synthesis must be performed in several steps. The hydrogenation of Rb to RbH under mechanochemical conditions was performed at low revolutions per minute (250 rpm). Otherwise, the metal is smeared over the wall of the milling vial and no complete hydrogenation can be observed [
19]. In the next step, the mechanochemically induced hydrogenation of the RbH-Al mixture can be done with 450 rpm to produce RbAlH
4 by ball-milling under 20 MPa H
2 pressure for 5 h according to
Scheme 4. However, this synthesis requires the addition of 5 mol% TiCl
3 for a successful synthesis.
All operations were performed under an inert argon atmosphere by using a glove box or common Schlenk techniques.
2.2. Powder X-ray Diffraction
Prior to analysis, all samples were filled into 0.5 mm Ø glass capillaries and sealed to prevent any contact with air. Powder X-ray diffraction (PXD) experiments were carried out in Debye-Scherrer transmission geometry on a STOE STADI P diffractometer using Cu Kα1 (λ = 1.54056 Å) radiation. Data were collected with a position sensitive detector in the 6–80° 2θ range with an acquisition time of 60 s per measuring point. Rietveld refinements were performed with TOPAS Version 5 (Bruker AXS, Karlsruhe, Germany).
For the decomposition studies, samples were filled in a glove box into 0.5 mm Ø quartz glass capillaries. The capillaries were transported under protective atmosphere to a capillary furnace. Since hydrogen evolution during decomposition reaction can lead to the destruction of the quartz glass capillaries the capillaries were not sealed but kept under a continuous Ar flow. In a first series of experiments (not shown here), samples were heated from room temperature to 350 °C in temperature steps of 50 and 25 °C. Based on the screening experiments, defined temperatures were selected that showed significant changes in the diffraction patterns. A sample was heated to 300 °C with a heating rate of 1 °C min−1. After cooling to room temperature, data were collected on a STOE STADI P diffractometer (STOE, Darmstadt, Germany) using Cu Kα1 radiation. Afterwards the same sample was heated to 325 and 350 °C. After reaching the temperature, the samples were cooled down to room temperature and X-ray powder patterns were collected.
2.3. Powder Neutron Diffraction
The powder neutron diffraction (PND) experiment was performed with the PUS diffractometer at the JEEP II reactor at the Institute for Energy Technology (IFE), Kjeller, Norway [
20]. Neutrons with the wavelength 1.5539 Å were provided by a Ge(511) monochromator. The sample was contained in a cylindrical vanadium sample holder with 6 mm diameter. The diffracted neutrons were detected with two detector banks, each with 7 vertically stacked
3He position-sensitive detector tubes covering a 20° scattering angle. The 2θ range from 10 to 130° was thus covered by moving each detector bank to three different positions. Rietveld refinements on the PND data were performed with
GSAS [
21] and expgui [
22] employing a Pseudo-Voigt function according to Thompson et al. [
23]. 2400 data points were refined using 38 parameters.
2.4. Thermal Analysis
Thermal analysis measurements (thermal gravimetry (TG) and differential scanning calorimetry (DSC)) were performed on a Mettler-Toledo TGA/DSC 1 instrument (Mettler-Toledo, Gießen, Germany) using a fully automated program for the evaluation of the data. The measurements were carried out in aluminum crucibles, which were filled in a glove box. For transportation, crucibles were closed to prevent any contact with air. For the measurements, the crucibles were opened under protective argon using typically 5–8 mg of sample. Heating rates were 10 °C min−1.
3. Results and Discussion
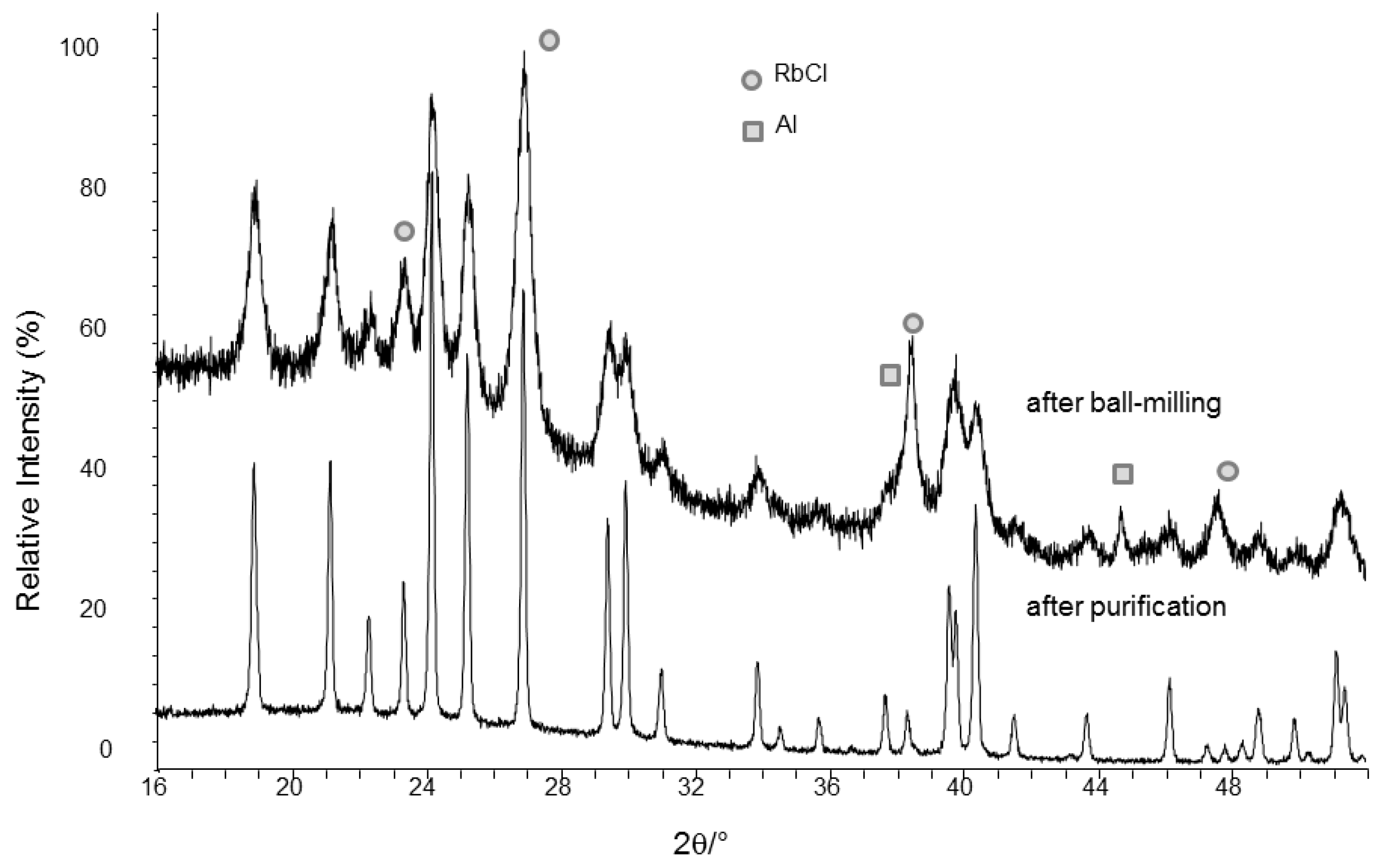
The PXD patterns obtained after direct synthesis and after purification are shown in
Figure 2. The successful synthesis of RbAlH
4 from the elements is a proof of reversibility of de-and re-hydrogenation of this compound.
Rietveld refinements using the laboratory PXD data are based on the DFT structure predicted by Vajeeston et al. [
16]. Refinement of the Rb and Al atomic positions results in a very good agreement between observed and calculated data (
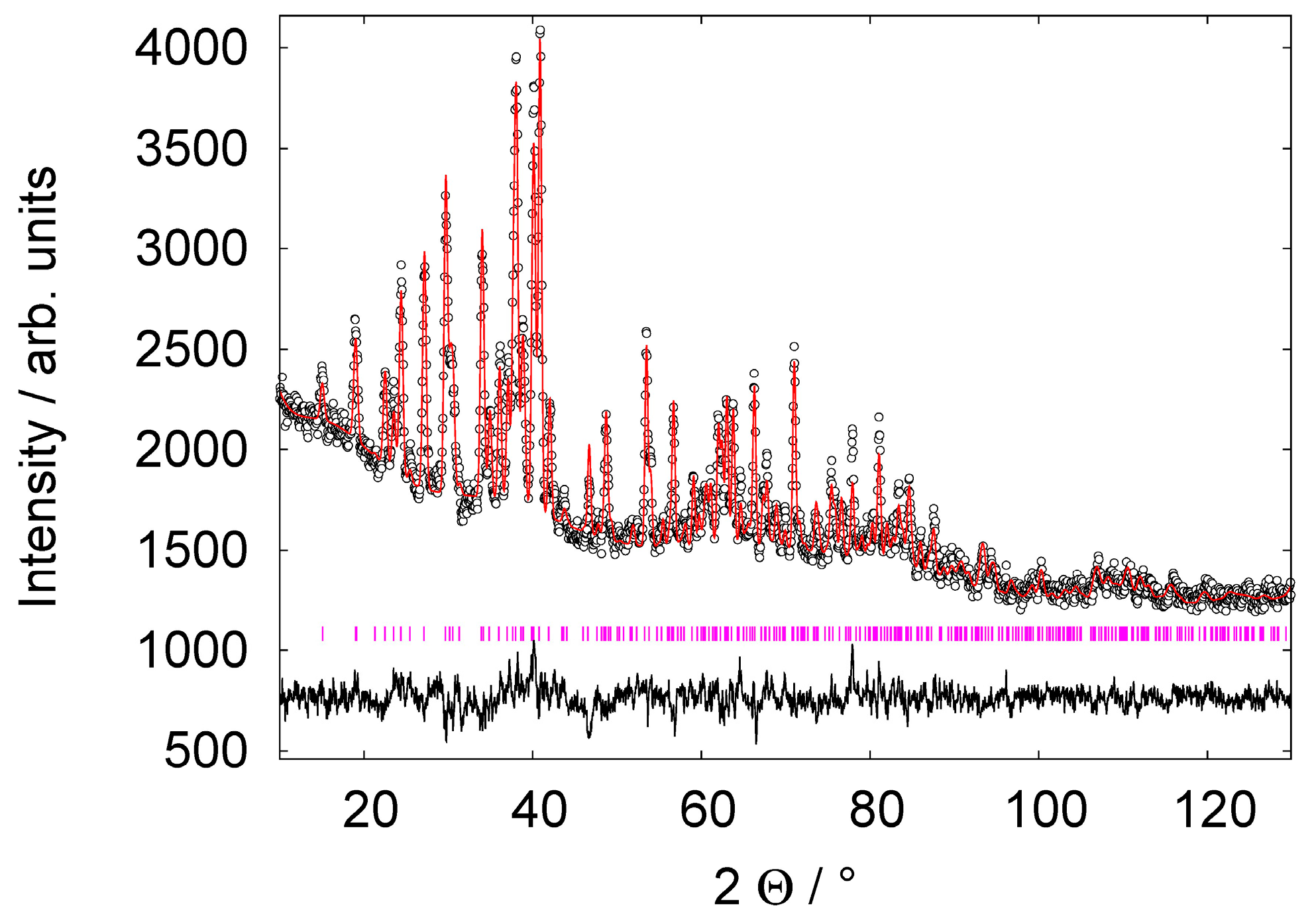
Figure S1, Supporting Information). Rietveld refinements (
Figure 3) of PND data of RbAlD
4 enable also the refinement of the deuterium positions. The refinements started with fixed isotropic thermal displacement parameters (U
iso) with 0.01 Å
2 for Rb and Al, and 0.05 Å
2 for D. The Al-D bond distance was restrained to 1.64 Å. All restraints were removed at the final stage of the refinement and the thermal displacement factors were also refined. Crystallographic data of the predicted and the refined crystal structure are summarized in
Table 1.
The refined atomic positions match quite well with the predicted values. The refined lattice parameters match the values published by Bastide et al. [
15]. However, the lattice parameters predicted by DFT calculations differ significantly from the experimental data. The crystal structure of RbAlD
4 is isostructural with KAlD
4 [
24], which is built from isolated [AlD
4]
− tetrahedra. The Rb cation is coordinated by 7 [AlD
4]
− tetrahedra within a distance to the center Al cation in a range between 3.721 and 4.413 Å (
Figure 4a).
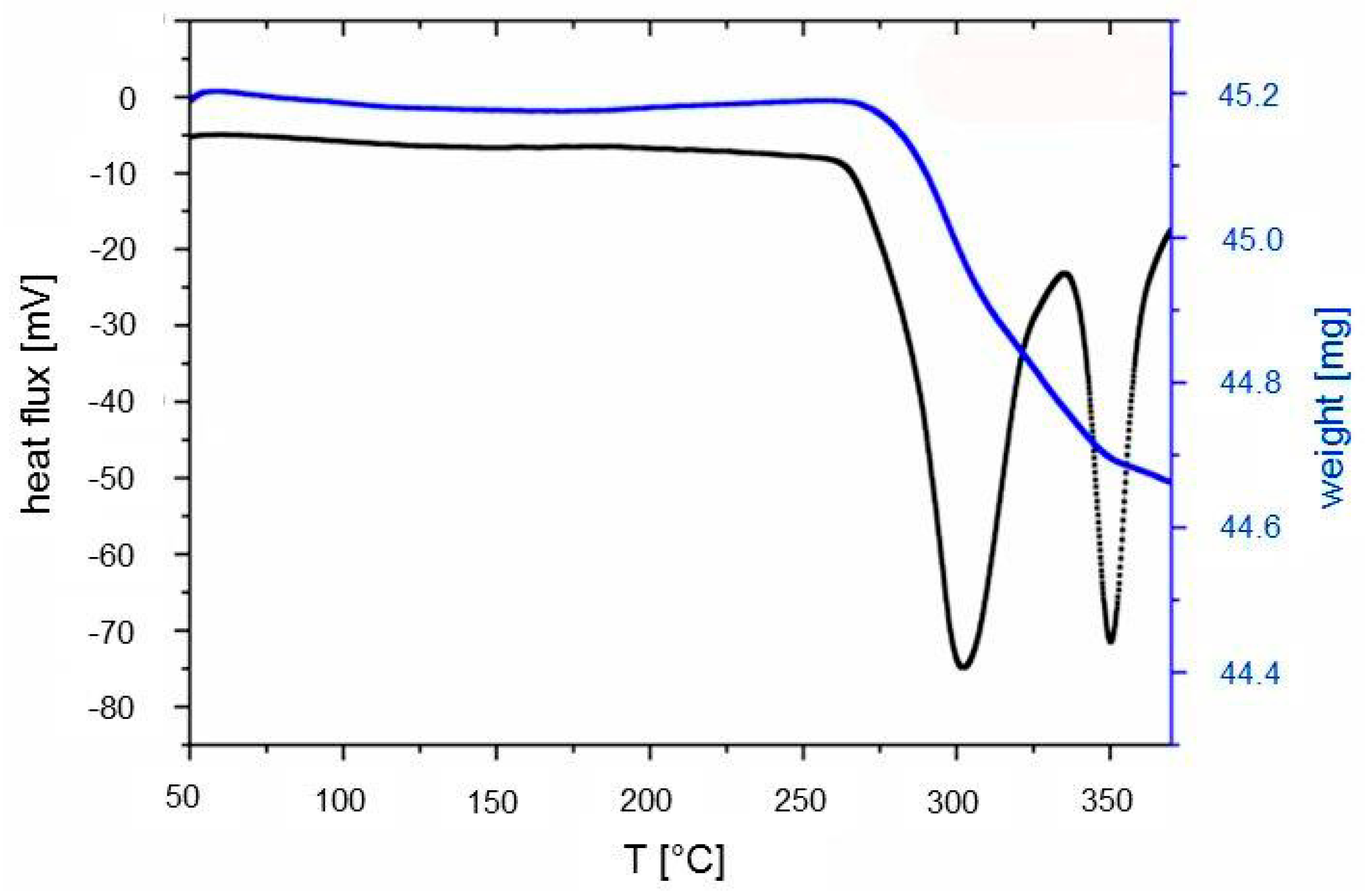
The decomposition properties were studied by thermal analysis (TG-DSC) and by PXD experiments. The samples were heated from room temperature only to 375 °C, because at temperatures above 400 °C, the Rb metal formed during decomposition starts to evaporate (melting point of Rb: 39 °C). The TG-DSC measurement shows two endothermic events, the first one starting around 275 °C and the second one at around 330 °C (
Figure 5). A weight loss of 1.6 wt % was observed for both decomposition steps.
This corresponds to only 45% of the theoretical weight loss if a complete decomposition to Rb- and Al-metal as final products is assumed. During the first thermal event, a multistep decomposition must take place, producing RbH and Al-metal. The second decomposition step can be assigned to hydrogen release from RbH (decomposition temperature of RbH 364 °C [
25]). The low amount of released hydrogen indicates either that the decomposition process was not completed under the experimental conditions or that other intermediates with higher hydrogen contents were produced during decomposition.
Dymova et al. studied the decomposition of RbAlH
4 during heating under hydrogen pressure [
26]. The decomposition of RbAlH
4 to Rb
3AlH
6 was proposed in the temperature range between 317 and 334 °C. A second decomposition between 390 and 417 °C produces RbH from Rb
3AlH
6. No further information about the structural parameters of intermediate compounds such as Rb
3AlH
6 or precise experimental parameters was given.
The proposed decomposition mechanism is based on expected similarities to other decomposition reactions of complex aluminum hydrides as given in
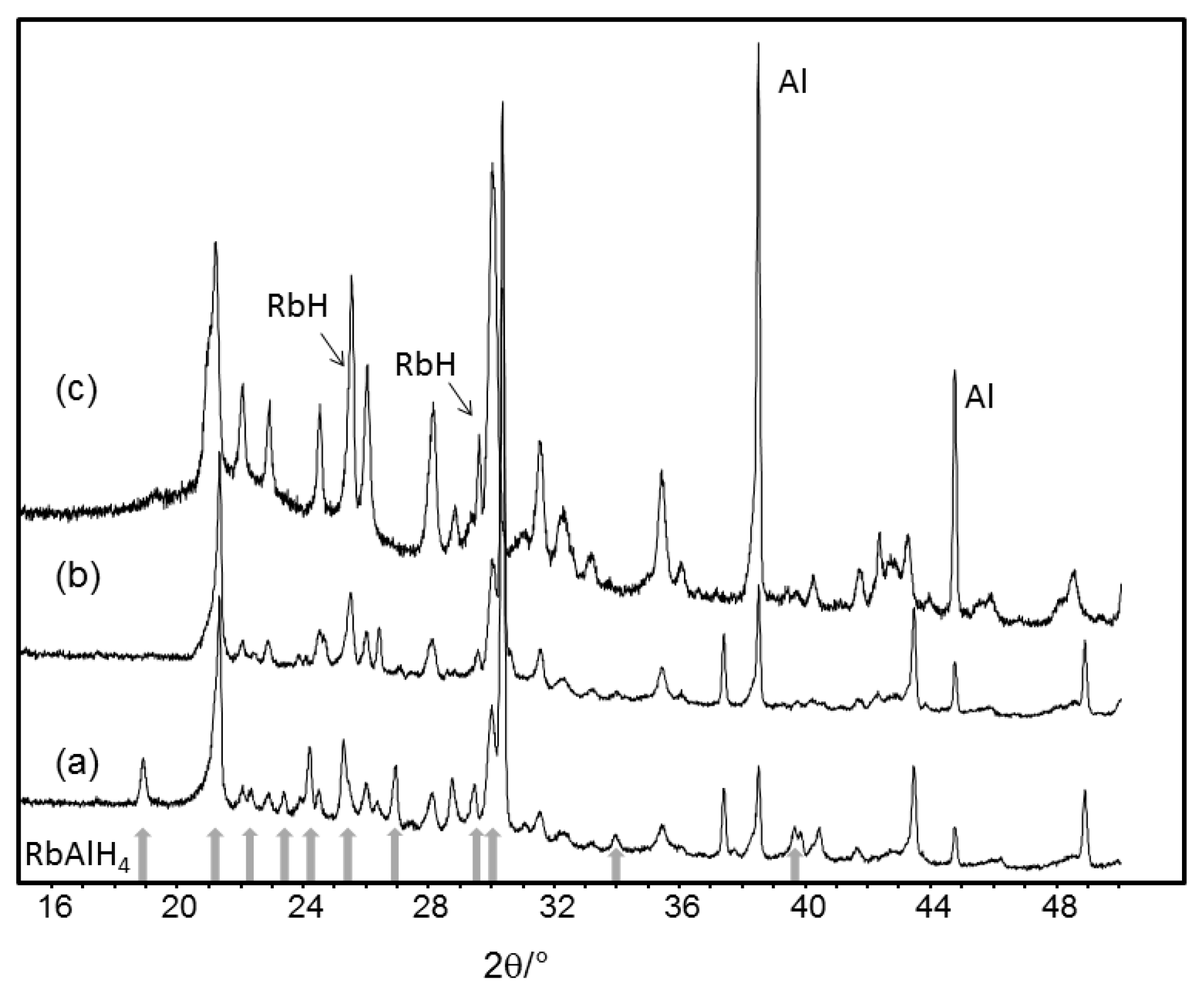
Scheme 1. However, powder X-ray diffraction patterns collected at room temperature after heating to 300, 325 and 350 °C (
Figure 6) revealed an unexpectedly complicated decomposition behavior. According to the decomposition behavior of other complex aluminum hydrides such as LiAlH
4 or KAlH
4, a two-step decomposition with a hexahydride as intermediate was to be expected (
Scheme 1). The sample studied after temperature treatment at 300 °C still contained some RbAlH
4, but the presence of small amounts of RbH and Al indicated partial decomposition. However, the main reflection at about 30.3° 2θ could not be explained by RbH or Al. At least one intermediate decomposition product had formed, together with RbH and Al. After heating to 325 °C, RbAlH
4 decomposed, and more RbH and Al were formed. The unidentified phase with the main reflection at 30.3° 2θ was the main crystalline phase. At 350 °C, the unidentified phase had disappeared, and new reflections at 21.2, 25.5, and 30.0° 2θ had appeared. This indicates the formation of an additional decomposition product. The crystal structure(s) of the intermediate phase(s) could not be determined, and are currently under investigation. We have first indications that kinetics is an important issue for decomposition, and that heating treatment is decisive whether intermediate decomposition phases can be monitored or not.