Structure and Raman Spectra of C60 and C70 Fullerenes Encased into Single-Walled Boron Nitride Nanotubes: A Theoretical Study
Abstract
:1. Introduction
2. Models and Simulation Method
2.1. Structure and Dynamics of C@SWBNNT and C@SWBNNT Peapods
2.2. Methods
3. Results and Discussion
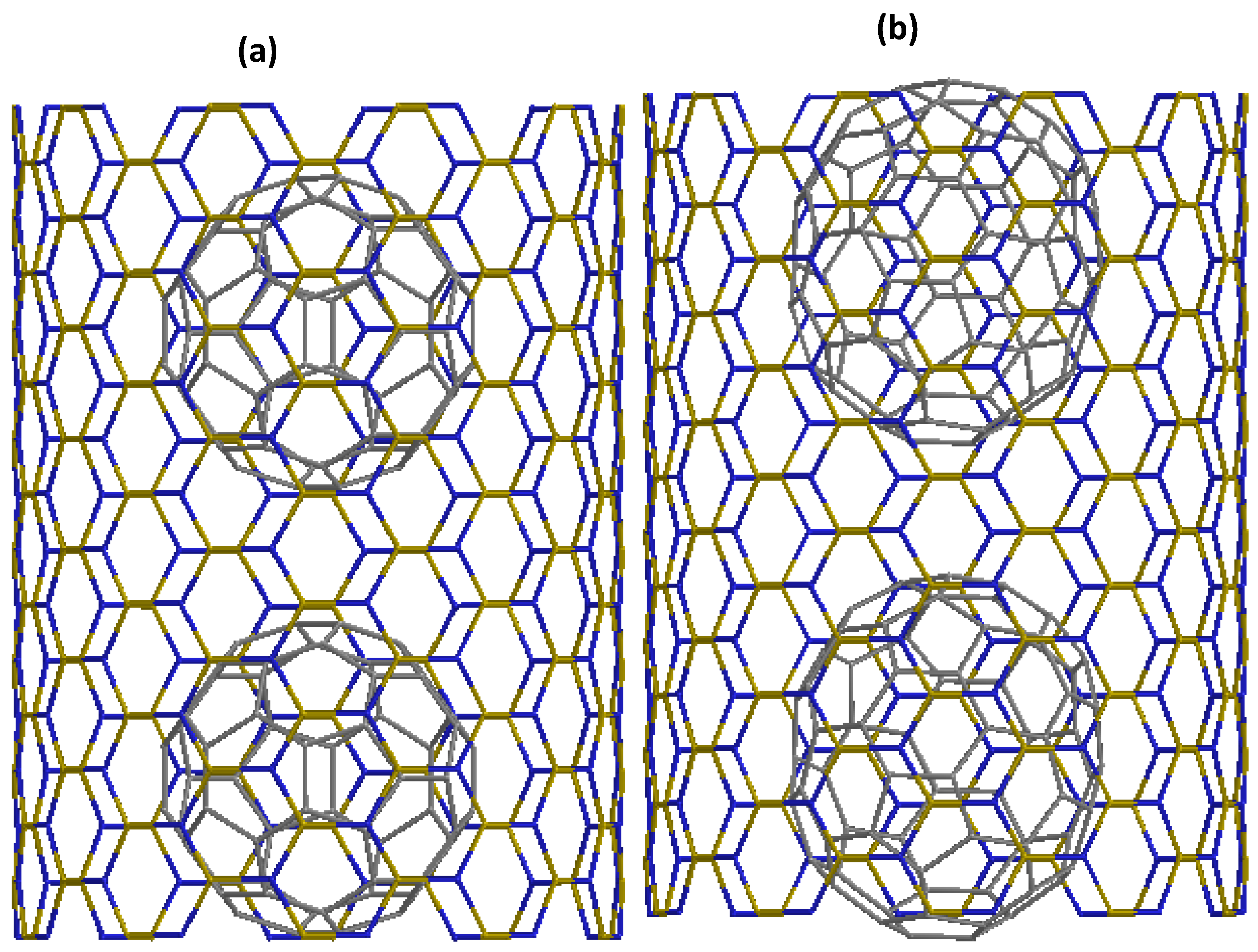
3.1. Optimized Structure of C@SWBNNT and C@SWBNNT
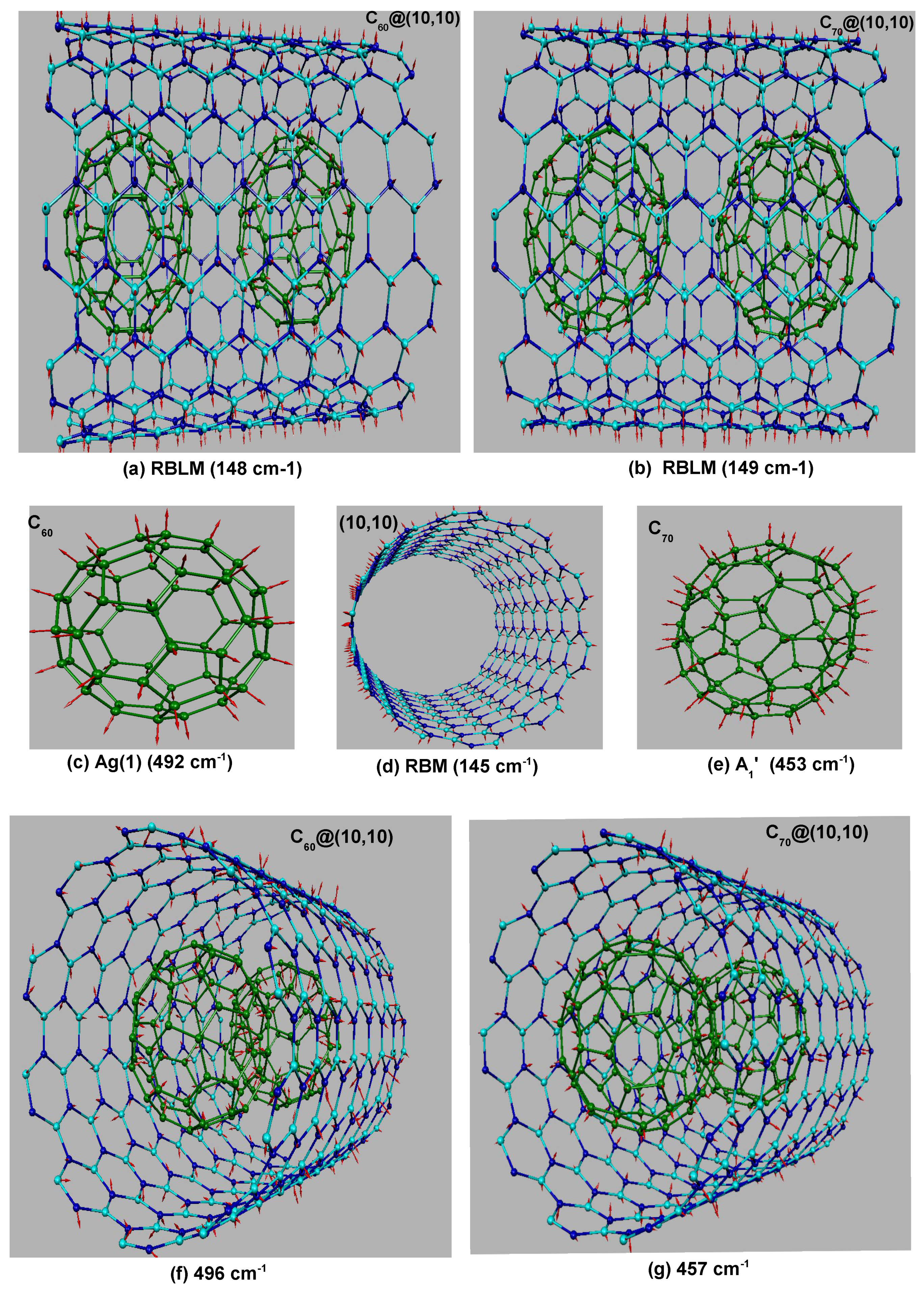
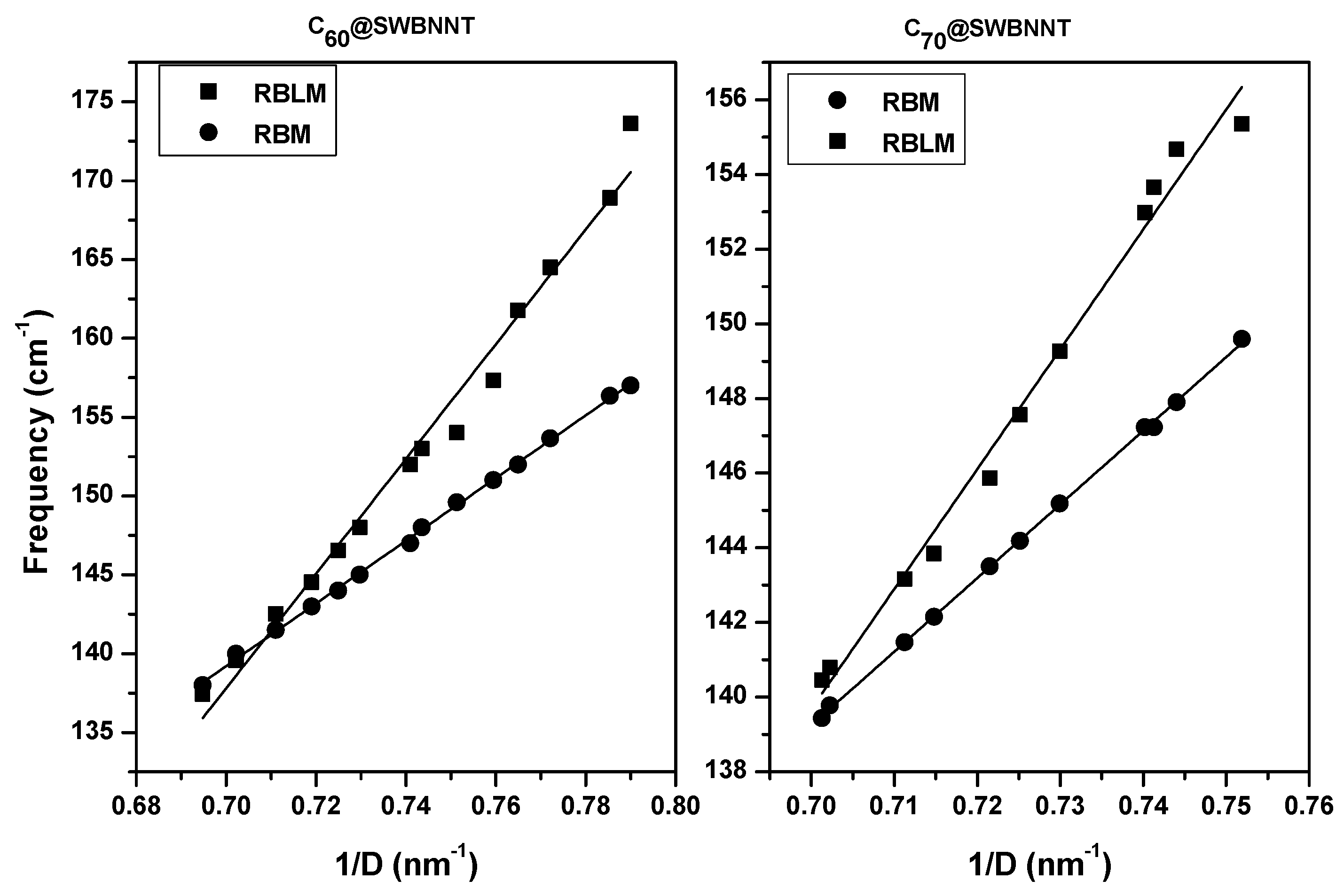
3.2. Raman Spectra of Completely Filled Peapods
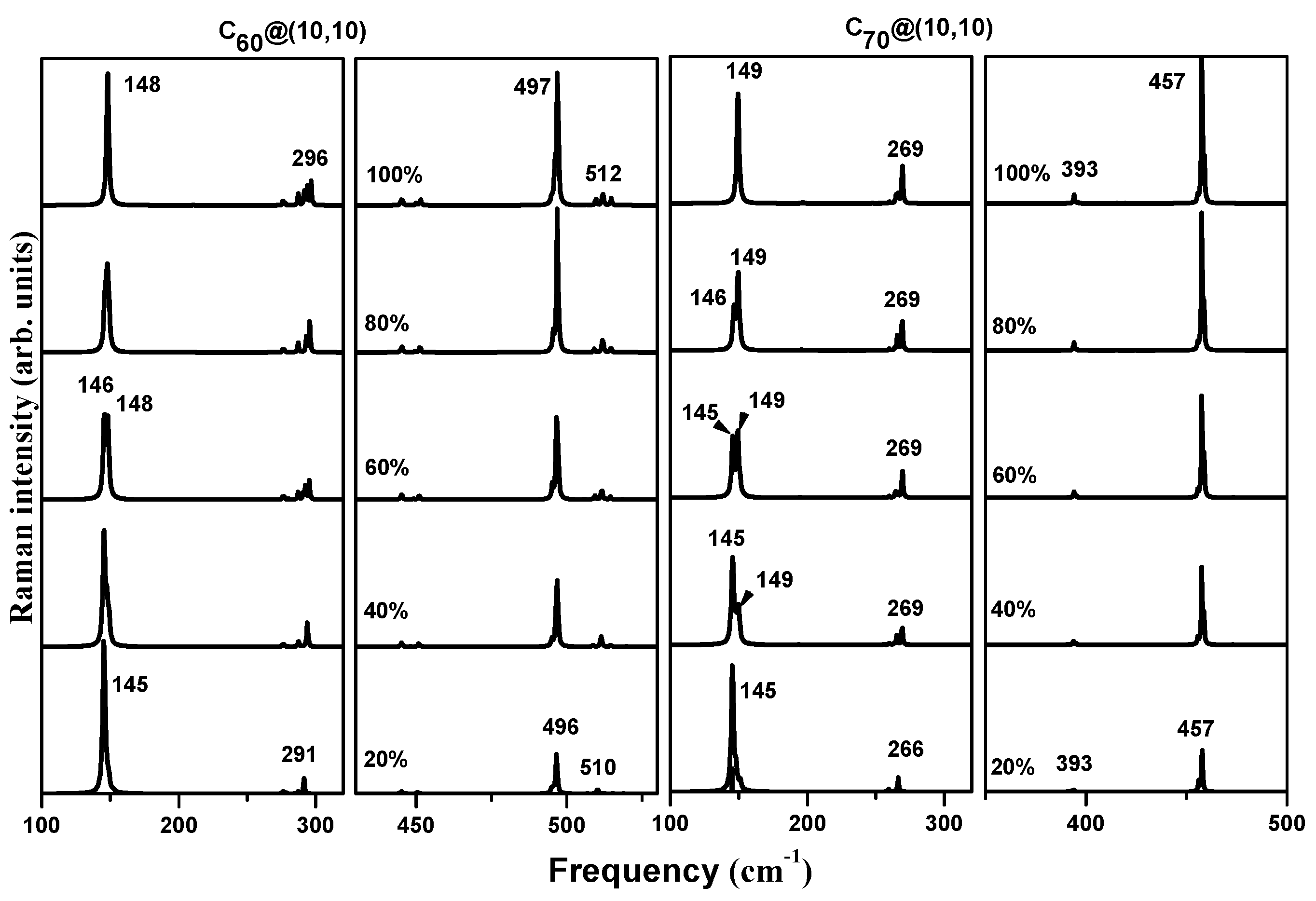
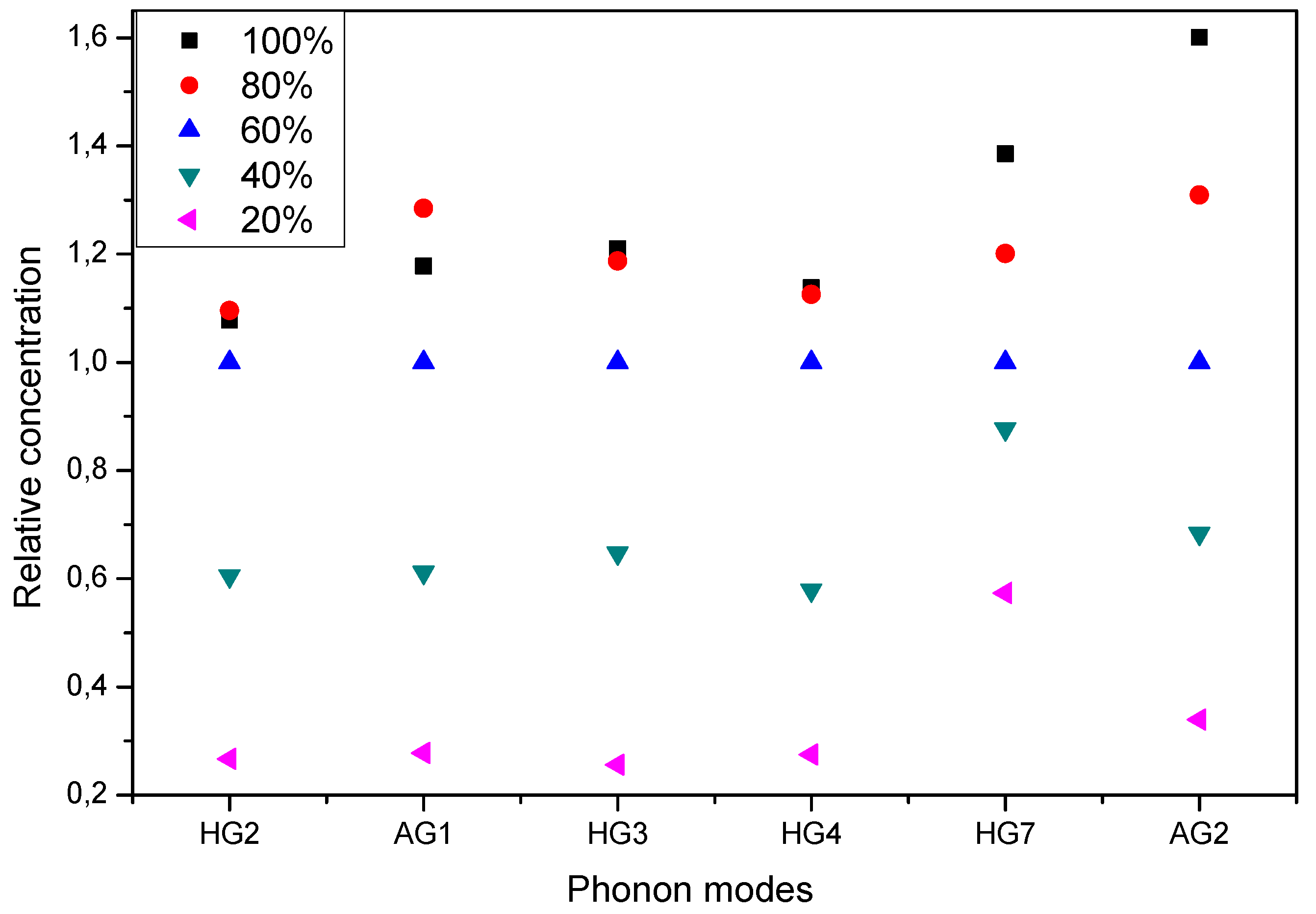
3.3. Raman Spectra of Incompletely Filled Peapods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1995, 318, 162–163. [Google Scholar] [CrossRef]
- Ijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron nitride nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.W.; Monthioux, M.; Luzzi, D. Encapsulated C60 in carbon nanotubes. Nature 1998, 396, 323–324. [Google Scholar] [CrossRef]
- Smith, B.W.; Luzzi, D.E. Formation mechanism of fullerene peapods and coaxial tubes: A path to large scale synthesis. Chem. Phys. Lett. 2000, 321, 169–174. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Zou, D.J.; Zhang, X.; Yan, Q.; Rodriguez-Gil, D.J.; Eisner, C.; Wells, E.; Greer, C.A.; Wang, T.; Firestein, S.; et al. Functional expression of the olfactory signaling system in the kidney. Proc. Natl Acad. Sci. USA 2009, 106, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.S.; Ito, Y.; Robertson, A.W.; Shinohara, H.; Warner, J.H. Two-dimensional coalescence dynamics of encapsulated metallofullerenes in carbon nanotubes. ACS Nano 2011, 5, 10084. [Google Scholar] [CrossRef] [PubMed]
- Terrones, H. Beyond Carbon Nanopeapods. ChemPhysChem 2012, 13, 2273–2276. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.K.; Tomanek, D.; Iijima, S. “Bucky Shuttle” memory device: Synthetic approach and molecular dynamics simulations. Phys. Rev. Lett. 1999, 82, 1470–1476. [Google Scholar] [CrossRef]
- Cox, B.J.; Thamwattana, N.; Hill, J.M. Orientation of spheroidal fullerenes inside carbon nanotubes with potential applications as memory devices in nano-computing. Physica A 2008, 41, 235209. [Google Scholar] [CrossRef]
- Service, R.F. Nanotube ’Peapods’ Show Electrifying Promise. Science 2001, 292, 45. [Google Scholar] [CrossRef]
- Okada, S.; Saito, S.; Oshiyama, A. Semiconducting form of the first-row elements: C60 chain encapsulated in BN nanotubes. Phys. Rev. B 2001, 64, 201303(R). [Google Scholar] [CrossRef] [Green Version]
- Mickelson, W.; Aloni, S.; Han, W.Q.; Cumings, J.; Zettl, A. Packing C60 in boron nitride nanotubes. Science 2003, 5300, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Hasi, F.; Simon, F.; Kuzmany, H.; Arenal De La Concha, R.; Loiseau, A. Raman spectroscopy of boron nitride nanotubes and boron nitride-Carbon composites. AIP Conf. Proc. 2005, 786, 340–344. [Google Scholar] [CrossRef]
- Thamwattana, N.; Hill, J.M. Continuum modelling for carbon and boron nitride nanostructures. J. Phys. Condens. Matter 2007, 19, 406209. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Hwang, H.J. Comparison of C60 encapsulations into carbon and boron nitride nanotubes. J. Phys. Condens. Mat. 2004, 16, 3901. [Google Scholar] [CrossRef]
- Trave, A.; Ribeiro, F.; Louie, S.; Cohen, M. Energetics and structural characterization of C60 polymerization in BN and carbon nanopeapods. Phys. Rev. B 2004, 70, 205418. [Google Scholar] [CrossRef]
- Zettl, A.; Cumings, J.; Weiqiang, H.; Mickelson, W. Boron nitride nanotube peapods. AIP Conf. Proc. 2002, 633, 140. [Google Scholar] [CrossRef]
- Burteaux, B.; Claye, A.; Smith, B.W.; Monthioux, M.; Luzzi, D.E.; Fischer, J.E. Abundance of encapsulated C60 in single-wall carbon nanotubes. Chem. Phys, Lett. 1999, 310, 21–24. [Google Scholar] [CrossRef]
- Moon, W.H.; Son, M.S.; Lee, J.H.; Hwang, H.J. Molecular dynamics simulation of C60 encapsulated in boron nitride nanotubes. Phys. Status Solidi B 2004, 241, 1783. [Google Scholar] [CrossRef]
- Hodak, M.; Girifalco, L.A. Systems of C60 molecules inside (10,10) and (15,15) nanotube: A Monte Carlo study. Phys. Rev. B 2003, 68, 085405. [Google Scholar] [CrossRef]
- Hodak, M.; Girifalco, L.A. Ordered phases of fullerene molecules formed inside carbon nanotubes. Phys. Rev. B 2003, 67, 075419. [Google Scholar] [CrossRef]
- Chadli, H.; Rahmani, A.; Sbai, K.; Hermet, P.; Rols, S.; Sauvajol, J.-L. Calculation of Raman-active modes in linear and zigzag phases of fullerene peapods. Phys. Rev. B 2006, 74, 205412–205419. [Google Scholar] [CrossRef]
- Chadli, H.; Rahmani, A.; Sbai, K.; Sauvajol, J.L. Raman active modes in carbon peapods. Physica A 2005, 358, 226–236. [Google Scholar] [CrossRef]
- Barajas-Barraza, R.E.; Guirado-Lopez, R.A. Clustering of H2 molecules encapsulated in fullerene structures. Phys. Rev. B 2002, 66, 155426–155437. [Google Scholar] [CrossRef]
- Guan, L.; Li, H.; Shi, Z.; You, L.; Gu, Z. Standing or lying C70s encapsulated in carbon nanotubes with different diameters. Solid State Commun. 2005, 133, 333–337. [Google Scholar] [CrossRef]
- Fergani, F.; Chadli, H.; Belhboub, A.; Hermet, P.; Rahmani, A. Theoretical Study of the Raman Spectra of C70 Fullerene Carbon Peapods. J. Phys. Chem. C 2015, 119, 5679–5686. [Google Scholar] [CrossRef]
- Fergani, F.; Abdelkader, S.A.A.; Chadli, H.; Fakrach, B.; Rahmani, A.H.; Hermet, P.; Rahmani, A. C60 filling rate in carbon peapods: A nonresonant raman spectra analysis. Hindawi J. Nanomater. 2017, 2017, 9248153–9248159. [Google Scholar] [CrossRef]
- Saito, R.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Electronic structure of graphene tubules based on C60. Phys. Rev. B 1992, 46, 1804–1811. [Google Scholar] [CrossRef]
- Hamada, N.; Sawada, S.I.; Oshiyama, A. New one-dimensional conductors: Graphitic microtubules. Phys. Rev. Lett. 1992, 68, 1579–1581. [Google Scholar] [CrossRef] [PubMed]
- Darkrim, F. Levesque, Monte Carlo simulations of hydrogen adsorption in single-walled carbon nanotubes. J. Chem. Phys. 1998, 19, 4981. [Google Scholar] [CrossRef]
- Fakrach, B.; Rahmani, A.; Chadli, H.; Sbai, K.; Bentaleb, M.; Bantignies, J.L.; Sauvajol, J.L. Infrared spectrum of single-walled boron nitride nanotubes. Phys. Rev. B 2012, 85, 115437–115446. [Google Scholar] [CrossRef]
- Jishi, R.A.; Mirie, R.M.; Dresselhaus, M.S. Force-constant model for the vibrational modes in C60. Phys. Rev. B 1992, 45, 13685–13689. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; GarcÃ, A.; Junquera, J.; Ordejon, P.; Sanchez-Portal, J. The SIESTA method for ab initio order-N materials simulation. Phys. Condens. Matter 2002, 14, 2745–2779. [Google Scholar] [CrossRef]
- Guha, S.; Menendez, J.; Page, J.B.; Adams, G.B. Empirical bond polarizability model for fullerenes. Phys. Rev. B 1996, 53, 13106–13114. [Google Scholar] [CrossRef]
- Benoit, C.; Royer, E.; Poussigue, G. The spectral moments method. J. Phys. Condens. Matter 1992, 4, 3125–13114. [Google Scholar] [CrossRef]
- Rahmani, A.; Benoit, C.; Poussigue, G. Vibrational properties of random percolating networks. J. Phys. Condens. Matter 1993, 5, 7941. [Google Scholar] [CrossRef]
- Chadli, H.; Rahmani, A.; Sauvajol, J.-L. Raman spectra of C60 dimer and C60 polymer confined inside a (10,10) single-walled carbon nanotube. J. Phys. Condens. Matter 2010, 22, 145303–145312. [Google Scholar] [CrossRef]
- Chadli, H.; Fergani, F.; Bentaleb, M.; Fakrach, B.; Sbai, K.; Rahmani, A.; Bantignies, J.-L.; Sauvajol, J.-L. Influence of packing on the vibrations of homogeneous bundles of C60 peapods. Physica E 2015, 71, 31–38. [Google Scholar] [CrossRef]
- Bradley, C.J.; Cracknell, A.P. The Mathematical Theory of Symmetry in Solids: Representation Theory for Point Groups and Space Groups; Clarendon Press: Oxford, UK, 1972; ISBN-13: 978-0199582587. [Google Scholar]
- Golberg, D.; Bando, Y.; Kurashima, K.; Soto, T. Synthesis and characterization of ropes made of BN multiwalled nanotubes. Scr. Mater. 2001, 44, 1561–1565. [Google Scholar] [CrossRef]
- Lin, F.H.; Hsu, C.K.; Tang, T.P.; Kang, P.L.; Yang, F.F. Thermal-heating CVD synthesis of BN nanotubes from trimethyl borate and nitrogen gas. Mater. Chem. Phys. 2008, 107, 115–121. [Google Scholar] [CrossRef]
- Lee, C.H.; Wang, J.; Kayatsha, V.K.; Huang, J.Y.; Yap, Y.K. Junctions between a boron nitridenanotube and a boron nitride sheet. Nanotechnology 2008, 19, 455605–455616. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Luo, J.; Wei, J.; Lin, J. Synthesis of boron nitride nanotubes and its hydrogen uptake. Catal. Today 2007, 120, 346–350. [Google Scholar] [CrossRef]
- Smith, M.W.; Jordan, K.C.; Park, C.; Kim, J.W.; Lillehei, P.T.; Crooks, R.; Harrison, J.S. Very long single and few-walled boron nitride nanotubes via the pressurized vapor/condenser method. Nanotechnology 2009, 20, 505604–505609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, W.; Dai, Y. Stability and electronic properties of small boron nitride nanotubes. J. Appl. Phys. 2009, 105, 084312–084319. [Google Scholar] [CrossRef]
- Fakrach, B.; Rahmani, A.; Chadli, H.; Sbai, K.; Sauvajol, J.L. Raman spectrum of single-walled boron nitride nanotube. Physica E 2009, 41, 1800–1805. [Google Scholar] [CrossRef]
- Popov, V.N. Lattice dynamics of single-walled boron nitride nanotubes. Phys. Rev. B 2003, 67, 085408–085413. [Google Scholar] [CrossRef]
- Wirtz, L.; Rubio, A.; de la Concha, R.A.; Loiseau, A. Ab initio calculations of the lattice dynamics of boron nitride nanotubes. Phys. Rev. B 2003, 68, 045425–045437. [Google Scholar] [CrossRef]
- Akdim, R.; Pachter, R.; Xiaofeng, D.; Adams, W.W. Comparative theoretical study of single-wall carbon and boron-nitride nanotubes. Phys. Rev. B 2003, 67, 245404–245411. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. High-Yield Fullerene Encapsulation in Single-Wall Carbon Nanotubes. Synth. Metals 2001, 121, 1195–1196. [Google Scholar] [CrossRef]
- Chorro, M.; Delhey, A.; No, L.; Monthioux, M.; Launois, P. Orientation of C70 molecules in peapods as a function of the nanotube diameter. Phys. Rev. B 2007, 75, 035416. [Google Scholar] [CrossRef]
- Liu, X.; Pichler, T.; Knupfer, M.; Golden, M.S.; Fink, J.; Kataura, H.; Achiba, Y.; Hirahara, K.; Ijima, S. Filling factors, structural, and electronic properties of C60 molecules in single-wall carbon nanotubes. Phys. Rev. B 2002, 65, 045419–045424. [Google Scholar] [CrossRef]
- Bandow, S.; Takizawa, M.; Kato, H.; Okazaki, T.; Shinohara, H.; Iijima, S. Smallest limit of tube diameters for encasing of particular fullerenes determined by radial breathing mode raman scattering. Chem. Phys. Lett. 2001, 347, 23–28. [Google Scholar] [CrossRef]

| SWBNNT | SWBNNT | SWBNNT | -SWBNNT | - |
|---|---|---|---|---|
| Index | Diameter | Chirality | Distance | Distance |
| (n,m) | (nm) | (deg) | (nm) | (nm) |
| (11,8) | 1.307 | 24.80 | 0.303 | 1.010 |
| (12,7) | 1.316 | 21.36 | 0.307 | 1.007 |
| (13,6) | 1.330 | 18 | 0.314 | 1.007 |
| (17,0) | 1.344 | 0 | 0.321 | 1.010 |
| (14,5) | 1.351 | 14.70 | 0.324 | 1.000 |
| (10,10) | 1.370 | 30 | 0.334 | 1.008 |
| (15,4) | 1.372 | 11.52 | 0.335 | 1.010 |
| (12,8) | 1.379 | 23.41 | 0.339 | 1.009 |
| (14,6) | 1.406 | 17 | 0.351 | 1.009 |
| (18,0) | 1.424 | 0 | 0.350 | 1.003 |
| (11,10) | 1.439 | 28.42 | 0.349 | 1.005 |
| (13,8) | 1.452 | 22.17 | 0.334 | 1.012 |
| SWBNNT | SWBNNT | SWBNNT | -SWBNNT | Angle | - |
|---|---|---|---|---|---|
| Index | Diameter | Chirality | Distance | Distance | |
| (n,m) | (nm) | (deg) | (nm) | (deg) | (nm) |
| (13,6) | 1.330 | 18 | 0.307 | 0 | 1.1273 |
| (17,0) | 1.344 | 0 | 0.314 | 0 | 1.1276 |
| (14,5) | 1.351 | 14.70 | 0.317 | 0 | 1.1280 |
| (10,10) | 1.370 | 30 | 0.327 | 0 | 1.1281 |
| (15,4) | 1.372 | 11.52 | 0.3028 | 0 | 1.1280 |
| (14,6) | 1.406 | 17 | 0.305 | 2 | 1.1260 |
| (18,0) | 1.424 | 0 | 0.309 | 40 | 1.1079 |
| (11,10) | 1.439 | 28.42 | 0.301 | 47 | 1.1060 |
| (18,1) | 1.465 | 2.68 | 0.304 | 65 | 1.0821 |
| (17,3) | 1.478 | 8 | 0.325 | 90 | 1.0039 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakrach, B.; Fergani, F.; Boutahir, M.; Rahmani, A.; Chadli, H.; Hermet, P.; Rahmani, A. Structure and Raman Spectra of C60 and C70 Fullerenes Encased into Single-Walled Boron Nitride Nanotubes: A Theoretical Study. Crystals 2018, 8, 118. https://doi.org/10.3390/cryst8030118
Fakrach B, Fergani F, Boutahir M, Rahmani A, Chadli H, Hermet P, Rahmani A. Structure and Raman Spectra of C60 and C70 Fullerenes Encased into Single-Walled Boron Nitride Nanotubes: A Theoretical Study. Crystals. 2018; 8(3):118. https://doi.org/10.3390/cryst8030118
Chicago/Turabian StyleFakrach, Brahim, Fatima Fergani, Mourad Boutahir, Abdelhai Rahmani, Hassane Chadli, Patrick Hermet, and Abdelali Rahmani. 2018. "Structure and Raman Spectra of C60 and C70 Fullerenes Encased into Single-Walled Boron Nitride Nanotubes: A Theoretical Study" Crystals 8, no. 3: 118. https://doi.org/10.3390/cryst8030118





