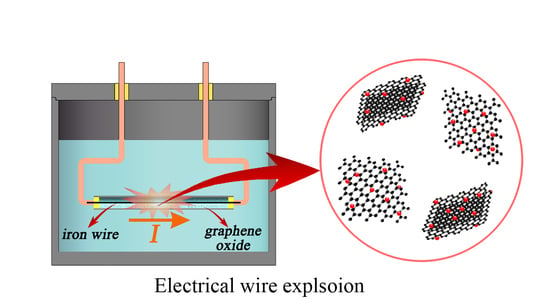
One Step Preparation of Fe–FeO–Graphene Nanocomposite through Pulsed Wire Discharge
Abstract
:1. Introduction
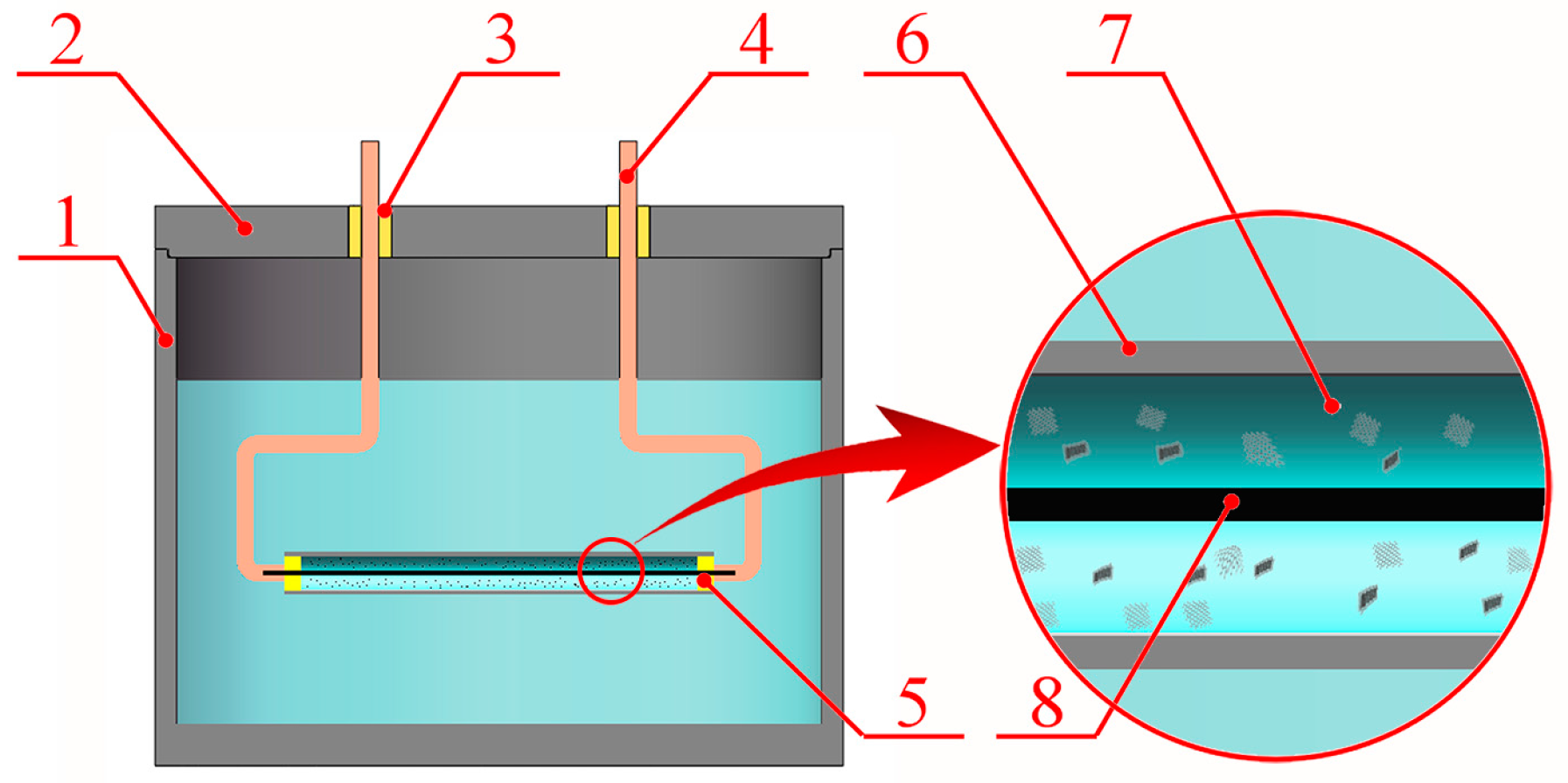
2. Materials and Methods
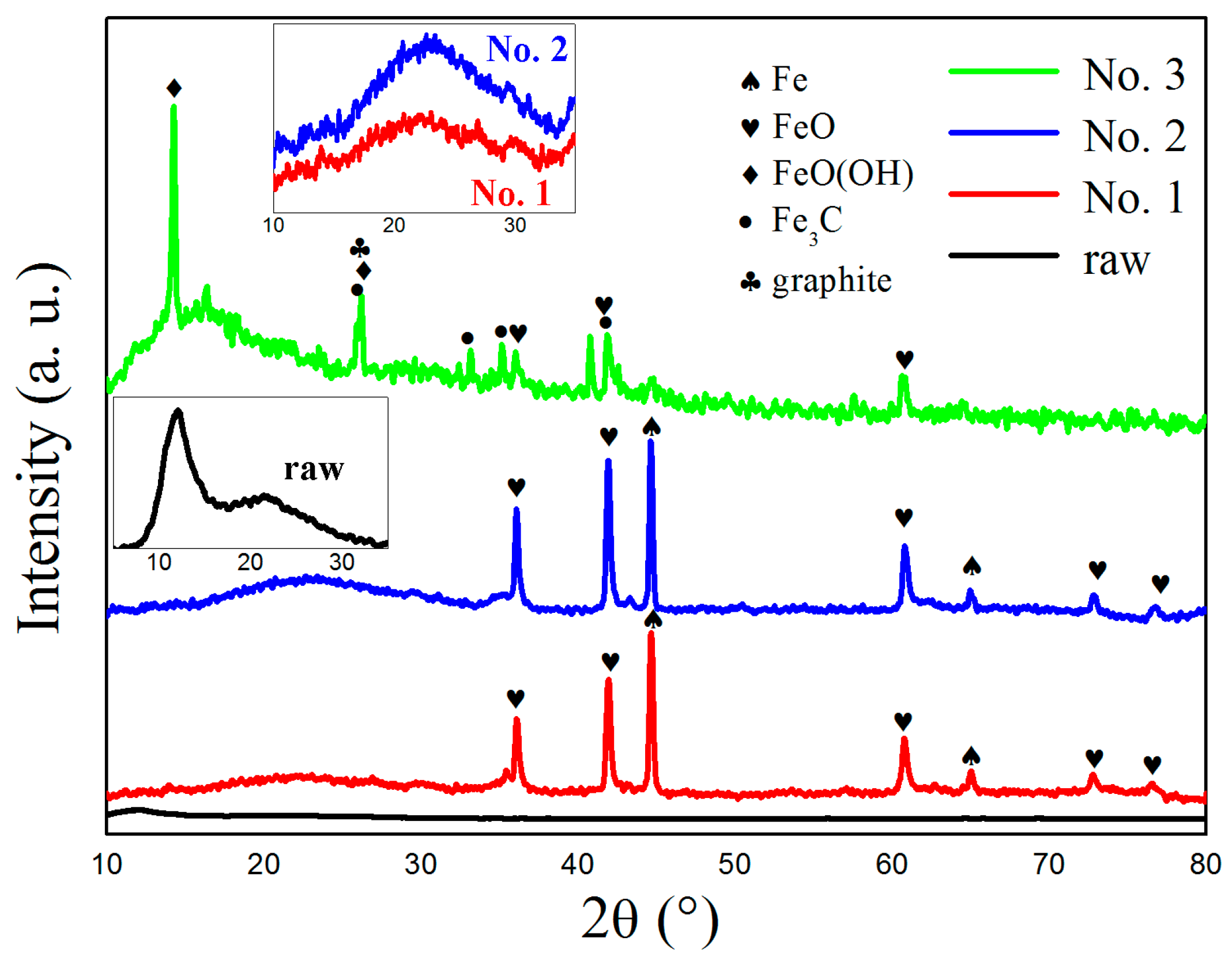
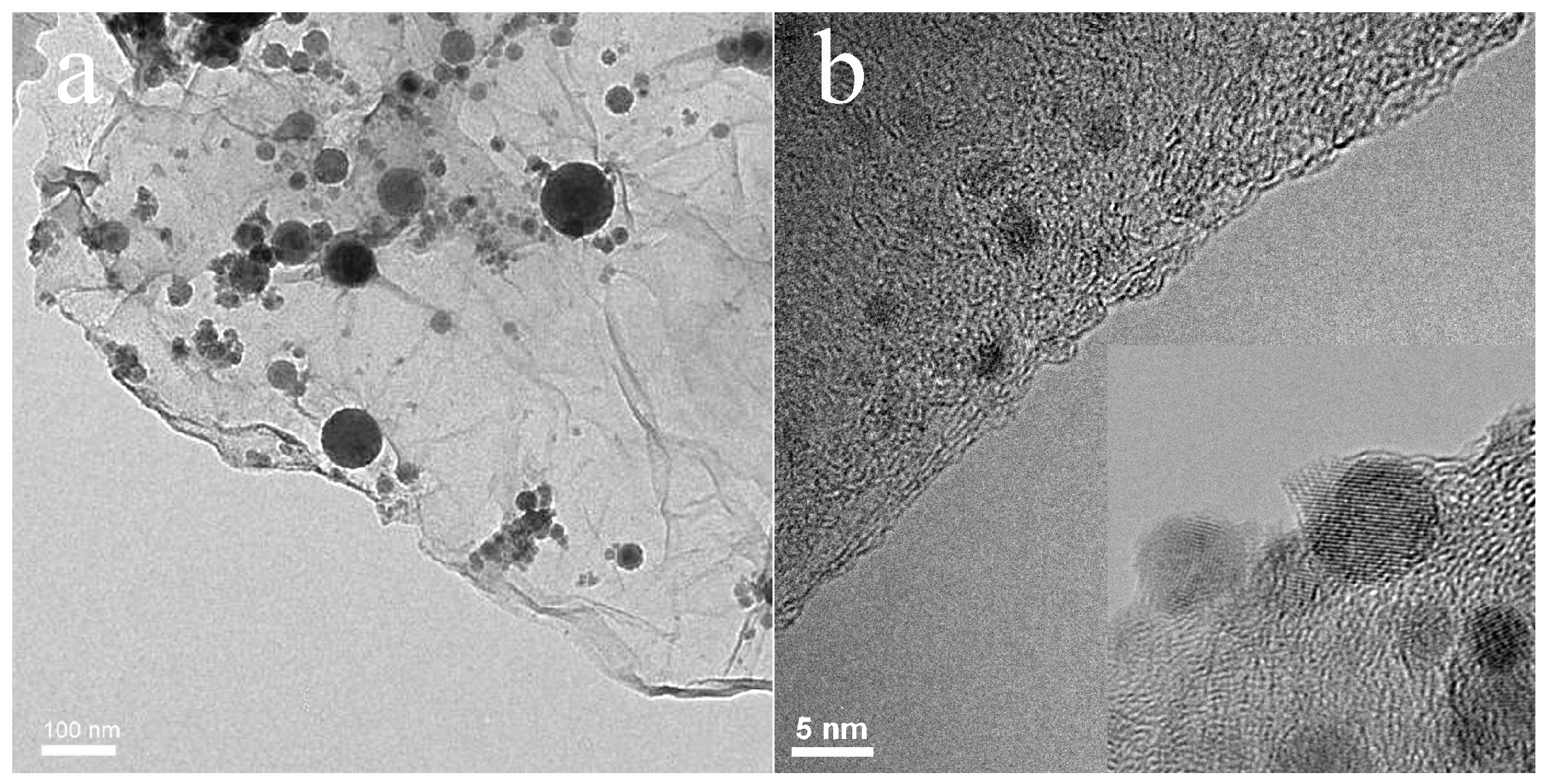
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, M.; Zimney, E.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Singha, V.; Jounga, D.; Zhaia, L.; Dasa, S.; Khondakera, S.I. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Wang, X.X.; Yu, M.X.; Zhang, W.; Zhang, B.Q.; Dong, L.F. Synthesis and microwave absorption properties of graphene/nickel composite materials. Appl. Phys. A 2015, 118, 1053–1058. [Google Scholar] [CrossRef]
- Wu, Z.S.; Zhou, G.M.; Yin, L.C.; Ren, W.C.; Li, F.; Cheng, H.M. Graphene/metal oxide composite electrodes materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Cadmium (Cd(2+)) removal by nano zerovalent iron: Surface analysis, effects of solution chemistry and surface complexation modeling. Environ. Sci. Pollut. Res. 2013, 20, 6210–6221. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, S.; Rezaee, A.; Moheb-Ai, G.A.; Jaafarzadeh, N.A. Pyrene removal from contaminated soils by modified fenton oxidation using iron nano particles. J. Environ. Health Sci. Eng. 2013, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kanel, S.R.; Greneche, J.M.; Choi, H. Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Zhang, Y.; Voit, W.; Rao, K.V.; Kehr, J.; Bjelke, B.; Muhammed, M. Superparamagnetic iron oxide nanoparticles for bio-medical applications. Scr. Mater. 2001, 44, 1713–1717. [Google Scholar] [CrossRef]
- Ma, X.X.; Tao, H.Q.; Yang, K.; Feng, L.Z.; Cheng, L.; Shi, X.Z.; Li, Y.G.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Jabeen, H.; Chandra, V.; Jung, S.; Lee, J.W.; Kim, K.S.; Kim, S.B. Enhanced Cr(VI) removal using iron nanoparticle decorated graphene. Nanoscle 2011, 3, 3583–3585. [Google Scholar] [CrossRef] [PubMed]
- Kotov, Y.A. Electric explosion of wires as a method for preparation of nanopowders. J. Nanopart. Res. 2003, 5, 539–550. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Q.G.; Yan, W.Y.; Liu, X.D.; Zhang, J.; Zhao, J.P.; Pang, L. Effect of medium on deposited energy in microsecond electrical explosion of wires. IEEE Trans. Plasma Sci. 2012, 40, 2198–2204. [Google Scholar] [CrossRef]
- Wada, N.; Akiyoshi, K.; Morita, K.; Hokamoto, K. Reaction synthesis of several titanium oxides through electrical wire explosion in air and in water. Ceram. Int. 2013, 39, 7927–7933. [Google Scholar] [CrossRef]
- Abdelkader, E.M.; Jelliss, P.A.; Buckner, S.W. Metal and metal carbide nanoparticle synthesis using electrical explosion of wires coupled with epoxide polymerization capping. Inorg. Chem. 2015, 54, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- He, L.F.; Shirahata, J.; Suematsu, H.; Nakayama, T.; Suzuki, T.; Jaing, W.; Niihara, K. Synthesis of BN nanosheet/nanotube-Fe nanocomposites by pulsed wire discharge and high-temperature annealing. Mater. Lett. 2014, 117, 120–123. [Google Scholar] [CrossRef]
- Kobayashi, R.; Nishimura, S.; Suzuki, T.; Nakayama, T.; Suematsu, H.; Jiang, W.H.; Niihara, K. Pressure dependence of phases in carbon nanosized powders for investigating cooling rate of pulsed wire discharge plasma. Adv. Mater. Res. 2006, 11, 307–310. [Google Scholar] [CrossRef]
- Suematsu, H.; Minami, C.; Kobayashi, R.; Kinemuchi, Y.; Hirata, T.; Hatakeyama, R.; Yang, S.C.; Jiang, W.H.; Yatsui, K. Preparation of fullerene by pulsed wire discharge. Jpn. J. Appl. Phys. 2003, 42, L1028–L1031. [Google Scholar] [CrossRef]
- Kobayashi, R.; Nishimura, S.; Suzuki, T.; Suematsu, H.; Jiang, W.H.; Yatsui, K. Synthesis of single-walled carbon nanotubes by pulsed wire discharge. Jpn. J. Appl. Phys. 2005, 44, 742–744. [Google Scholar] [CrossRef]
- Baklar, V.Y. Optimization of the synthesis of different carbon allotropes by electric explosion of graphite conductors. Surf. Eng. Appl. Electrochem. 2011, 47, 61–65. [Google Scholar] [CrossRef]
- Gao, X.; Xu, C.X.; Yin, H.; Wang, X.G.; Song, Q.Z.; Chen, P.W. Preparation of graphene by electrical explosion of graphite sticks. Nanoscale 2017, 9, 10639–10646. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Shim, H.W.; Kim, J.C.; Kim, D.W. Mo-MoO3-graphene nanocomposites as anode materials forlithium-ion batteries: Scalable, facile preparation and characterization. Electrochim. Acta 2017, 251, 81–90. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.C.; Shim, H.W.; Kim, D.W. Highly Reversible Li storage in hybrid NiO/Ni/graphene nanocomposites prepared by an electrical wire explosion process. ACS Appl. Mater. Interfaces 2014, 6, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Du, Q.J.; Wang, J.J.; Liu, T.H.; Sun, J.K.; Wangy, Y.H.; Wang, Z.H.; Xia, Y.Z.; Xia, L.H. Defluoridation from aqueous solution by manganese oxide coated graphene oxide. J. Fluor. Chem. 2013, 148, 67–73. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszyc, A.; Mazurkiewiczc, M.; Mierzwaa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Song, H.H.; Ma, L.L.; Chen, X.H. Magnetite/graphene nanosheet composites: Interfacial interaction and its impact on the durable high-rate performance in lithium-ion batteries. RSC Adv. 2011, 1, 782–791. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J.W.; Yang, X.J.; Lu, L.D. Deposition of Co3O4 nanoparticles onto exfoliated graphite oxide sheets. J. Mater. Chem. 2008, 18, 5625–5629. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhovalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Jansson, U.; Lu, J.; Hultman, L.; Magnuson, M. Structure and bonding in amorphous iron carbide thin films. J. Phys. Condens. Matter 2015, 27, 45002–45009. [Google Scholar] [CrossRef] [PubMed]
- Combellas, C.; Delamar, M.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Spontaneous grafting of iron surfaces by reduction of aryldiazonium salts in acidic or neutral aqueous solution. Application to the protection of iron against corrosion. Chem. Mater. 2005, 17, 3968–3975. [Google Scholar] [CrossRef]
- Chen, L.X.; He, F.; Zhao, N.Q.; Guo, R.S. Fabrication of 3D quasi-hierarchical Z-scheme RGO-Fe2O3-MoS2 nanoheterostructures for highly enhanced visible-light-driven photocatalytic degradation. Appl. Surf. Sci. 2017, 420, 669–680. [Google Scholar] [CrossRef]
- Bhargava, G.; Gouzman, I.; Chun, C.M.; Ramanarayanan, T.A.; Bernasek, S.L. Characterization of the ‘‘native’’ surface thin film on pure polycrystalline iron: A high resolution XPS and TEM study. Appl. Surf. Sci. 2007, 253, 4322–4329. [Google Scholar] [CrossRef]
- Leedahl, B.; Korolev, A.V.; Zhidkov, I.S.; Skornyakov, S.L.; Anisimov, V.I.; Belozerov, A.S.; Kukharenko, A.I.; Kurmaev, E.Z.; Grokhovskii, V.I.; Cholakh, S.O.; et al. Searching for pure iron in nature: The chelyabinsk meteorite. RSC Adv. 2016, 6, 85844–85851. [Google Scholar] [CrossRef]
- Taylor, M.J. Formation of plasma around wire fragments created by electrically exploded copper wire. J. Phys. D Appl. Phys. 2012, 24, 544–548. [Google Scholar] [CrossRef]
- Wong, C.S.; Bora, B.; Yap, S.L.; Lee, Y.S.; Bhuyan, H.; Farve, M. Effect of ambient gas species on the formation of Cu nanoparticles in wire explosion process. Curr. Appl. Phys. 2012, 12, 1345–1348. [Google Scholar] [CrossRef]


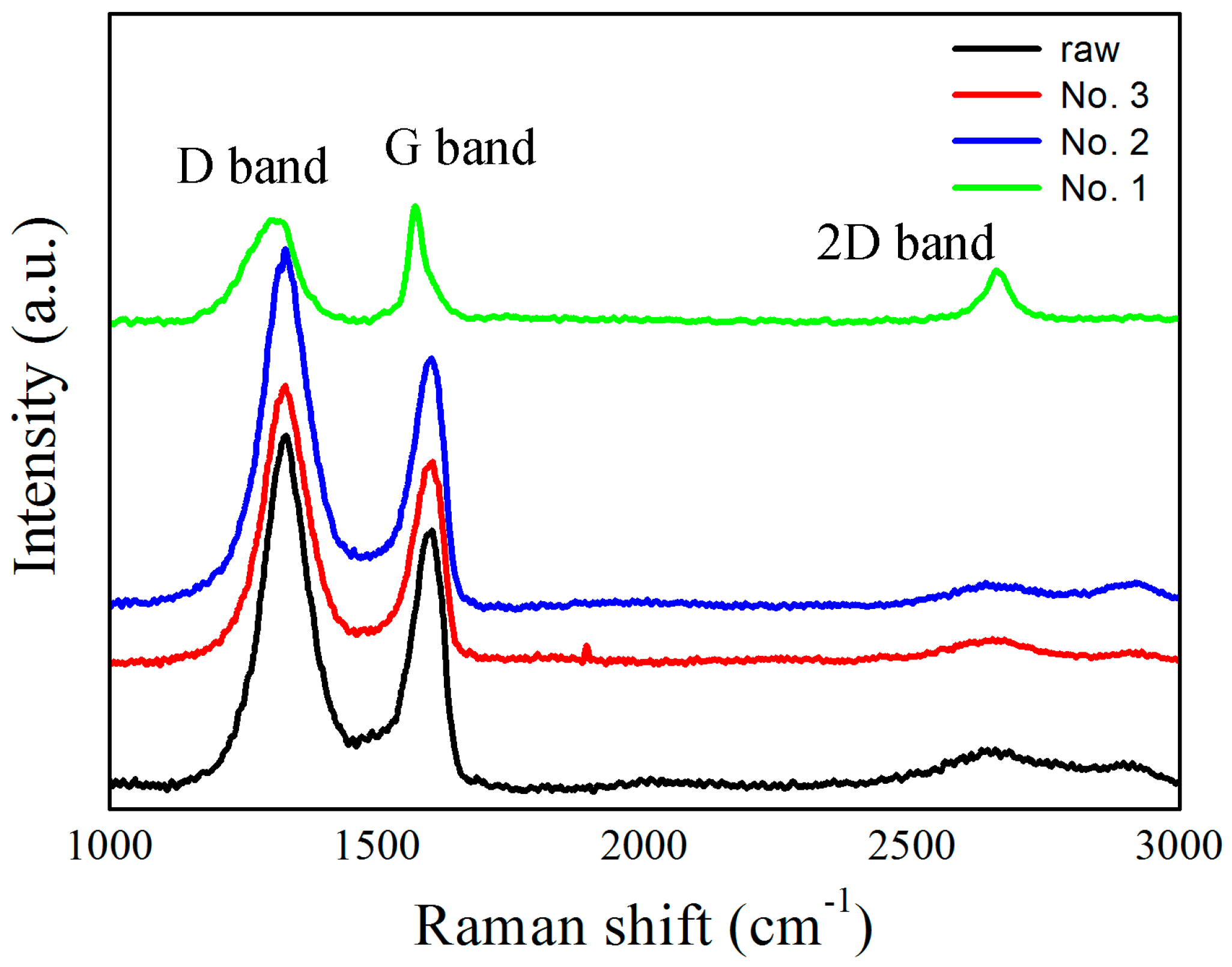
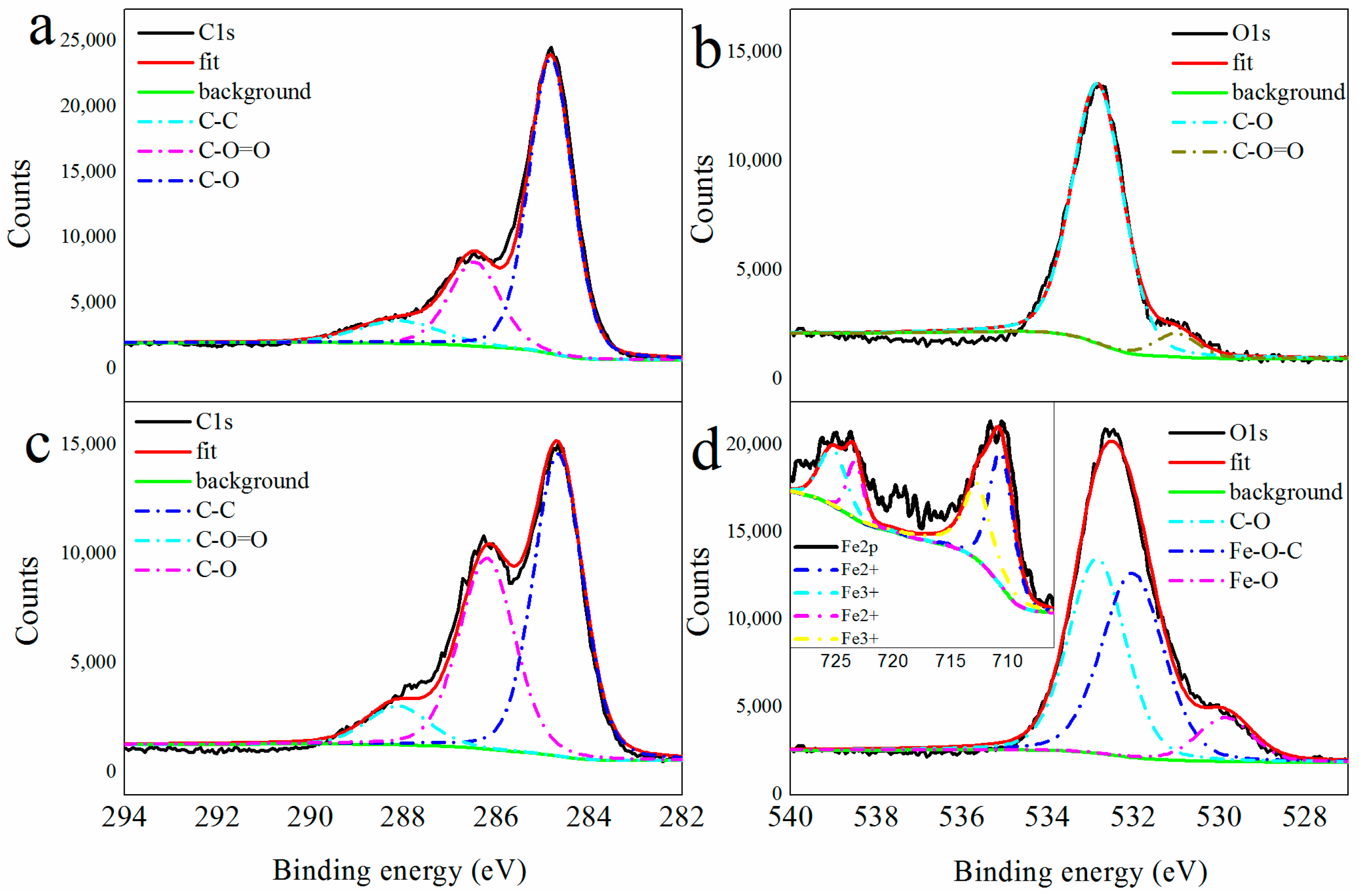
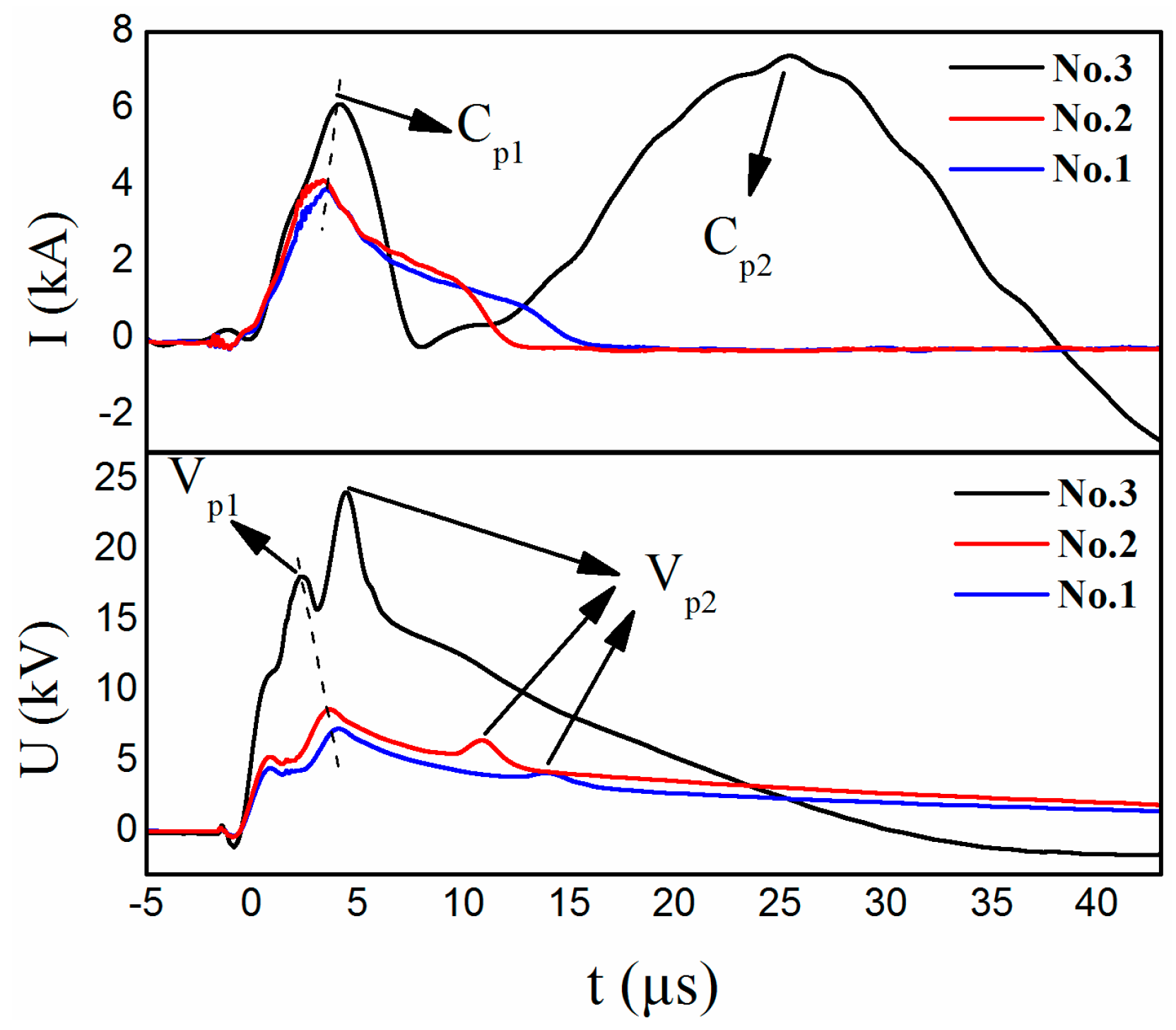
| No. | U (kV) | E (J) | Raman Spectra G Band (cm−1) | Main Products |
|---|---|---|---|---|
| 1 | 8 | 400 | 1600.5 | Fe, FeO, graphene |
| 2 | 10 | 625 | 1595.7 | Fe, FeO, graphene |
| 3 | 20 | 2500 | 1580.1 | FeO, Fe3C, FeO(OH), graphite particles |
| No. | Phase Content (%) | |||
|---|---|---|---|---|
| Fe | FeO | α-FeO (OH) | Fe3C | |
| 1 | 43 | 57 | 0 | 0 |
| 2 | 32 | 68 | 0 | 0 |
| 3 | 0 | 49 | 19 | 32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Yokota, N.; Oda, H.; Tanaka, S.; Hokamoto, K.; Chen, P. One Step Preparation of Fe–FeO–Graphene Nanocomposite through Pulsed Wire Discharge. Crystals 2018, 8, 104. https://doi.org/10.3390/cryst8020104
Gao X, Yokota N, Oda H, Tanaka S, Hokamoto K, Chen P. One Step Preparation of Fe–FeO–Graphene Nanocomposite through Pulsed Wire Discharge. Crystals. 2018; 8(2):104. https://doi.org/10.3390/cryst8020104
Chicago/Turabian StyleGao, Xin, Naoaki Yokota, Hayato Oda, Shigeru Tanaka, Kazuyuki Hokamoto, and Pengwan Chen. 2018. "One Step Preparation of Fe–FeO–Graphene Nanocomposite through Pulsed Wire Discharge" Crystals 8, no. 2: 104. https://doi.org/10.3390/cryst8020104