Thermoluminescent Properties of Cerium-Doped Lu2SO5 and Y2SiO5 Single Crystalline Films Scintillators Grown from PbO-B2O3 and Bi2O3 Fluxes
Abstract
:1. Introduction
2. SCF Preparation and Investigation Methods
3. Results
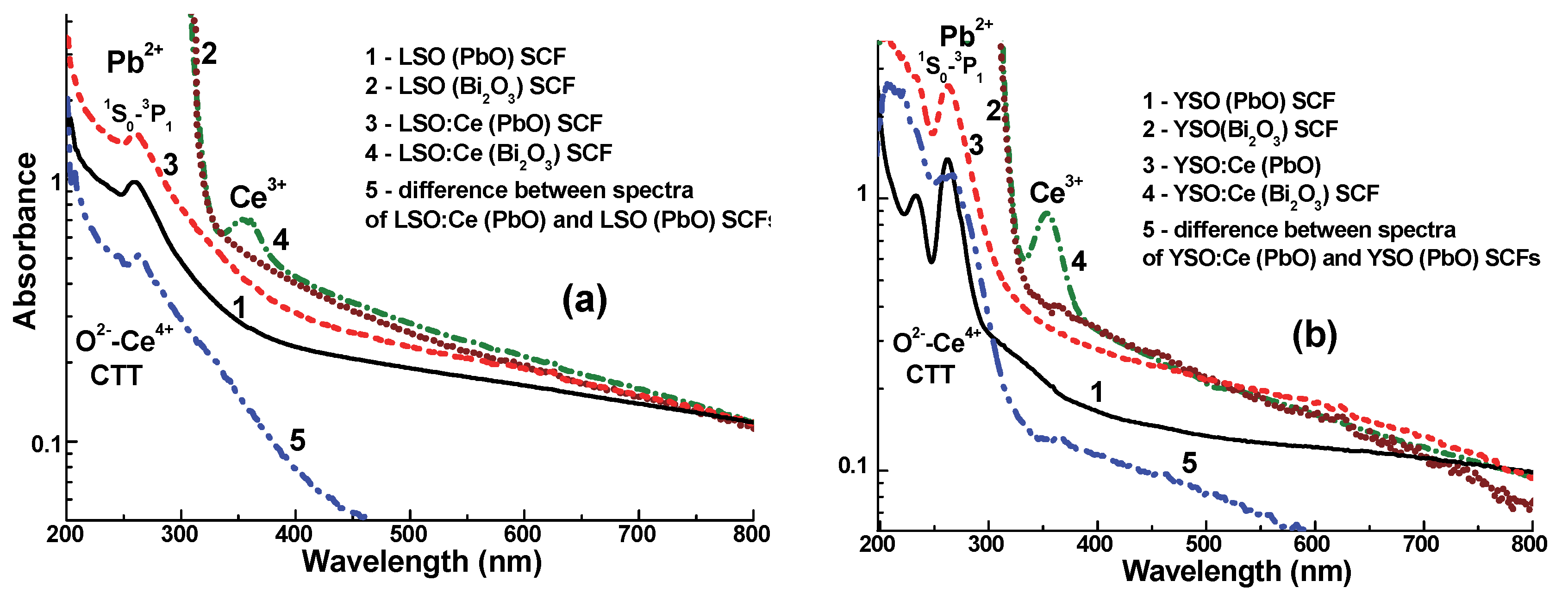
3.1. Absorption Spectra
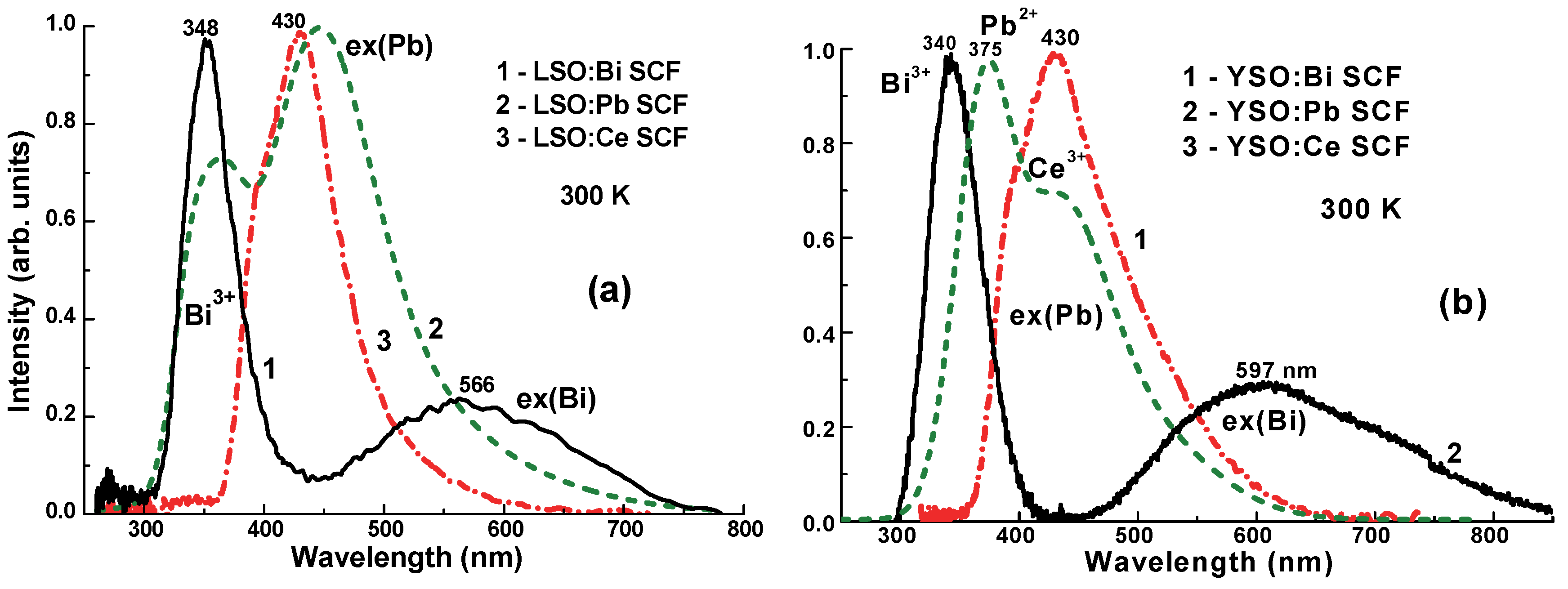
3.2. Cathodoluminescence (CL) Spectra
3.3. Spectrally-Resolved Thermoluminescence
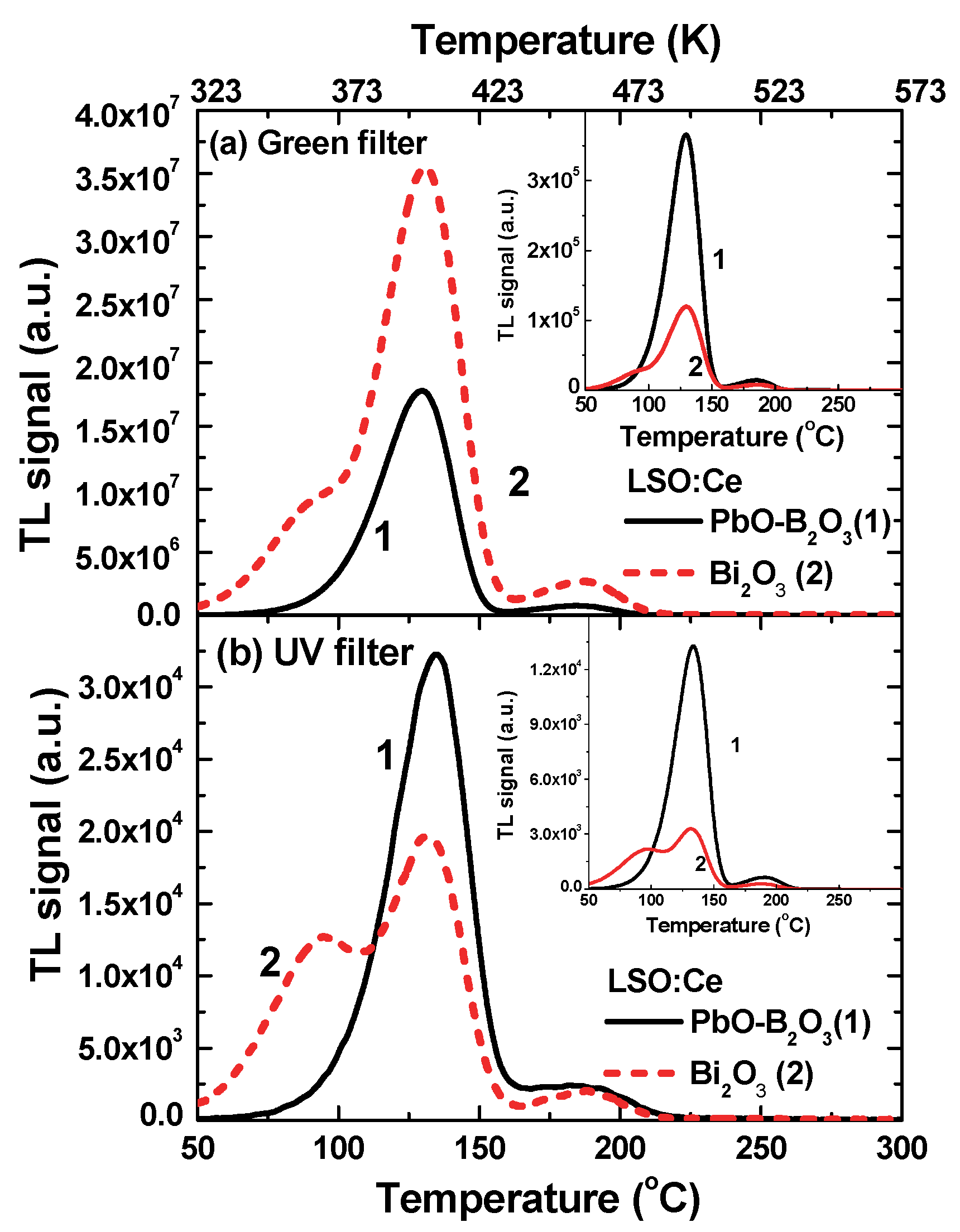
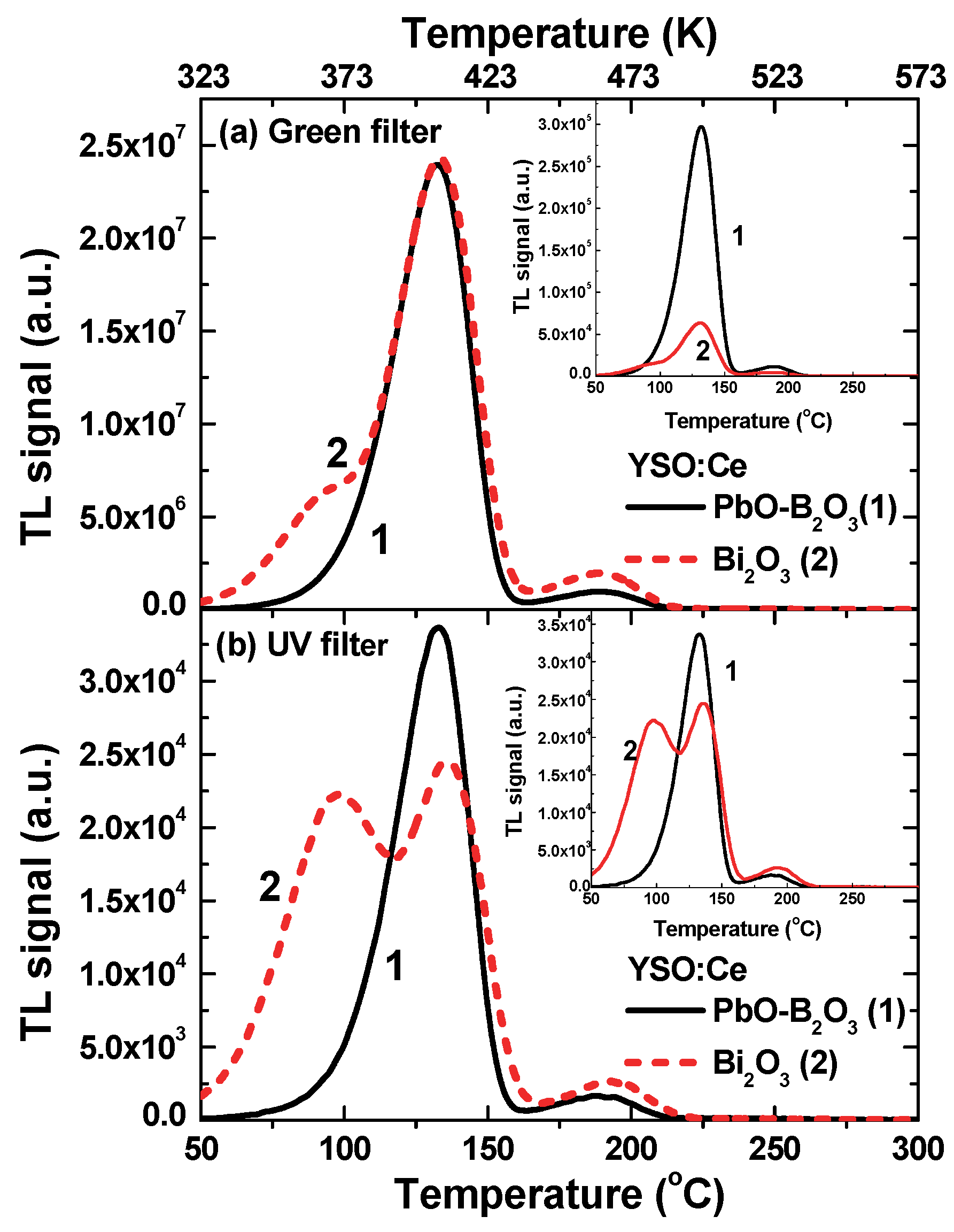
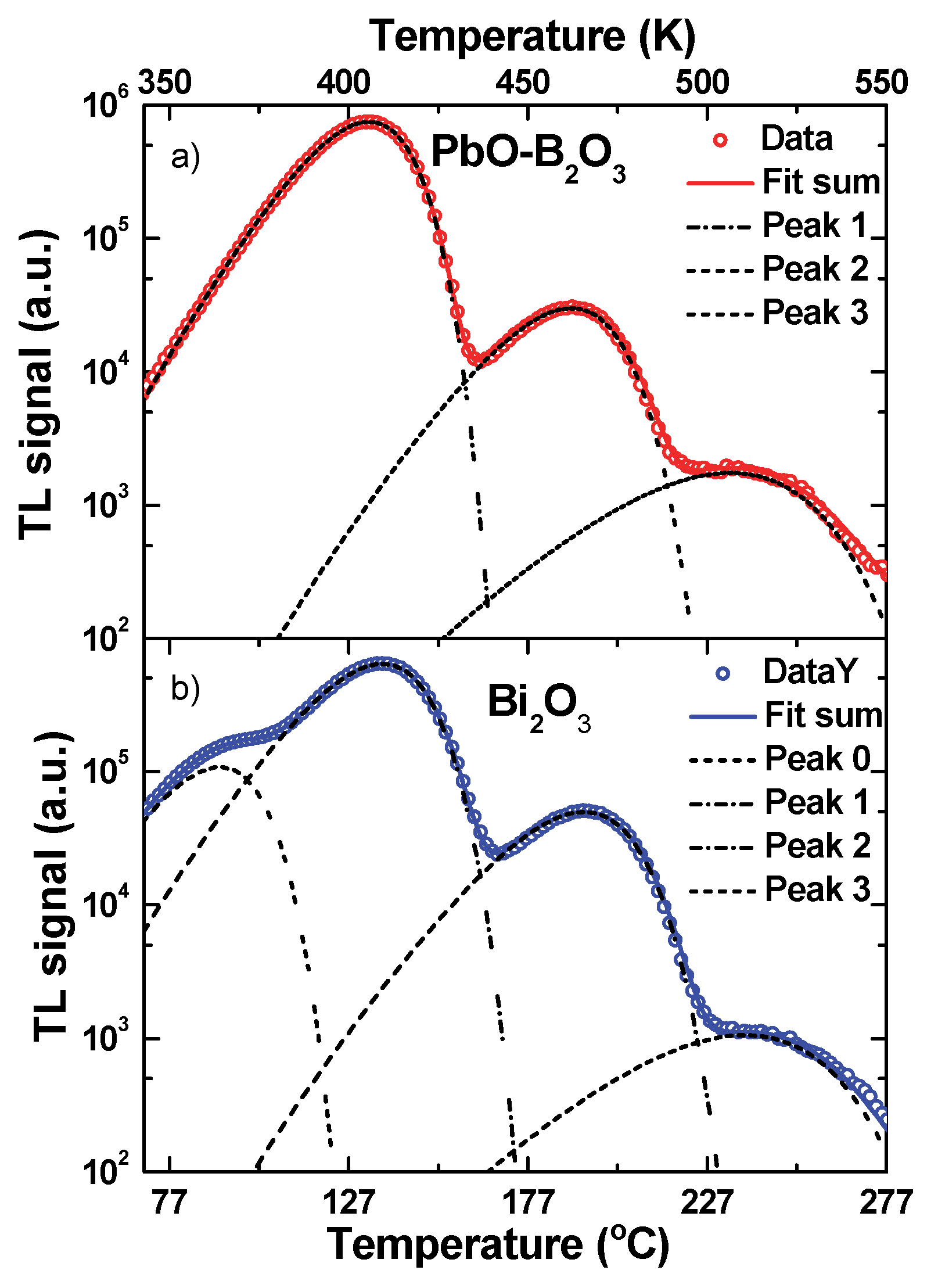
3.4. Thermoluminescence Glow Curves
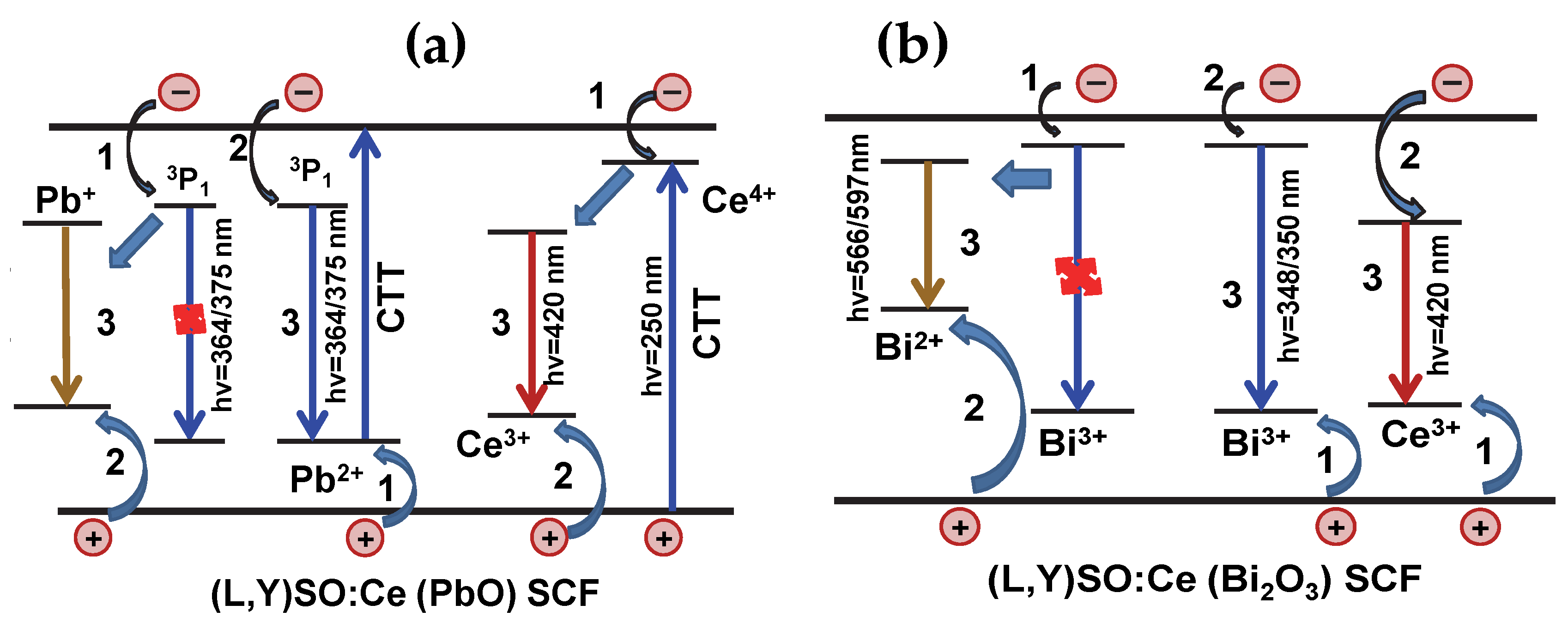
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Melcher, C.L.; Manente, R.A.; Peterson, C.A.; Schweizer, J.S. Czochralski growth of rare earth oxyorthosilicate single crystals. J. Cryst. Growth 1993, 128, 1001–1005. [Google Scholar] [CrossRef]
- Мartin, Т.; Koch, A. Recent development in X-ray imaging with micrometer spatial resolution. J. Synchrotron Radiat. 2006, 13, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Zorenko, Y.; Gorbenko, V.; Konstankevych, I.; Grinev, B.; Globus, M. Scintillation properties of Lu3Al5O12:Ce single crystalline films. Nucl. Instrum. Methods Phys. Res. A 2002, 486, 309–314. [Google Scholar] [CrossRef]
- Martin, T.; Douissard, P.-A.; Couchaud, M.; Cecilia, A.; Baumbach, T.; Dupré, K.; Rack, A. LSO-based single crystal film scintillator for synchrotron-based hard X-ray micro-imaging. IEEE Trans. Nucl. Sci. 2009, 56, 1412–1418. [Google Scholar] [CrossRef]
- Douissard, P.A.; Cecilia, A.; Martin, T.; Chevalier, V.; Couchaud, M.; Baumbach, T.; Dupré, K.; Kühbacher, M.; Rack, A. A novel epitaxially grown LSO-based thin-film scintillator for micro-imaging using hard synchrotron radiation. J. Synchrotron Radiat. 2010, 17, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Zorenko, Y.; Nikl, M.; Gorbenko, V.; Savchun, V.; Voznyak, T.; Kucerlova, R.; Sidletskiy, O.; Grynyov, B.; Fedorov, A. Growth and luminescent properties of Lu2SiO5 and Lu2SiO5:Ce single crystalline films. Opt. Mater. 2011, 33, 846–852. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Savchyn, V.; Voznyak, T.; Gorbenko, V.V.; Nikl, M.; Mares, J.A.; Sidletskiy, O.; Grynyov, B.; Fedorov, A.; et al. Scintillation and luminescent properties of undoped and Ce3+ doped Y2SiO5 and Lu2SiO5 single crystalline films grown by LPE method. Opt. Mater. 2012, 34, 1969–1974. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Savchyn, V.; Zorenko, T.; Grinyov, B.; Sidletskiy, O.; Fedorov, A. Growth and luminescent properties of Ce and Ce-Tb doped (Y,Lu,Gd)2SiO5:Ce single crystalline films. J. Cryst. Growth 2014, 401, 577–583. [Google Scholar] [CrossRef]
- Douissard, P.A.; Cecilia, A.; Rochet, X.; Chapel, X.; Martin, T.; Van De Kamp, T.; Helfen, L.; Baumbach, T.; Luquot, L.; Xiao, X.; et al. A versatile indirect detector design for hard X-ray microimaging. J. Instrum. 2012, 7, P09016. [Google Scholar] [CrossRef]
- Cecilia, A.; Rack, A.; Douissard, P.-A.; Martin, T.; dos Santos Rolo, T.; Vagovič, P.; Hamann, E.; van de Kampa, T.; Riedel, A.; Fiederle, M.; et al. LPE grown LSO: Tb scintillator films for high-resolution X-ray imaging applications at synchrotron light sources. Nucl. Instrum. Methods Phys. Res. A 2011, 648, S321–S323. [Google Scholar] [CrossRef]
- Twardak, A.; Bilski, P.; Zorenko, Y.; Gorbenko, V.; Mandowski, A.; Mandowska, E.; Sidletskiy, O. Comparative study of TSL and OSL properties of LSO and LSO:Ce single crystals and single crystalline films. Radiat. Meas. 2013, 56, 196–199. [Google Scholar] [CrossRef]
- Bilski, P.; Twardak, A.; Zorenko, Y.; Zorenko, T.; Gorbenko, V.; Mandowska, E.; Mandowski, A.; Sidletskiy, O.; Mares, J. Thermoluminescent properties of undoped and Ce-doped LSO and YSO single crystals and single crystalline films scintillators. IEEE Trans. Nucl. Sci. 2014, 61, 276–281. [Google Scholar]
- Zorenko, Y.; Gorbenko, V.; Bilski, P.; Twardak, A.; Mandowska, E.; Mandowski, A.; Sidletskiy, O. Comparative analysis of the scintillation and thermoluminescent properties of Ce-doped LSO and YSO crystals and films. Opt. Mater. 2014, 36, 1715–1719. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Zorenko, T.; Malinowski, P.; Jary, V.; Kucerkova, R.; Beitlerova, A.; Mares, J.A.; Nikl, M. Luminescent and scintillation properties of Bi3+ doped Y2SiO5 and Lu2SiO5 single crystalline films. J. Lumin. 2014, 154, 525–530. [Google Scholar] [CrossRef]
- Zazubovich, S.; Krasnikov, A.; Zorenko, Y.; Gorbenko, V.; Babin, V.; Mihokova, E.; Nikl, M. Luminescence of Pb- and Bi- Related Centers in Aluminum Garnet, Perovskite, and Orthosilicate Single-Crystalline Films. In Nanocomposite, Ceramic, and Thin Film Scintillators; Pan Stanford Publishing Pte. Ltd: Singapore, 2016; pp. 227–287, ISBN 978–981–4745–22–2 (Hardcover); ISBN 978–981–4745–23–9 (eBook). [Google Scholar]
- Gorbenko, V.; Krasnikov, A.; Mihokova, E.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Luminescence of lead-related centers in single crystalline films of Lu2SiO5. J. Phys. D Appl. Phys. 2012, 45, 355304. [Google Scholar] [CrossRef]
- Scott, G.B.; Page, J.L. Pb valence in iron garnets. J. Appl. Phys. 1977, 48, 1342. [Google Scholar] [CrossRef]
- Gorbenko, V.; Krasnikov, A.; Mihokova, E.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Photoluminescence and excited state structure of Bi3+-related centers in Lu2SiO5:Bi single crystalline films. J. Lumin. 2013, 134, 469–476. [Google Scholar] [CrossRef]
- Babin, V.; Gorbenko, V.; Krasnikov, A.; Mihokova, E.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Photoluminescence and excited state structure in Bi3+-doped Y2SiO5 single crystalline films. Radiat. Meas. 2013, 56, 90–93. [Google Scholar] [CrossRef]
- Ilmer, M.; Grabmaier, B.C.; Blasse, G. Luminescence of Bi3+ in gallate garnets. Chem. Mater. 1994, 6, 204–206. [Google Scholar] [CrossRef]
- Lacklinson, D.E.; Scott, G.B.; Page, G.L. Absorption spectra of Bi3+ and Fe3+ in Y3Ga5O12. Solid State Commun. 1974, 14, 861–863. [Google Scholar] [CrossRef]
- Kärner, T.; Laguta, V.V.; Nikl, M.; Shalapska, T.; Zazubovich, S. On the origin of cerium-related centres in lead-containing single crystalline films of Y2SiO5: Ce and Lu2SiO5: Ce. J. Phys. D Appl. Phys. 2014, 47, 065303. [Google Scholar] [CrossRef]
- Awater, R.H.P.; Dorenbos, P. X-ray Induced Valence Change and Vacuum Referred Binding Energies of Bi3+ and Bi2+ in Li2BaP2O7. J. Phys. Chem. C 2016, 120, 15114–15188. [Google Scholar] [CrossRef]
- Awater, R.H.P.; Dorenbos, P. Towards a general concentration quenching model of Bi3+ luminescence. J. Lumin. 2017, 188, 487–489. [Google Scholar] [CrossRef]
- Pidol, L.; Bruno Viana, B.; Kahn-Harari, A.; Ferrand, B.; Dorenbos, P.; van Eijk, C.W.E. Scintillation and thermoluminescence properties of Lu2Si2O7:Ce3+ crystals. Nucl. Instrum. Methods A 2005, 537, 256–260. [Google Scholar] [CrossRef]
- Feng, H.; Jary, V.; Mihokova, E.; Ding, D.; Nikl, M.; Ren, G.; Li, H.; Pan, S.; Beitlerova, A.; Kucerkova, R. Temperature dependence of luminescence characteristics of Lu2(1−x)Y2xSiO5:Ce3+ scintillator grown by the Czochralski method. J. Appl. Phys. 2010, 108, 033519–033524. [Google Scholar] [CrossRef]
- Puchalska, M.; Bilski, P. GlowFit—A new tool for thermoluminescence glow-curve deconvolution. Radiat. Meas. 2006, 41, 659–664. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter. Nucl. Instrum. Methods B 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Nikl, M.; Kamada, K.; Babin, V.; Pejchal, J.; Pilarova, K.; Mihokova, E.; Beitlerova, A.; Bartosiewicz, K.; Kurosawa, S.; Yoshikawa, A. Defect Engineering in Ce-Doped Aluminum Garnet Single Crystal Scintillators. Cryst. Growth Des. 2014, 14, 4827–4833. [Google Scholar] [CrossRef]
- Nikl, M.; Yoshikawa, A. Recent R&D Trends in Inorganic Single-Crystal Scintillator Materials. Adv. Optical Mater. 2015, 3, 463–481. [Google Scholar]

| ID | Material | Flux | Pb/Bi Content, at % | Ce Content, at % | h, μm | Tg, °C |
|---|---|---|---|---|---|---|
| 1 | LSO:Ce | PbO-B2O3 | 0.19 | 0.002 | 21 | 990 |
| 2 | LSO:Ce | Bi2O3 | 2.30 | 0.032 | 15 | 935 |
| 3 | YSO:Ce | PbO-B2O3 | 0.03 | 0.052 | 6.1 | 983 |
| 4 | YSO:Ce | Bi2O3 | 0.03 | 0.002 | 15 | 918 |
| 5 | LSO | PbO-B2O3 | 0.04 | - | 9 | 993 |
| 6 | LSO | Bi2O3 | 1.46 | - | 64 | 940 |
| 7 | YSO | PbO-B2O3 | 0.035 | - | 15.5 | 986 |
| 8 | YSO | Bi2O3 | 1.46 | - | 10 | 945 |
| ID | Peak 0 | Peak 1 | Peak 2 | Peak 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Intensity (%) | Tmax (K) | E (eV) | Relative Intensity (%) | Tmax (K) | E (eV) | Relative Intensity (%) | Tmax (K) | E (eV) | Relative Intensity (%) | Tmax (K) | E (eV) | |
| 1 | - | - | - | 100 | 403 | 1.11 | 4.1 | 458 | 1.29 | 0.18 | 478 | 0.52 |
| 2 | 17 | 360 | 0.88 | 100 | 404 | 1.00 | 7.4 | 459 | 1.18 | 0.14 | 474 | 0.50 |
| 3 | - | - | - | 100 | 406 | 1.10 | 4.0 | 462 | 1.23 | 0.23 | 507 | 0.87 |
| 4 | 17 | 364 | 0.87 | 100 | 409 | 1.01 | 7.8 | 466 | 1.16 | 0.17 | 510 | 0.87 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilian, A.; Bilski, P.; Gorbenko, V.; Zorenko, T.; Witkiewicz, S.; Paprocki, K.; Zorenko, Y. Thermoluminescent Properties of Cerium-Doped Lu2SO5 and Y2SiO5 Single Crystalline Films Scintillators Grown from PbO-B2O3 and Bi2O3 Fluxes. Crystals 2018, 8, 120. https://doi.org/10.3390/cryst8030120
Kilian A, Bilski P, Gorbenko V, Zorenko T, Witkiewicz S, Paprocki K, Zorenko Y. Thermoluminescent Properties of Cerium-Doped Lu2SO5 and Y2SiO5 Single Crystalline Films Scintillators Grown from PbO-B2O3 and Bi2O3 Fluxes. Crystals. 2018; 8(3):120. https://doi.org/10.3390/cryst8030120
Chicago/Turabian StyleKilian, Anna, Paweł Bilski, Vitalii Gorbenko, Tetiana Zorenko, Sandra Witkiewicz, Kazimierz Paprocki, and Yuriy Zorenko. 2018. "Thermoluminescent Properties of Cerium-Doped Lu2SO5 and Y2SiO5 Single Crystalline Films Scintillators Grown from PbO-B2O3 and Bi2O3 Fluxes" Crystals 8, no. 3: 120. https://doi.org/10.3390/cryst8030120







