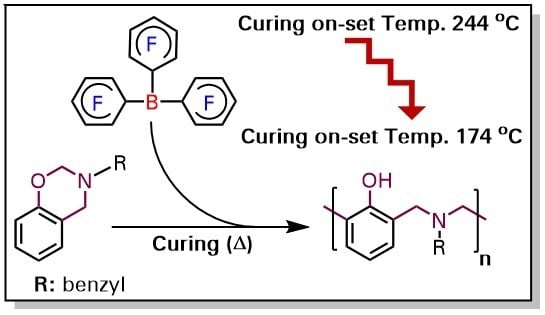
Ring-Opening Polymerization of 1,3-Benzoxazines via Borane Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
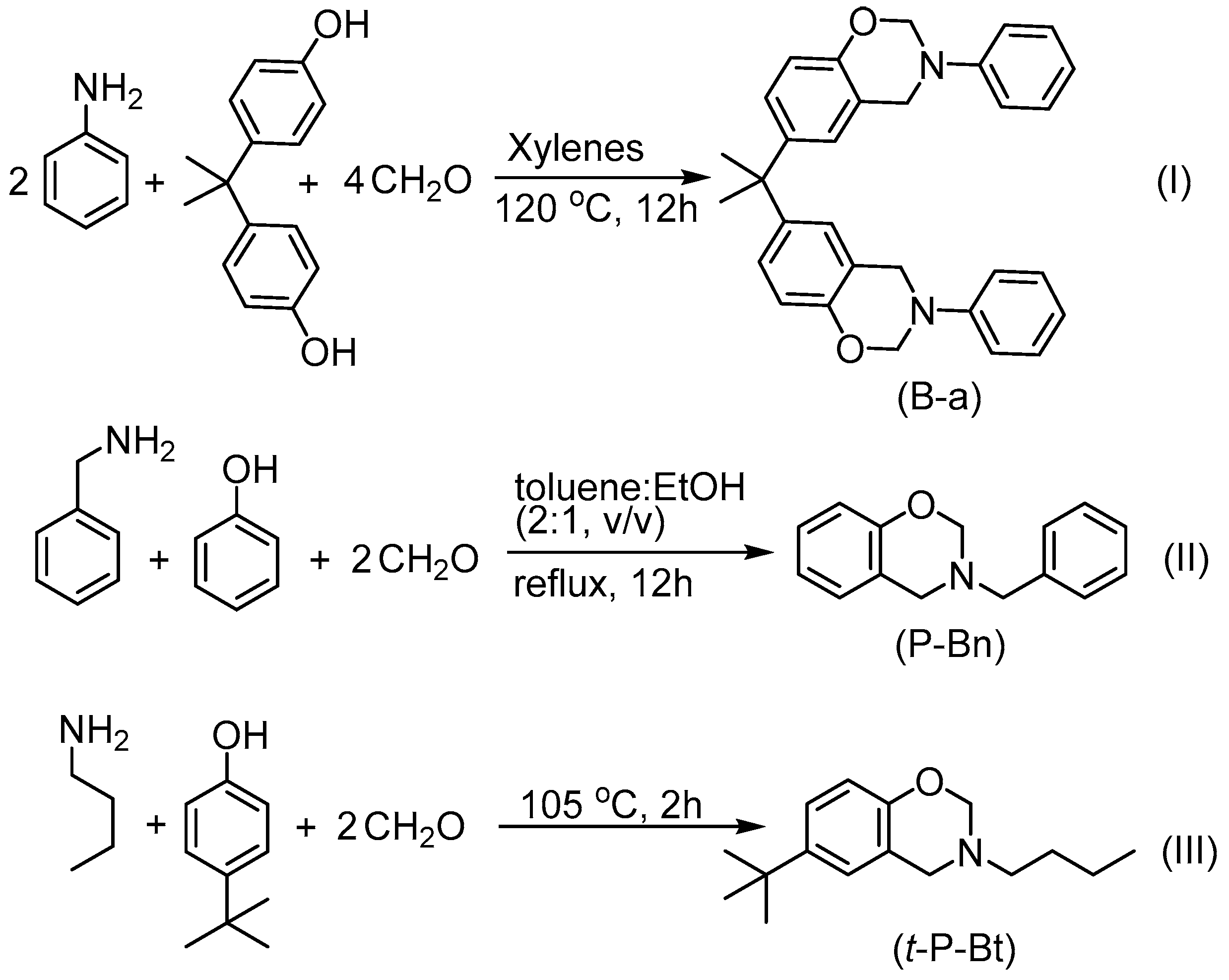
2.3. Synthesis of Aniline Based Bisbenzoxazine (B-a)
2.4. Synthesis of Benzylamine Based Monobenzoxazine (P-Bn)
2.5. Synthesis of 4-t-butylphenol Based Monobenzoxazine (t-P-Bt)
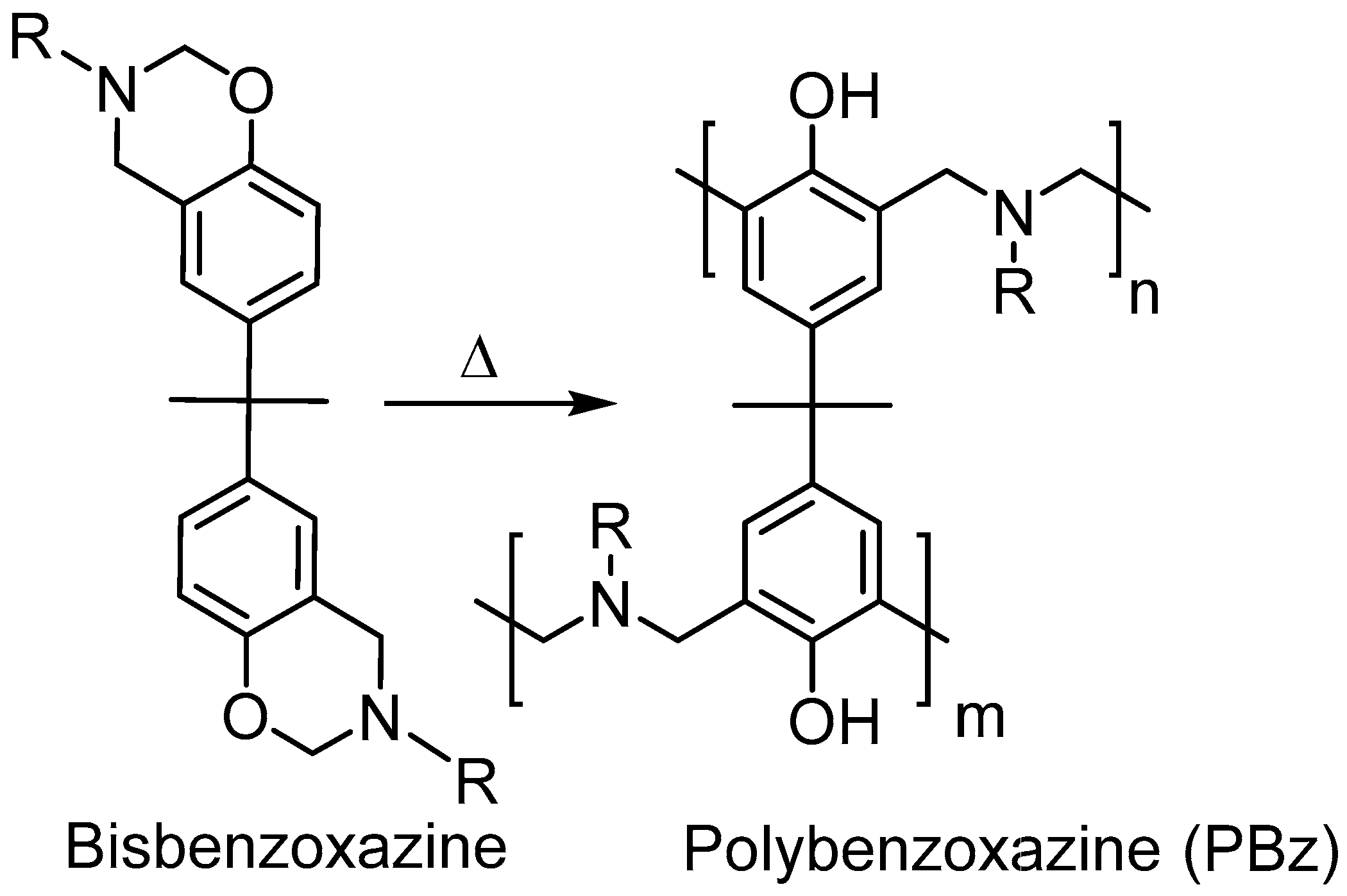
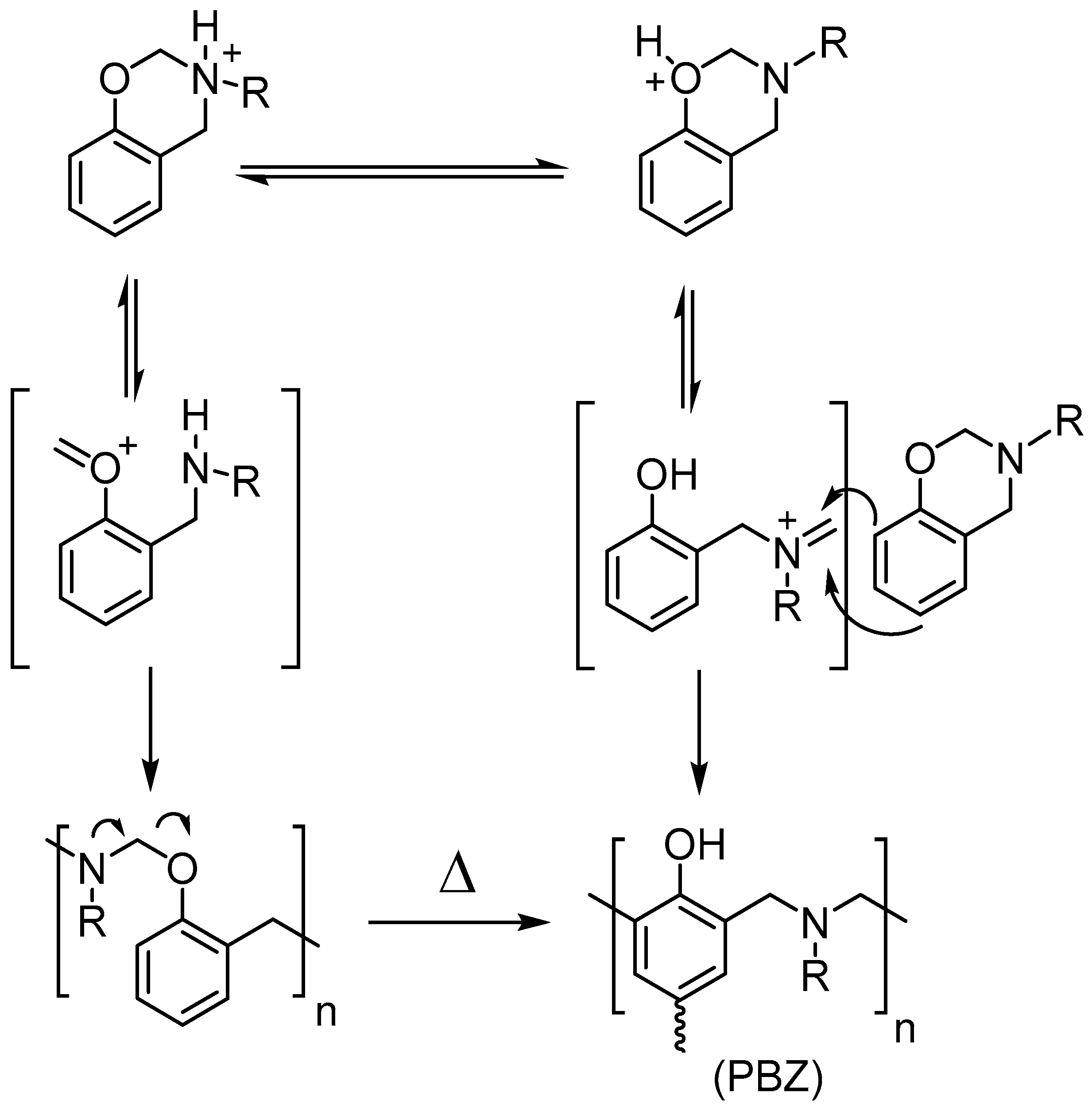
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Wirasate, S.; Dhumrongvaraporn, S.; Allen, D.J.; Ishida, H. Molecular origin of unusual physical and mechanical properties in novel phenolic materials based on benzoxazine chemistry. J. Appl. Polym. Sci. 1998, 70, 1299–1306. [Google Scholar] [CrossRef]
- Kiskan, B. Adapting benzoxazine chemistry for unconventional applications. React. Funct. Polym. 2017. [Google Scholar] [CrossRef]
- Kukut, M.; Kiskan, B.; Yagci, Y. Self-curable benzoxazine functional polybutadienes synthesized by click chemistry. Des. Monomers Polym. 2009, 12, 167–176. [Google Scholar] [CrossRef]
- Lin, R.-C.; Mohamed, M.; Chen, T.; Kuo, S.-W. Coumarin- and carboxyl-functionalized supramolecular polybenzoxazines form miscible blends with polyvinylpyrrolidone. Polymers 2017, 9, 146. [Google Scholar] [CrossRef]
- Hu, W.-H.; Huang, K.-W.; Kuo, S.-W. Heteronucleobase-functionalized benzoxazine: Synthesis, thermal properties, and self-assembled structure formed through multiple hydrogen bonding interactions. Polym. Chem. 2012, 3, 1546–1554. [Google Scholar] [CrossRef]
- Kim, H.D.; Ishida, H. A study on hydrogen-bonded network structure of polybenzoxazines. J. Phys. Chem. A 2002, 106, 3271–3280. [Google Scholar] [CrossRef]
- Dong, H.; Xin, Z.; Lu, X.; Lv, Y. Effect of n-substituents on the surface characteristics and hydrogen bonding network of polybenzoxazines. Polymer 2011, 52, 1092–1101. [Google Scholar] [CrossRef]
- Hu, W.H.; Huang, K.W.; Chiou, C.W.; Kuo, S.W. Complementary multiple hydrogen bonding interactions induce the self-assembly of supramolecular structures from heteronucleobase-functionalized benzoxazine and polyhedral oligomeric silsesquioxane nanoparticles. Macromolecules 2012, 45, 9020–9028. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Physical and mechanical characterization of near-zero shrinkage polybenzoxazines. J. Polym. Sci. B 1996, 34, 1019–1030. [Google Scholar] [CrossRef]
- Andronescu, C.; Gârea, S.A.; Deleanu, C.; Iovu, H. Characterization and curing kinetics of new benzoxazine monomer based on aromatic diamines. Thermochim. Acta 2012, 530, 42–51. [Google Scholar] [CrossRef]
- Hamerton, I.; McNamara, L.T.; Howlin, B.J.; Smith, P.A.; Cross, P.; Ward, S. Examining the initiation of the polymerization mechanism and network development in aromatic polybenzoxazines. Macromolecules 2013, 46, 5117–5132. [Google Scholar] [CrossRef] [PubMed]
- Baqar, M.; Agag, T.; Huang, R.; Maia, J.; Qutubuddin, S.; Ishida, H. Mechanistic pathways for the polymerization of methylol-functional benzoxazine monomers. Macromolecules 2012, 45, 8119–8125. [Google Scholar] [CrossRef]
- Chutayothin, P.; Ishida, H. Cationic ring-opening polymerization of 1,3-benzoxazines: Mechanistic study using model compounds. Macromolecules 2010, 43, 4562–4572. [Google Scholar] [CrossRef]
- Sudo, A.; Kudoh, R.; Nakayama, H.; Arima, K.; Endo, T. Selective formation of poly(n,o-acetal) by polymerization of 1,3-benzoxazine and its main chain rearrangement. Macromolecules 2008, 41, 9030–9034. [Google Scholar] [CrossRef]
- Endo, T. Toward elucidating the role of number of oxazine rings and intermediates in the benzoxazine backbone on their thermal characteristics. Macromolecules 2016, 49, 8466–8478. [Google Scholar]
- Andreu, R.; Reina, J.A.; Ronda, J.C. Carboxylic acid-containing benzoxazines as efficient catalysts in the thermal polymerization of benzoxazines. J. Polym. Sci. A 2008, 46, 6091–6101. [Google Scholar] [CrossRef]
- Zuniga, C.; Larrechi, M.S.; Lligadas, G.; Ronda, J.C.; Galia, M.; Cadiz, V. Polybenzoxazines from renewable diphenolic acid. J. Polym. Sci. A 2011, 49, 1219–1227. [Google Scholar] [CrossRef]
- Kiskan, B.; Demirel, A.L.; Kamer, O.; Yagci, Y. Synthesis and characterization of nanomagnetite thermosets based on benzoxazines. J. Polym. A 2008, 46, 6780–6788. [Google Scholar] [CrossRef]
- Andreu, R.; Reina, J.A.; Ronda, J.C. Studies on the thermal polymerization of substituted benzoxazine monomers: Electronic effects. J. Polym. Sci. A Chem. 2008, 46, 3353–3366. [Google Scholar] [CrossRef]
- Soto, M.; Hiller, M.; Oschkinat, H.; Koschek, K. Multifunctional benzoxazines feature low polymerization temperature and diverse polymer structures. Polymers 2016, 8, 278. [Google Scholar] [CrossRef]
- Zuniga, C.; Lligadas, G.; Carlos Ronda, J.; Galia, M.; Cadiz, V. Self-foaming diphenolic acid benzoxazine. Polymer 2012, 53, 3089–3095. [Google Scholar] [CrossRef]
- Lin, C.H.; Feng, Y.R.; Dai, K.H.; Chang, H.C.; Juang, T.Y. Synthesis of a benzoxazine with precisely two phenolic oh linkages and the properties of its high-performance copolymers. J. Polym. Sci. A 2013, 51, 2686–2694. [Google Scholar]
- Zhang, W.; Froimowicz, P.; Arza, C.R.; Ohashi, S.; Xin, Z.; Ishida, H. Latent catalyst-containing naphthoxazine: Synthesis and effects on ring-opening polymerization. Macromolecules 2016, 49, 7129–7140. [Google Scholar] [CrossRef]
- Kocaarslan, A.; Kiskan, B.; Yagci, Y. Ammonium salt catalyzed ring-opening polymerization of 1,3-benzoxazines. Polymer 2017, 122, 340–346. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Combining elemental sulfur with polybenzoxazines via inverse vulcanization. Macromolecules 2016, 49, 767–773. [Google Scholar] [CrossRef]
- Semerci, E.; Kiskan, B.; Yagci, Y. Thiol reactive polybenzoxazine precursors: A novel route to functional polymers by thiol-oxazine chemistry. Eur. Polym. J. 2015, 69, 636–641. [Google Scholar] [CrossRef]
- Bektas, S.; Kiskan, B.; Orakdogen, N.; Yagci, Y. Synthesis and properties of organo-gels by thiol-benzoxazine chemistry. Polymer 2015, 75, 44–50. [Google Scholar] [CrossRef]
- Sudo, A.; Yamashita, H.; Endo, T. Ring-opening polymerization of 1,3-benzoxazines by p-toluenesulfonates as thermally latent initiators. J. Polym. Sci. A 2011, 49, 3631–3636. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.Z.; Fu, Y.F.; Liu, X.D. Latent curing systems stabilized by reaction equilibrium in homogeneous mixtures of benzoxazine and amine. Sci. Rep. 2016, 6, 38584. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, W.; Xu, Y.; Qu, J.; Liu, X.; Endo, T. A curing system of benzoxazine with amine: Reactivity, reaction mechanism and material properties. RSC Adv. 2015, 5, 19048–19057. [Google Scholar] [CrossRef]
- Gorodisher, I.; DeVoe, R.J.; Webb, R.J. Catalytic opening of lateral benzoxazine rings by thiols. Handb. Benzoxazine Resins 2011, 211–234. [Google Scholar] [CrossRef]
- Dunkers, J.; Ishida, H. Reaction of benzoxazine-based phenolic resins with strong and weak carboxylic acids and phenols as catalysts. J. Polym. Sci. A 1999, 37, 1913–1921. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y.; Sahmethogilu, E.; Toppare, L. Preparation of conductive polybenzoxazines by oxidative polymerization. J. Polym. Sci. A Chem. 2007, 45, 999–1006. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ishida, H. Cationic ring-opening polymerization of benzoxazines. Polymer 1999, 40, 4563–4570. [Google Scholar] [CrossRef]
- Cid, J.A.; Wang, Y.X.; Ishida, H. Cationic polymerization of benzoxazine monomers by boron trifluoride complex. Polym. Polym. Compos. 1999, 7, 409–420. [Google Scholar]
- Sudo, A.; Hirayama, S.; Endo, T. Highly efficient catalysts-acetylacetonato complexes of transition metals in the 4th period for ring-opening polymerization of 1,3-benzoxazine. J. Polym. Sci. A 2010, 48, 479–484. [Google Scholar] [CrossRef]
- Lawson, J.R.; Melen, R.L. Tris(pentafluorophenyl)borane and beyond: Modern advances in borylation chemistry. Inorg. Chem. 2017, 56, 8627–8643. [Google Scholar] [CrossRef] [PubMed]
- Fasano, V.; Radcliffe, J.E.; Ingleson, M.J. Mechanistic insights into the b(c6f5)3-initiated aldehyde–aniline–alkyne reaction to form substituted quinolines. Organometallics 2017, 36, 1623–1629. [Google Scholar] [CrossRef]
- Erker, G. Tris(pentafluorophenyl)borane: A special boron lewis acid for special reactions. Dalton Trans. 2005, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, M.M.; López-Andarias, A.; Rettenmeier, E.; Egler-Lucas, C.; Rominger, F.; Hashmi, A.S.K.; Romero-Nieto, C. B(c6f5)3: A lewis acid that brings the light to the solid state. Angew. Chem. Int. Edit. 2016, 55, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Besar, K.; LeCover, R.; Rule, A.M.; Breysse, P.N.; Katz, H.E. Highly sensitive nh3 detection based on organic field-effect transistors with tris(pentafluorophenyl)borane as receptor. J. Am. Chem. Soc. 2012, 134, 14650–14653. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B-n compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Kiskan, B.; Yagci, Y. Concise synthesis and characterization of unsymmetric 1,3-benzoxazines by tandem reactions. Tetrahedron Lett. 2013, 54, 4966–4969. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Gu, Y. Influence of electronic effects from bridging groups on synthetic reaction and thermally activated polymerization of bisphenol-based benzoxazines. J. Polym. Sci. A Chem. 2011, 49, 1443–1452. [Google Scholar] [CrossRef]
- Demir, K.D.; Kiskan, B.; Yagci, Y. Thermally curable acetylene-containing main-chain benzoxazine polymers via sonogashira coupling reaction. Macromolecules 2011, 44, 1801–1807. [Google Scholar] [CrossRef]
- Han, L.; Iguchi, D.; Gil, P.; Heyl, T.R.; Sedwick, V.M.; Arza, C.R.; Ohashi, S.; Lacks, D.J.; Ishida, H. Oxazine ring-related vibrational modes of benzoxazine monomers using fully aromatically substituted, deuterated, 15n isotope exchanged, and oxazine-ring-substituted compounds and theoretical calculations. J. Phys. Chem. A 2017, 121, 6269–6282. [Google Scholar] [CrossRef] [PubMed]
- Achiha, T.; Nakajima, T.; Ohzawa, Y.; Koh, M.; Yamauchi, A.; Kagawa, M.; Aoyama, H. Thermal stability and electrochemical properties of fluorine compounds as nonflammable solvents for lithium-ion batteries. J. Electrochem. Soc. 2010, 157, A707–A712. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
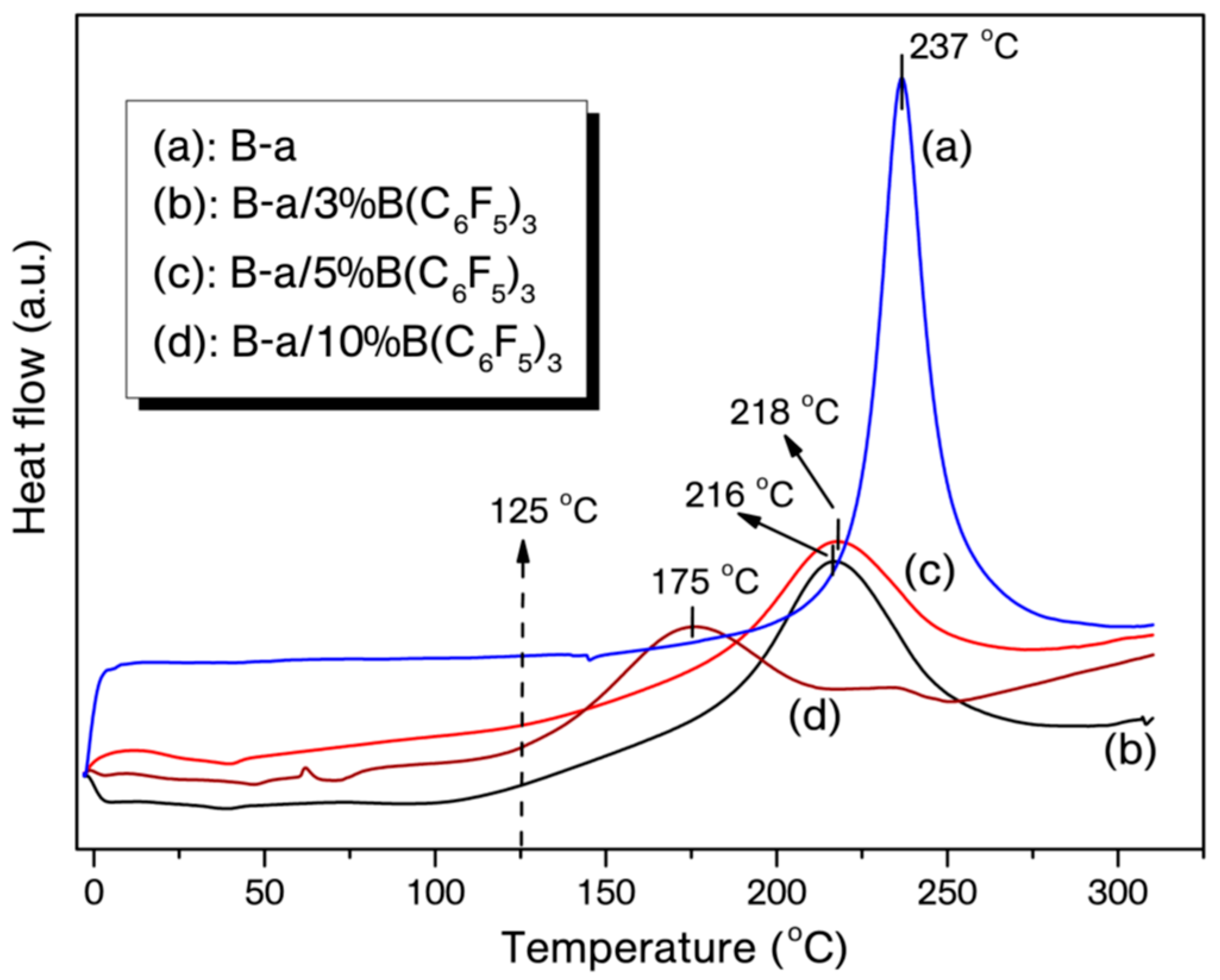
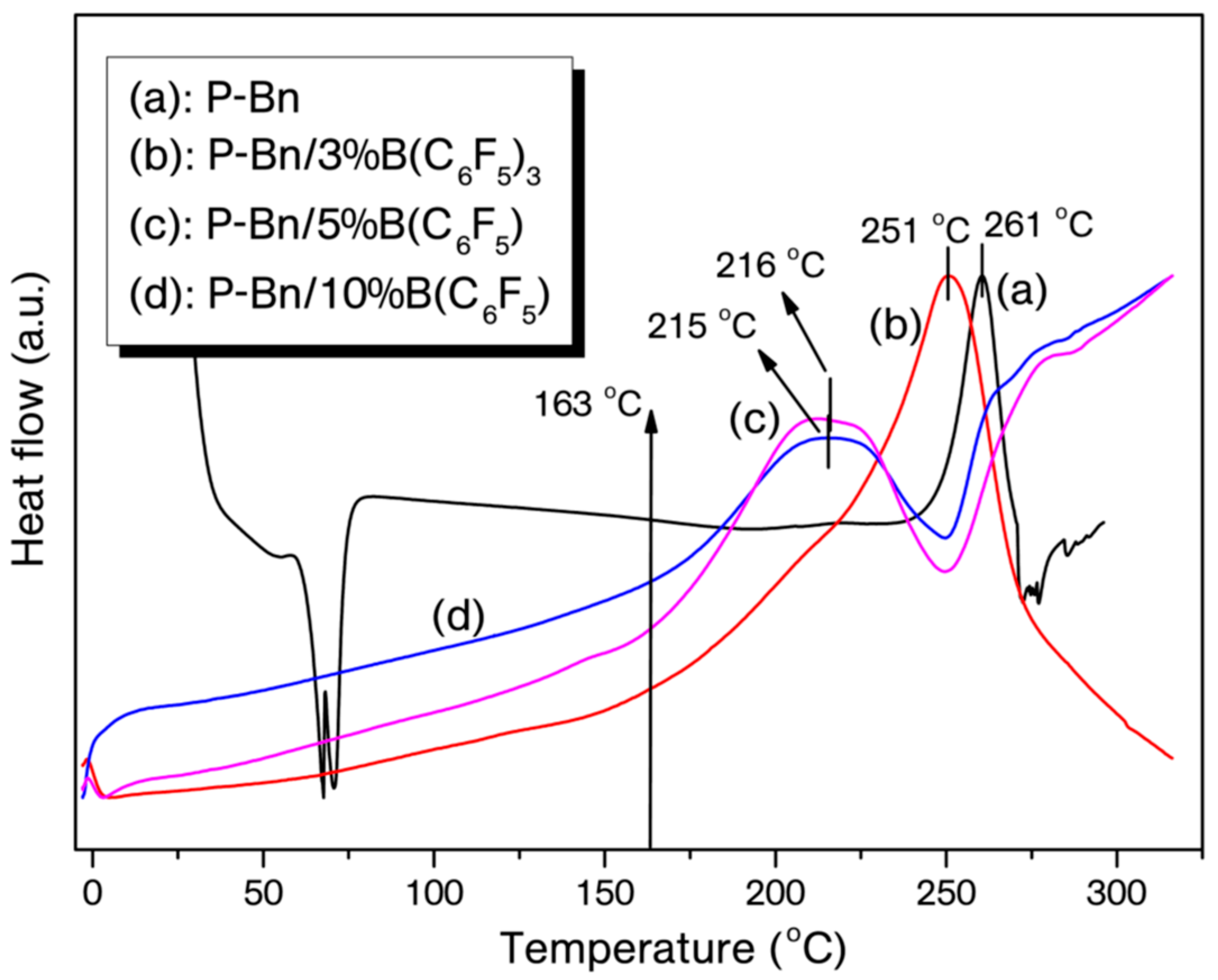


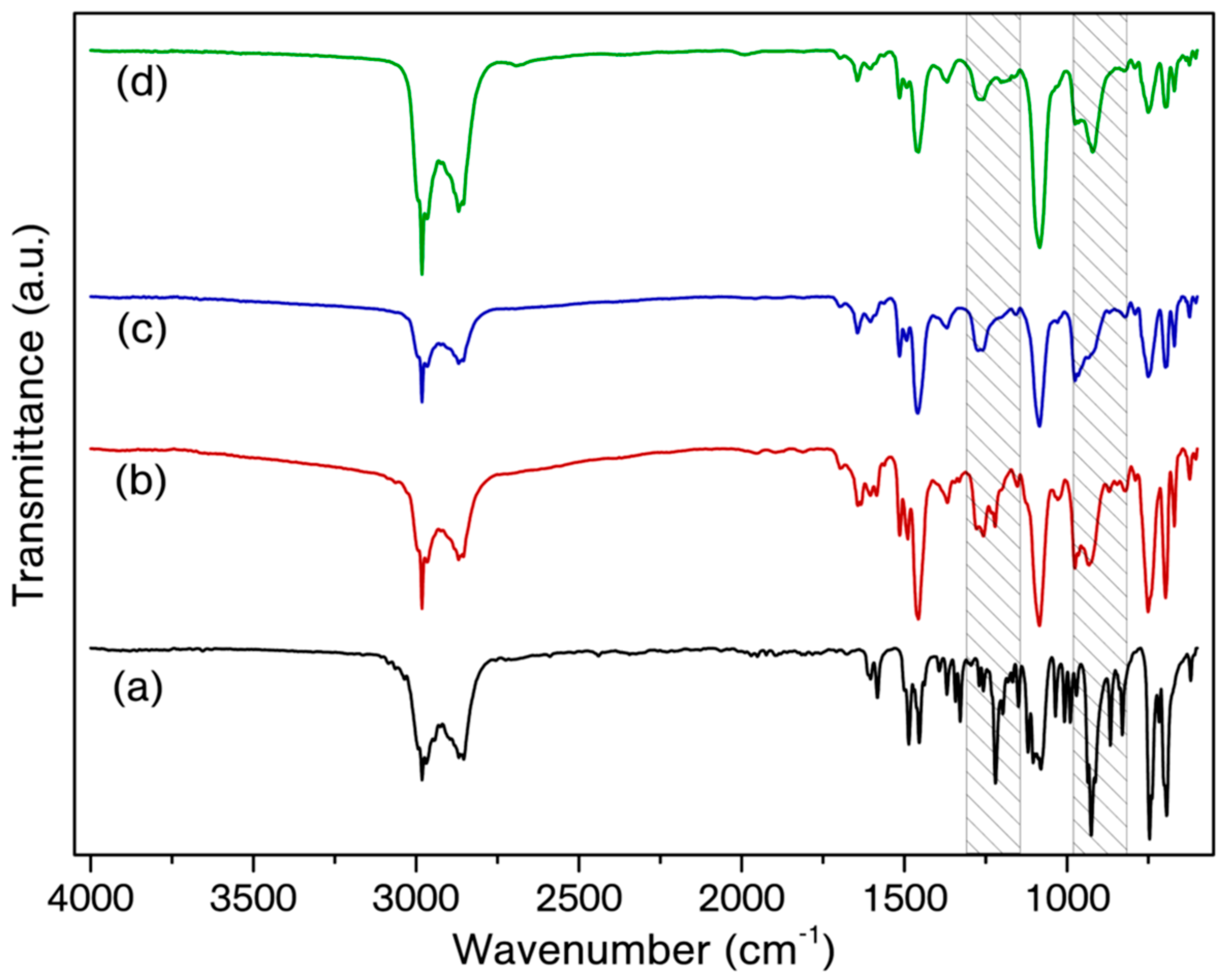

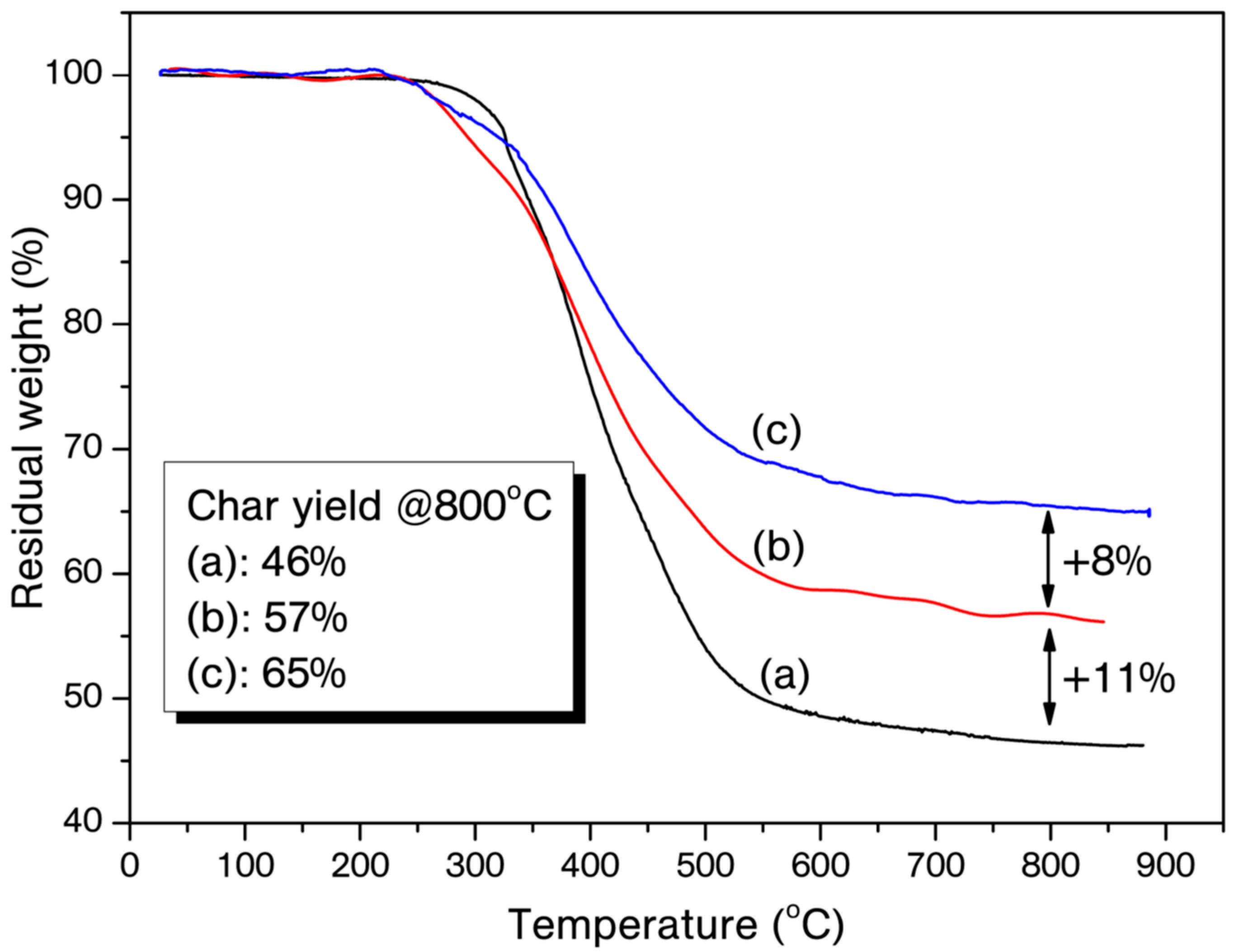
| Entry | On-Set of Curing (°C) | End-Set of Curing (°C) | Maximum Curing (°C) | ΔH (J/g) |
|---|---|---|---|---|
| B-a | 212 | 266 | 237 | −201 |
| B-a/3% B(C6F5)3 | 180 | 251 | 216 | −156 |
| B-a/5% B(C6F5)3 | 180 | 252 | 217 | −117 |
| B-a/10% B(C6F5)3 | 125 | 216 | 174 | −103 |
| P-Bn | 242 | 272 | 261 | −35 |
| P-Bn/3% B(C6F5)3 | 217 | 270 | 251 | −130 |
| P-Bn/5% B(C6F5)3 | 174 | 248 | 216 | −45 |
| P-Bn/10% B(C6F5)3 | 163 | 247 | 215 | −74 |
| Sample | T5% (°C) | T10% (°C) | Tmax (°C) a | Tc (%) |
|---|---|---|---|---|
| P-Bn | 257 | 267 | 270 *, 466 | 35 |
| P-Bn/3% B(C6F5)3 | 258 | 263 | 272 *, 492 | 48 |
| P-Bn/5% B(C6F5)3 | 244 | 297 | 268 *, 557 | 63 |
| B-a | 326 | 364 | 390 | 46 |
| B-a/3% B(C6F5)3 | 294 | 347 | 284 *, 392 | 57 |
| B-a/5% B(C6F5)3 | 323 | 340 | 271 *, 391 | 65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, M.; Kiskan, B.; Yagci, Y. Ring-Opening Polymerization of 1,3-Benzoxazines via Borane Catalyst. Polymers 2018, 10, 239. https://doi.org/10.3390/polym10030239
Arslan M, Kiskan B, Yagci Y. Ring-Opening Polymerization of 1,3-Benzoxazines via Borane Catalyst. Polymers. 2018; 10(3):239. https://doi.org/10.3390/polym10030239
Chicago/Turabian StyleArslan, Mustafa, Baris Kiskan, and Yusuf Yagci. 2018. "Ring-Opening Polymerization of 1,3-Benzoxazines via Borane Catalyst" Polymers 10, no. 3: 239. https://doi.org/10.3390/polym10030239