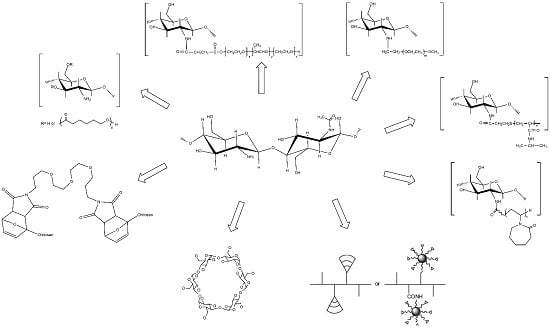
Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials †
Abstract
:1. Introduction
- Chitin and chitosan are, in fact, a family of polymers, differing in terms of not only the molecular weight and extent of acetylation but also in the dispersion of the degree of polymerization and the distribution of the acetylated and deacetylated units along the polymer chain. All these parameters will depend mainly on the natural source and isolation processes. Therefore, it is very important to know these intrinsic characteristics, as far as they shall affect the properties of the derivatives.
- Due to their insolubility in certain solvents (particularly chitin), some chemical reactions are carried out under heterogeneous conditions. This will have a determinant influence on the structure of the obtained derivatives. Using words from Kurita, “reactions under heterogeneous conditions are usually accompanied by problems including poor extents of reaction, difficulty in regioselective substitution, structurally ununiformly products, and partial degradation due to severe reaction conditions” [1]. Nowadays, these drawbacks could be circumvented using some novel solvent systems like ionic liquids. This topic will be revised herein as well.
- Usually, non-selective chemical derivatization could lead to the development of products with an irregular distribution and uncontrolled growth of the substituent groups in the main chain, or undesired depolymerization of the polysaccharide.
- Although chitosan presents valuable functional groups for derivatization reactions, often it is necessary to obtain some precursors to facilitate subsequent reactions or, in other cases, to protect the reactive amine in order to favor the chemoselectivity of the modification. Due to their frequent use in chitosan functionalization processes, we will first refer to those reactions whose use is more or less recurrent under diverse experimental conditions.
1.1. Some Frequent Reactions in Chitosan Chemistry
1.2. Chitosan Precursors with Protected Amino Groups
- the N-phthaloylation of chitosan affects the solubility of chitosan in aqueous solutions. It is only soluble in aprotic polar organic solvents, which have been attributed to an increase in the crystallinity of the derivative as compared with the pristine chitosan [22,23]. Obviously, the solubility of the precursor in some organic solvents could be advantageous when the O-substitution reaction needs to be carried out in the latter.
2. Click Chemistry Reactions
- cycloaddition reactions, including those from the 1,3-dipolar family (like Huisgen reaction), and hetero-Diels-Alder reactions,
- nucleophilic ring-opening reactions in strained heterocyclic electrophiles,
- carbonyl chemistry of the non-aldol type, and
- additions to carbon-carbon multiple bonds.
3. Graft Copolymerization
3.1. Chitin “Grafting from” Copolymers
3.2. Chitosan “Grafting from” Copolymers
3.3. Chitosan “Grafting onto” Copolymers
3.4. Chitosan Network Systems Prepared by Radiation
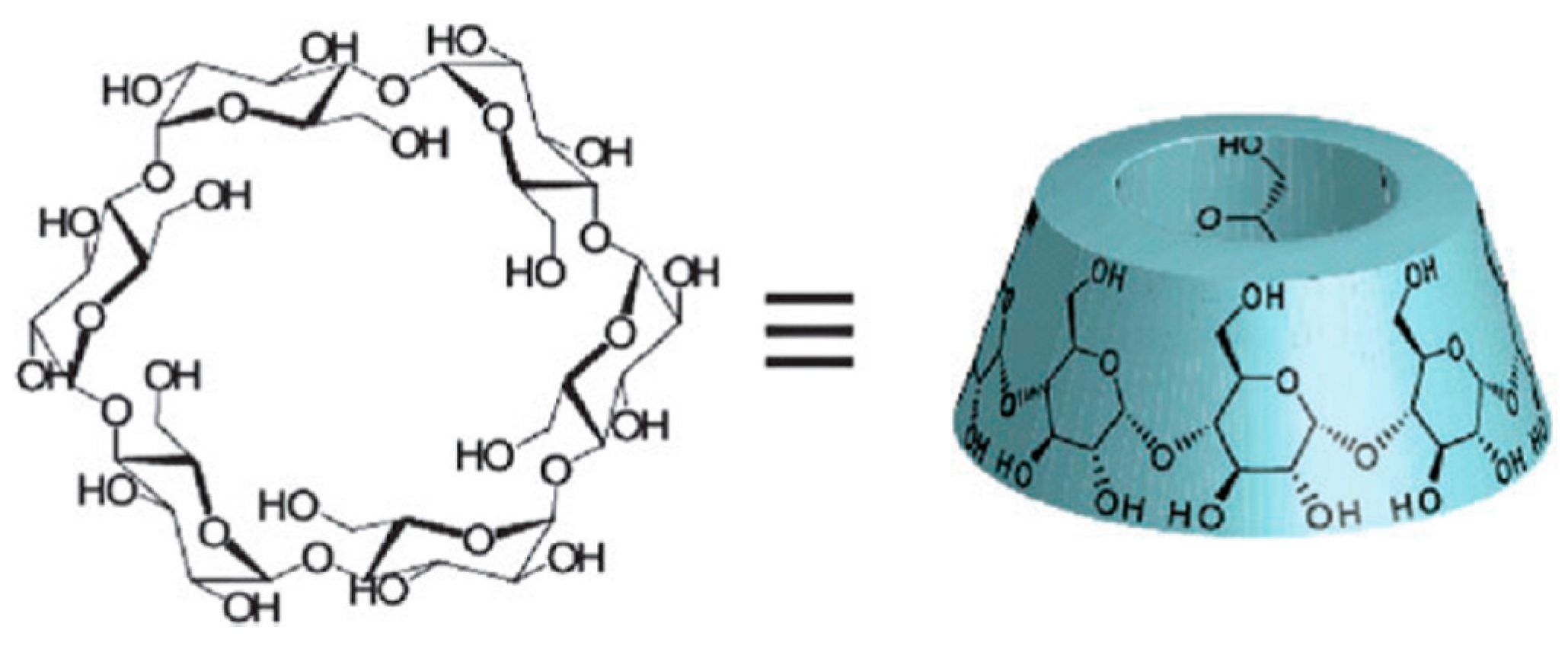
4. Chitosan-grafted-Cyclodextrin Derivatives
- Reductive amination reaction. Usually, the CD is modified in order to attach an aldehyde group. The inclusion of the CD moieties into the chitosan backbone is carried out by the formation of a Schiff base, followed by the reduction with a proper agent. The reductive amination procedure is one of the most used, because it is a simple, easy, and slightly degradative method [169,177,178,179].
- The second most important method is via amidation of CDs modified with a carboxylic group with the amino groups of chitosan. In this case, two strategies have been applied: (i) the classic condensation reaction [180,181], and (ii) by amidation using coupling activators of the carboxylic acid group, like EDC/NHS [175,182,183,184,185,186]. The former reaction requires high temperatures due to the high activation energies involved, while the use of condensation agents in the later selectively promotes the formation of the amide bond in aqueous solution under mild and controllable conditions.
- A method so far little used but which, in the future, can provide derivatives with a high regioselectivity, is anchoring β-cyclodextrin onto chitosan by click chemistry. In this way, using the Huisgen cycloaddition reaction, β-CD chains have been grafted onto the chitosan backbone through the amino group (position 2) [59] or to the O-6 [31].
5. Dendronized Chitosan
6. Chitosan Modification Using Ionic Liquids
6.1. Acylation
6.2. Alkylation
6.3. Grafting
6.4. Other Derivatizations
6.5. Degradation
6.6. Biocatalyzed Reactions
7. Conclusions
Conflicts of Interest
References
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. ISBN 978-0-08-045316-3. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical Characteristics and Functional Properties of Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 3–31. ISBN 978-0-12-802735-6. [Google Scholar]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Sabaa, M.W. Chitosan-g-Copolymers: Synthesis, Properties, and Applications. In Polysaccharide Based Graft Copolymers; Kalia, S., Sabaa, M.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 111–147. ISBN 978-3-642-36565-2. [Google Scholar]
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2018, 179, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Jenkins, D.W.; Hudson, S.M. Review of Vinyl Graft Copolymerization Featuring Recent Advances toward Controlled Radical-Based Reactions and Illustrated with Chitin/Chitosan Trunk Polymers. Chem. Rev. 2001, 101, 3245–3274. [Google Scholar] [CrossRef] [PubMed]
- Sashiwa, H.; Aiba, S. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.; Recillas-Mota, M.; Fernández-Quiroz, D. Chitosan-Based Thermosensitive Materials. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E.A., Ed.; InTech: Rijeka, Croatia, 2017; pp. 279–302. ISBN 978-953-51-2859-5. [Google Scholar]
- Zhang, Y.; Chan, J.W.; Moretti, A.; Uhrich, K.E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control. Release 2015, 219, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Kecen, X.; Xiaosai, Q. Grafting Modification of Chitosan. In Biopolymer Grafting; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–364. ISBN 978-0-323-48104-5. [Google Scholar]
- Roberts, G.A.F. Chitin Chemistry; Macmillan: London, UK, 1992; ISBN 978-0-333-52417-6. [Google Scholar]
- Uragami, T.; Tokura, S. (Eds.) Material Science of Chitin and Chitosan, 2006 ed.; Springer: Tokyo, Japan; Berlin, Germany; New York, NY, USA, 2006; ISBN 978-3-540-32813-1. [Google Scholar]
- Kim, S.-K. (Ed.) Chitin and Chitosan Derivatives: Advances in Drug Discovery and Developments, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4665-6628-6. [Google Scholar]
- Carvalho, L.C.R.; Queda, F.; Santos, C.V.A.; Marques, M.M.B. Selective Modification of Chitin and Chitosan: En Route to Tailored Oligosaccharides. Chem. Asian J. 2016, 11, 3468–3481. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Kohgo, O.; Kurita, K.; Kuzuhara, H. Chemospecific manipulations of a rigid polysaccharide: Syntheses of novel chitosan derivatives with excellent solubility in common organic solvents by regioselective chemical modifications. Macromolecules 1991, 24, 4745–4748. [Google Scholar] [CrossRef]
- Kurita, K.; Ikeda, H.; Yoshida, Y.; Shimojoh, M.; Harata, M. Chemoselective Protection of the Amino Groups of Chitosan by Controlled Phthaloylation: Facile Preparation of a Precursor Useful for Chemical Modifications. Biomacromolecules 2002, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Ikeda, H.; Shimojoh, M.; Yang, J. N-Phthaloylated Chitosan as an Essential Precursor for Controlled Chemical Modifications of Chitosan: Synthesis and Evaluation. Polym. J. 2007, 39, 945–952. [Google Scholar] [CrossRef]
- Makuška, R.; Gorochovceva, N. Regioselective grafting of poly(ethylene glycol) onto chitosan through C-6 position of glucosamine units. Carbohydr. Polym. 2006, 64, 319–327. [Google Scholar] [CrossRef]
- Hiroyuki, I.; Yoshie, T.; Jin, Y.; Keisuke, K. Phthaloylated Chitosan: Protection-Deprotection and the Influence on the Molecular Weight. Chitin Chitosan Res. 2009, 15, 7–12. [Google Scholar]
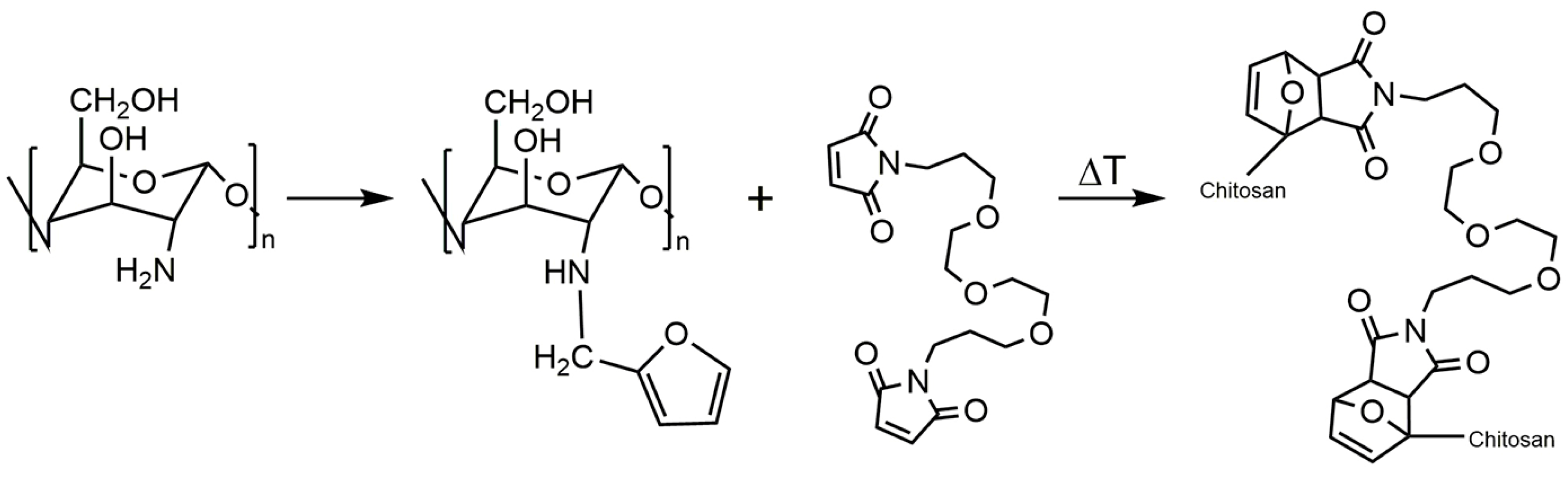
- Montiel-Herrera, M.; Gandini, A.; Goycoolea, F.M.; Jacobsen, N.E.; Lizardi-Mendoza, J.; Recillas-Mota, M.T.; Argüelles-Monal, W.M. Furan–chitosan hydrogels based on click chemistry. Iran. Polym. J. 2015, 24, 349–357. [Google Scholar] [CrossRef]
- Plisko, E.A.; Nud’ga, L.A.; Danilov, S.N. Chitin and Its Chemical Transformations. Russ. Chem. Rev. 1977, 46, 764. [Google Scholar] [CrossRef]
- Moore, G.K.; Roberts, G.A.F. Reactions of chitosan: 3. Preparation and reactivity of Schiff’s base derivatives of chitosan. Int. J. Biol. Macromol. 1981, 3, 337–340. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Tang, Y. Synthesis and adsorption properties for metal ions of mesocyclic diamine-grafted chitosan-crown ether. J. Appl. Polym. Sci. 2000, 75, 1255–1260. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y. Studies on the synthesis and properties of hydroxyl azacrown ether-grafted chitosan. J. Appl. Polym. Sci. 2001, 82, 1838–1843. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, Y.; Li, R.; Guo, Y.; Tan, H. Synthesis of chitosan 6-OH immobilized cyclodextrin derivates via click chemistry. Fibers Polym. 2013, 14, 1058–1065. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Yun, D.; Guo, Y.; Ye, Y.; Wang, Y.; Tan, H. Preparation of a C6 quaternary ammonium chitosan derivative through a chitosan schiff base with click chemistry. J. Appl. Polym. Sci. 2013, 129, 3185–3191. [Google Scholar] [CrossRef]
- Guinesi, L.S.; Cavalheiro, É.T.G. Influence of some reactional parameters on the substitution degree of biopolymeric Schiff bases prepared from chitosan and salicylaldehyde. Carbohydr. Polym. 2006, 65, 557–561. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Wang, Y.; He, B. Structural characterization of phosphorylated chitosan and their applications as effective additives of calcium phosphate cements. Biomaterials 2001, 22, 2247–2255. [Google Scholar] [CrossRef]
- Sashiwa, H.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Yamamoto, N.; Aiba, S. Chemical Modification of Chitosan. 14: Synthesis of Water-Soluble Chitosan Derivatives by Simple Acetylation. Biomacromolecules 2002, 3, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Chen, H.; Huang, J.; Yu, J.; Liu, S.; Wang, D.; Li, Y. One-step synthesis of amino-reserved chitosan-graft-polycaprolactone as a promising substance of biomaterial. Carbohydr. Polym. 2010, 80, 498–503. [Google Scholar] [CrossRef]
- Gu, C.; Le, V.; Lang, M.; Liu, J. Preparation of polysaccharide derivates chitosan-graft-poly(ε-caprolactone) amphiphilic copolymer micelles for 5-fluorouracil drug delivery. Colloids Surf. B Biointerfaces 2014, 116, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar cycloaddition–Introduction, survey, mechanism. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; John Wiley and Sons: New York, NY, USA, 1984; Volume 1, pp. 1–176. ISBN 978-0-471-08364-1. [Google Scholar]
- Wang, Z. Diels-Alder Reaction. In Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 886–891. ISBN 978-0-470-63885-9. [Google Scholar]
- Wang, Z. Retro-Diels-Alder Reaction. In Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010, 2010; pp. 2367–2372. ISBN 978-0-470-63885-9. [Google Scholar]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Elchinger, P.-H.; Faugeras, P.-A.; Boëns, B.; Brouillette, F.; Montplaisir, D.; Zerrouki, R.; Lucas, R. Polysaccharides: The “Click” Chemistry Impact. Polymers 2011, 3, 1607–1651. [Google Scholar] [CrossRef]
- Uliniuc, A.; Popa, M.; Hamaide, T.; Dobromir, M. New approaches in hydrogel synthesis—Click chemistry: A review. Cellul. Chem. Technol. 2012, 46, 1–11. [Google Scholar]
- Barbosa, M.; Martins, C.; Gomes, P. “Click” chemistry as a tool to create novel biomaterials: A short review. UPorto J. Eng. 2015, 1, 22–34. [Google Scholar]
- Meng, X.; Edgar, K.J. “Click” reactions in polysaccharide modification. Prog. Polym. Sci. 2016, 53, 52–85. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Skorik, Y.A. Click reactions in chitosan chemistry. Russ. Chem. Bull. 2017, 66, 769–781. [Google Scholar] [CrossRef]
- Bertoldo, M.; Nazzi, S.; Zampano, G.; Ciardelli, F. Synthesis and photochromic response of a new precisely functionalized chitosan with “clicked” spiropyran. Carbohydr. Polym. 2011, 85, 401–407. [Google Scholar] [CrossRef]
- Ifuku, S.; Wada, M.; Morimoto, M.; Saimoto, H. A short synthesis of highly soluble chemoselective chitosan derivatives via “click chemistry”. Carbohydr. Polym. 2012, 90, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, W.; Gu, S.; Ren, J. Synthesis and self-assembly of tunable thermosensitive chitosan amphiphilic copolymers by click chemistry. Mater. Lett. 2010, 64, 2663–2666. [Google Scholar] [CrossRef]
- Zampano, G.; Bertoldo, M.; Ciardelli, F. Defined Chitosan-based networks by C-6-Azide–alkyne “click” reaction. React. Funct. Polym. 2010, 70, 272–281. [Google Scholar] [CrossRef]
- Kulbokaite, R.; Ciuta, G.; Netopilik, M.; Makuska, R. N-PEG’ylation of chitosan via “click chemistry” reactions. React. Funct. Polym. 2009, 69, 771–778. [Google Scholar] [CrossRef]
- Truong, V.X.; Ablett, M.P.; Gilbert, H.T.J.; Bowen, J.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P. In situ-forming robust chitosan-poly(ethylene glycol) hydrogels prepared by copper-free azide–alkyne click reaction for tissue engineering. Biomater. Sci. 2013, 2, 167–175. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Martins, M.C.L.; Mafra, L.; Gomes, P. Synthesis of an O-alkynyl-chitosan and its chemoselective conjugation with a PEG-like amino-azide through click chemistry. Carbohydr. Polym. 2012, 87, 240–249. [Google Scholar] [CrossRef]
- Tirino, P.; Laurino, R.; Maglio, G.; Malinconico, M.; d’Ayala, G.G.; Laurienzo, P. Synthesis of chitosan–PEO hydrogels via mesylation and regioselective Cu(I)-catalyzed cycloaddition. Carbohydr. Polym. 2014, 112, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, L.; Leong, W.C.; Gan, L.H. Thermo-Responsive Association of Chitosan-graft-Poly(N-isopropylacrylamide) in Aqueous Solutions. J. Phys. Chem. B 2010, 114, 10666–10673. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, L.; Gan, L.H.; Ping, Y.; Li, J.; Ravi, P. Thermo-and pH-Responsive Association Behavior of Dual Hydrophilic Graft Chitosan Terpolymer Synthesized via ATRP and Click Chemistry. Macromolecules 2010, 43, 5679–5687. [Google Scholar] [CrossRef]
- Lu, L.; Shao, X.; Jiao, Y.; Zhou, C. Synthesis of chitosan-graft-β-cyclodextrin for improving the loading and release of doxorubicin in the nanopaticles. J. Appl. Polym. Sci. 2014, 131, 41034. [Google Scholar] [CrossRef]
- Guerry, A.; Bernard, J.; Samain, E.; Fleury, E.; Cottaz, S.; Halila, S. Aniline-Catalyzed Reductive Amination as a Powerful Method for the Preparation of Reducing End-“Clickable” Chitooligosaccharides. Bioconjug. Chem. 2013, 24, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Guerry, A.; Cottaz, S.; Fleury, E.; Bernard, J.; Halila, S. Redox-stimuli responsive micelles from DOX-encapsulating polycaprolactone-g-chitosan oligosaccharide. Carbohydr. Polym. 2014, 112, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Li, X.; Gu, S.; Cao, A.; Ren, J. Amphiphilic chitosan graft copolymer via combination of ROP, ATRP and click chemistry: Synthesis, self-assembly, thermosensitivity, fluorescence, and controlled drug release. Polymer 2011, 52, 658–666. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuang, P.; Wang, Z.; Li, Y.; Jiang, Z.; Hu, Q.; Liu, M.; Zhao, Q. One-pot synthesis of chitosan-g-(PEO-PLLA-PEO) via “click” chemistry and “SET-NRC” reaction. Carbohydr. Polym. 2012, 90, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Yi, H. Fabrication of Chitosan-Poly(ethylene glycol) Hybrid Hydrogel Microparticles via Replica Molding and Its Application toward Facile Conjugation of Biomolecules. Langmuir 2012, 28, 17061–17070. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Z.; Chen, L.; Gu, W.; Li, Y. Synthesis of 6-N,N,N-Trimethyltriazole Chitosan via “Click Chemistry” and Evaluation for Gene Delivery. Biomacromolecules 2009, 10, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Wada, M.; Morimoto, M.; Saimoto, H. Preparation of highly regioselective chitosan derivatives via “click chemistry”. Carbohydr. Polym. 2011, 85, 653–657. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.; Song, L.; Ji, Q.; Yao, Y.; Cui, Y.; Shen, J.; Wang, P.G.; Kong, D. Polysaccharide-based biomaterials with on-demand nitric oxide releasing property regulated by enzyme catalysis. Biomaterials 2013, 34, 8450–8458. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Matsumoto, C.; Wada, M.; Morimoto, M.; Saimoto, H. Preparation of highly regioselective amphiprotic chitosan derivative via “click chemistry”. Int. J. Biol. Macromol. 2013, 52, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Jirawutthiwongchai, J.; Krause, A.; Draeger, G.; Chirachanchai, S. Chitosan-Oxanorbornadiene: A Convenient Chitosan Derivative for Click Chemistry without Metal Catalyst Problem. ACS Macro Lett. 2013, 2, 177–180. [Google Scholar] [CrossRef]
- Jirawutthiwongchai, J.; Draeger, G.; Chirachanchai, S. Rapid Hybridization of Chitosan-Gold-Antibodies via Metal-free Click in Water-based Systems: A Model Approach for Naked-eye Detectable Antigen Sensors. Macromol. Rapid Commun. 2014, 35, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhao, P.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. Electrical signal guided click coating of chitosan hydrogel on conductive surface. RSC Adv. 2014, 4, 13477–13480. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Zhang, C.; Gu, G.; Guo, Z. Novel triazolyl-functionalized chitosan derivatives with different chain lengths of aliphatic alcohol substituent: Design, synthesis, and antifungal activity. Carbohydr. Res. 2015, 418, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective Sequential Modification of Chitosan via Azide-Alkyne Click Reaction: Synthesis, Characterization, and Antimicrobial Activity of Chitosan Derivatives and Nanoparticles. PLOS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef] [PubMed]
- Koshiji, K.; Nonaka, Y.; Iwamura, M.; Dai, F.; Matsuoka, R.; Hasegawa, T. C6-Modifications on chitosan to develop chitosan-based glycopolymers and their lectin-affinities with sigmoidal binding profiles. Carbohydr. Polym. 2016, 137, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Cao, X.; Peng, F.; Bian, J.; Xu, F.; Sun, R. Binding cellulose and chitosan via click chemistry: Synthesis, characterization, and formation of some hollow tubes. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 5201–5210. [Google Scholar] [CrossRef]
- Ryu, H.J.; Mahapatra, S.S.; Yadav, S.K.; Cho, J.W. Synthesis of click-coupled graphene sheet with chitosan: Effective exfoliation and enhanced properties of their nanocomposites. Eur. Polym. J. 2013, 49, 2627–2634. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mahapatra, S.S.; Yadav, M.K.; Dutta, P.K. Mechanically robust biocomposite films of chitosan grafted carbon nanotubes via the [2 + 1] cycloaddition of nitrenes. RSC Adv. 2013, 3, 23631–23637. [Google Scholar] [CrossRef]
- Gandini, A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Montiel-Herrera, M.; Gandini, A.; Goycoolea, F.M.; Jacobsen, N.E.; Lizardi-Mendoza, J.; Recillas-Mota, M.; Argüelles-Monal, W.M. N-(furfural) chitosan hydrogels based on Diels–Alder cycloadditions and application as microspheres for controlled drug release. Carbohydr. Polym. 2015, 128, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Odian, G. Principles of Polymerization, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-471-27400-1. [Google Scholar]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Kurita, K.; Kawata, M.; Koyama, Y.; Nishimura, S.-I. Graft copolymerization of vinyl monomers onto chitin with cerium (IV) ion. J. Appl. Polym. Sci. 1991, 42, 2885–2891. [Google Scholar] [CrossRef]
- Lagos, A.; Yazdani-Pedram, M.; Reyes, J.; Campos, N. Ceric Ion-Initiated Grafting of Poly(Methyl Acrylate) onto Chitin. J. Macromol. Sci. Part A 1992, 29, 1007–1015. [Google Scholar] [CrossRef]
- Ren, L.; Miura, Y.; Nishi, N.; Tokura, S. Modification of chitin by ceric salt-initiated graft polymerisation—Preparation of poly(methyl methacrylate)-grafted chitin derivatives that swell in organic solvents. Carbohydr. Polym. 1993, 21, 23–27. [Google Scholar] [CrossRef]
- Tanodekaew, S.; Prasitsilp, M.; Swasdison, S.; Thavornyutikarn, B.; Pothsree, T.; Pateepasen, R. Preparation of acrylic grafted chitin for wound dressing application. Biomaterials 2004, 25, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, T.; Naguib, H.; Sabaa, M.; Mokhtar, S. Graft copolymerization of itaconic acid onto chitin and its properties. Polym. Int. 2005, 54, 221–225. [Google Scholar] [CrossRef]
- Mokhtar, S.M.; Mostafa, T.B.; Hewedy, M.A. Chemical induced grafting of indole onto chitin & chitosan-and their antimicrobial activity. Aust. J. Basic Appl. Sci. 2010, 4, 3268–3279. [Google Scholar]
- Ifuku, S.; Iwasaki, M.; Morimoto, M.; Saimoto, H. Graft polymerization of acrylic acid onto chitin nanofiber to improve dispersibility in basic water. Carbohydr. Polym. 2012, 90, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Abu Naim, A.; Umar, A.; Sanagi, M.M.; Basaruddin, N. Chemical modification of chitin by grafting with polystyrene using ammonium persulfate initiator. Carbohydr. Polym. 2013, 98, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Tamura, H. Synthesis, characterization and thermal properties of chitin-g-poly(ε-caprolactone) copolymers by using chitin gel. Int. J. Biol. Macromol. 2008, 43, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Pilakasiri, K.; Molee, P.; Sringernyuang, D.; Sangjun, N.; Channasanon, S.; Tanodekaew, S. Efficacy of chitin-PAA-GTMAC gel in promoting wound healing: Animal study. J. Mater. Sci. Mater. Med. 2011, 22, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Uppanan, P.; Channasanon, S.; Veeranondh, S.; Tanodekaew, S. Synthesis of GTMAC modified chitin–PAA gel and evaluation of its biological properties. J. Biomed. Mater. Res. Part A 2011, 98, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-M.; Chen, L.-C.; Yang, H.-C.; Li, M.-H.; Pan, T.-C. Preparation of acrylic acid-modified chitin improved by an experimental design and its application in absorbing toxic organic compounds. J. Hazard. Mater. 2012, 241–242, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Praveen, P.; Latha, D. A novel method for synthesis of polypyrrole grafted chitin. J. Polym. Res. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Davidenko, N.; Sastre, R.; Gallardo, A.; San Román, J. Self-curing membranes of chitosan/PAA IPNs obtained by radical polymerization: Preparation, characterization and interpolymer complexation. Biomaterials 1999, 20, 1869–1878. [Google Scholar] [CrossRef]
- Recillas, M.; Silva, L.L.; Peniche, C.; Goycoolea, F.M.; Rinaudo, M.; Argüelles-Monal, W.M. Thermoresponsive behavior of chitosan-g-N-isopropylacrylamide copolymer solutions. Biomacromolecules 2009, 10, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Lavrič, P.K.; Warmoeskerken, M.M.C.G.; Jocic, D. Functionalization of cotton with poly-NiPAAm/chitosan microgel. Part I. Stimuli-responsive moisture management properties. Cellulose 2012, 19, 257–271. [Google Scholar] [CrossRef]
- Chen, C.; Liu, M.; Gao, C.; Lü, S.; Chen, J.; Yu, X.; Ding, E.; Yu, C.; Guo, J.; Cui, G. A convenient way to synthesize comb-shaped chitosan-graft-poly(N-isopropylacrylamide) copolymer. Carbohydr. Polym. 2013, 92, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Iemma, F.; Cirillo, G.; Altimari, I.; Puoci, F.; Picci, N. Temperature-sensitive hydrogels by graft polymerization of chitosan and N-isopropylacrylamide for drug release. Pharm. Dev. Technol. 2013, 18, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Wang, C.-F.; Don, T.-M.; Chiu, W.-Y. Preparation of pH- and thermo-sensitive chitosan-PNIPAAm core–shell nanoparticles and evaluation as drug carriers. Cellulose 2013, 20, 1791–1805. [Google Scholar] [CrossRef]
- Raskin, M.M.; Schlachet, I.; Sosnik, A. Mucoadhesive nanogels by ionotropic crosslinking of chitosan-g-oligo(NiPAam) polymeric micelles as novel drug nanocarriers. Nanomedicine 2016, 11, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Cui, X.; Guo, Y.; Zhang, X.; Hongyan, W. Synthesis of chitosan-based nanohydrogels for loading and release of 5-fluorouracil. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 91–97. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Wang, G.; Wang, J. A pH-sensitive porous chitosan membrane prepared via surface grafting copolymerization in supercritical carbon dioxide. Polym. Int. 2015, 64, 383–388. [Google Scholar] [CrossRef]
- Metzler, M.; Chylińska, M.; Kaczmarek, H. Preparation and characteristics of nanosilver composite based on chitosan-graft-acrylic acid copolymer. J. Polym. Res. 2015, 22, 146. [Google Scholar] [CrossRef]
- García-Valdez, O.; Champagne-Hartley, R.; Saldívar-Guerra, E.; Champagne, P.; Cunningham, M.F. Modification of chitosan with polystyrene and poly(N-butyl acrylate) via nitroxide-mediated polymerization and grafting from approach in homogeneous media. Polym. Chem. 2015, 6, 2827–2836. [Google Scholar] [CrossRef]
- Khan, A.; Othman, M.B.H.; Chang, B.P.; Akil, H.M. Preparation, physicochemical and stability studies of chitosan-PNIPAM based responsive microgels under various pH and temperature conditions. Iran. Polym. J. 2015, 24, 317–328. [Google Scholar] [CrossRef]
- Echeverria, C.; Soares, P.; Robalo, A.; Pereira, L.; Novo, C.M.M.; Ferreira, I.; Borges, J.P. One-pot synthesis of dual-stimuli responsive hybrid PNIPAAm-chitosan microgels. Mater. Des. 2015, 86, 745–751. [Google Scholar] [CrossRef]
- Fernández-Gutiérrez, M.; Fusco, S.; Mayol, L.; Román, J.S.; Borzacchiello, A.; Ambrosio, L. Stimuli-responsive chitosan/poly(N-isopropylacrylamide) semi-interpenetrating polymer networks: Effect of pH and temperature on their rheological and swelling properties. J. Mater. Sci. Mater. Med. 2016, 27, 109. [Google Scholar] [CrossRef] [PubMed]
- Anbinder, P.; Macchi, C.; Amalvy, J.; Somoza, A. Chitosan-graft-poly(N-butyl acrylate) copolymer: Synthesis and characterization of a natural/synthetic hybrid material. Carbohydr. Polym. 2016, 145, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Liao, Q.; Yang, W.; Ma, W.; Zhao, J.; Zheng, X.; Yang, Y.; Chen, R. Preparation, property of the complex of carboxymethyl chitosan grafted copolymer with iodine and application of it in cervical antibacterial biomembrane. Mater. Sci. Eng. C 2016, 67, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hong, Y.; Song, Q.; Zhang, Z.; Gao, J.; Tao, T. Highly efficient removal of copper ions from water using poly(acrylic acid)-grafted chitosan adsorbent. Colloid Polym. Sci. 2017, 295, 627–635. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Physico-chemical characterization of pH-sensitive N-Succinyl chitosan-g-poly(acrylamide-co-acrylic acid) hydrogels and in vitro drug release studies. Polym. Degrad. Stab. 2017, 139, 38–54. [Google Scholar] [CrossRef]
- Khalaj Moazen, M.; Ahmad Panahi, H. Magnetic iron oxide nanoparticles grafted N-isopropylacrylamide/chitosan copolymer for the extraction and determination of letrozole in human biological samples. J. Sep. Sci. 2017, 40, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Munro, N.H.; Hanton, L.R.; Moratti, S.C.; Robinson, B.H. Synthesis and characterisation of chitosan-graft-poly(OEGMA) copolymers prepared by ATRP. Carbohydr. Polym. 2009, 77, 496–505. [Google Scholar] [CrossRef]
- Aziz, M.S.A.; Naguib, H.F.; Saad, G.R. Nanocomposites Based on Chitosan-Graft-Poly(N-Vinyl-2-Pyrrolidone): Synthesis, Characterization, and Biological Activity. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 578–586. [Google Scholar] [CrossRef]
- Leiva, A.; Bonardd, S.; Pino, M.; Saldías, C.; Kortaberria, G.; Radić, D. Improving the performance of chitosan in the synthesis and stabilization of gold nanoparticles. Eur. Polym. J. 2015, 68, 419–431. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Shen, X.; Fang, Y. Preparation of chitosan-g-polycaprolactone copolymers through ring-opening polymerization of ϵ-caprolactone onto phthaloyl-protected chitosan. Biopolymers 2005, 78, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, L.; Fang, Y. Self-Catalysis of Phthaloylchitosan for Graft Copolymerization of ε-Caprolactone with Chitosan. Macromol. Rapid Commun. 2006, 27, 1988–1994. [Google Scholar] [CrossRef]
- Liu, L.; Shi, A.; Guo, S.; Fang, Y.; Chen, S.; Li, J. Preparation of chitosan-g-polylactide graft copolymers via self-catalysis of phthaloylchitosan and their complexation with DNA. React. Funct. Polym. 2010, 70, 301–305. [Google Scholar] [CrossRef]
- Mirabbasi, F.; Dorkoosh, F.A.; Moghimi, A.; Shahsavari, S.; Babanejad, N.; Seifirad, S. Preparation of Mesalamine Nanoparticles Using a Novel Polyurethane-Chitosan Graft Copolymer. Pharm. Nanotechnol. 2017, 5, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Cabuk, M.; Yavuz, M.; Unal, H.I. Electrokinetic properties of biodegradable conducting polyaniline-graft-chitosan copolymer in aqueous and non-aqueous media. Colloids Surf. Physicochem. Eng. Asp. 2014, 460, 494–501. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Recillas, M.; Silva, L.L.; Peniche, C.; Goycoolea, F.M.; Rinaudo, M.; Román, J.S.; Argüelles-Monal, W.M. Thermo- and pH-responsive polyelectrolyte complex membranes from chitosan-g-N-isopropylacrylamide and pectin. Carbohydr. Polym. 2011, 86, 1336–1343. [Google Scholar] [CrossRef]
- Marques, N.D.N.; Maia, A.M.D.S.; Balaban, R.D.C. Development of dual-sensitive smart polymers by grafting chitosan with poly (N-isopropylacrylamide): An overview. Polímeros 2015, 25, 237–246. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Liu, H.; Fang, Y. Synthesis and characterization of chitosan-graft-polycaprolactone copolymers. Eur. Polym. J. 2004, 40, 2739–2744. [Google Scholar] [CrossRef]
- Liu, L.; Xu, X.; Guo, S.; Han, W. Synthesis and self-assembly of chitosan-based copolymer with a pair of hydrophobic/hydrophilic grafts of polycaprolactone and poly(ethylene glycol). Carbohydr. Polym. 2009, 75, 401–407. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Lang, M. Preparation of mono-dispersed silver nanoparticles assisted by chitosan-g-poly(ε-caprolactone) micelles and their antimicrobial application. Appl. Surf. Sci. 2014, 301, 273–279. [Google Scholar] [CrossRef]
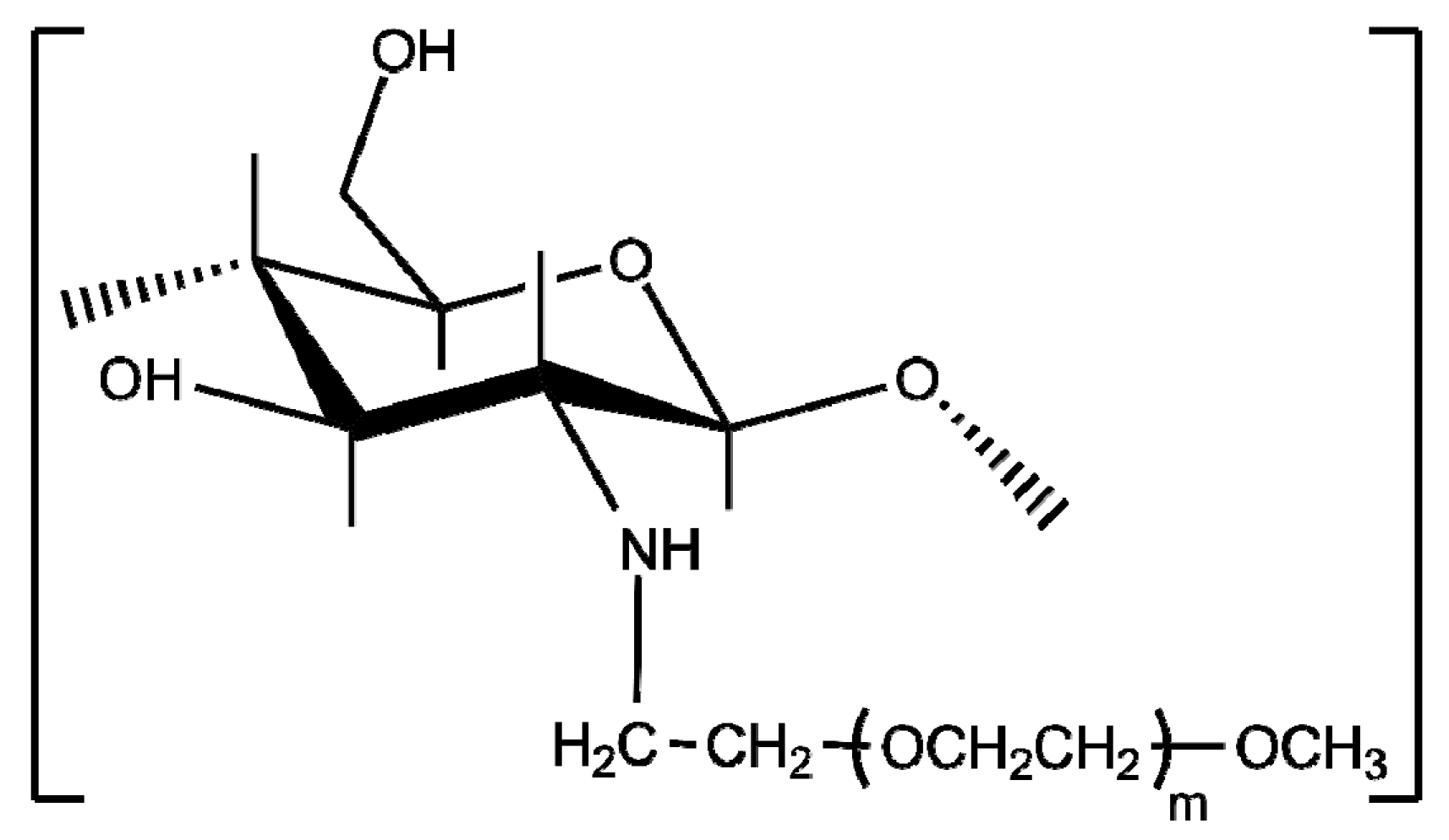
- Gorochovceva, N.; Makuška, R. Synthesis and study of water-soluble chitosan-O-poly(ethylene glycol) graft copolymers. Eur. Polym. J. 2004, 40, 685–691. [Google Scholar] [CrossRef]
- Bhattarai, N.; Matsen, F.A.; Zhang, M. PEG-Grafted Chitosan as an Injectable Thermoreversible Hydrogel. Macromol. Biosci. 2005, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dai, H.; Leong, K.W.; Goh, S.-H.; Mao, H.-Q.; Yang, Y.-Y. Chitosan-g-PEG/DNA complexes deliver gene to the rat liver via intrabiliary and intraportal infusions. J. Gene Med. 2006, 8, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Hsieh, Y.-L. PEGylation of chitosan for improved solubility and fiber formation via electrospinning. Cellulose 2007, 14, 543–552. [Google Scholar] [CrossRef]
- Bae, K.H.; Moon, C.W.; Lee, Y.; Park, T.G. Intracellular Delivery of Heparin Complexed with Chitosan-g-Poly(Ethylene Glycol) for Inducing Apoptosis. Pharm. Res. 2009, 26, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Deng, L.; Chen, C.; Zhang, J.; Zhou, R.; Li, X.; Hu, R.; Dong, A. Preparation and properties of thermoreversible hydrogels based on methoxy poly(ethylene glycol)-grafted chitosan nanoparticles for drug delivery systems. Carbohydr. Polym. 2011, 83, 1828–1833. [Google Scholar] [CrossRef]
- Papadimitriou, S.A.; Achilias, D.S.; Bikiaris, D.N. Chitosan-g-PEG nanoparticles ionically crosslinked with poly(glutamic acid) and tripolyphosphate as protein delivery systems. Int. J. Pharm. 2012, 430, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Sun, J.; Ding, F. PEG-g-chitosan thermosensitive hydrogel for implant drug delivery: Cytotoxicity, in vivo degradation and drug release. J. Biomater. Sci. Polym. Ed. 2014, 25, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, D.; Milusheva, R.; Baumann, P.; Constantin, D.; Chami, M.; Palivan, C.G. The Amine Content of PEGylated Chitosan Bombyx mori Nanoparticles Acts as a Trigger for Protein Delivery. Langmuir 2014, 30, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.; Le, T.N.T.; Nguyen, T.A.; Dang, M.C. Poly(ethylene glycol) grafted chitosan as new copolymer material for oral delivery of insulin. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 035004. [Google Scholar] [CrossRef]
- Chung, H.J.; Go, D.H.; Bae, J.W.; Jung, I.K.; Lee, J.W.; Park, K.D. Synthesis and characterization of Pluronic® grafted chitosan copolymer as a novel injectable biomaterial. Curr. Appl. Phys. 2005, 5, 485–488. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan–Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gilstrap, K.; Wu, L.; Bahadur, R.K.C.; Moss, M.A.; Wang, Q.; Lu, X.; He, X. Synthesis and Characterization of Thermally Responsive Pluronic F127−Chitosan Nanocapsules for Controlled Release and Intracellular Delivery of Small Molecules. ACS Nano 2010, 4, 6747–6759. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L. (Lucy); Ping, Q. Poly(N-isopropylacrylamide)–chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control. Release 2007, 120, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Ma, L.; Yan, J.; Yan, M.; Gao, C.; Shen, J. The gene transfection efficiency of thermoresponsive N,N,N-trimethyl chitosan chloride-g-poly(N-isopropylacrylamide) copolymer. Biomaterials 2007, 28, 4488–4500. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, Y.H. Synthesis and thermo-responsive properties of chitosan-g-poly (N-isopropylacrylamide) and HTCC-g-poly(N-isopropylacrylamide) copolymers. Fibers Polym. 2010, 11, 164–169. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int. J. Biol. Macromol. 2011, 49, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Antoniraj, M.G.; Kumar, C.S.; Kandasamy, R. Synthesis and characterization of poly (N-isopropylacrylamide)-g-carboxymethyl chitosan copolymer-based doxorubicin-loaded polymeric nanoparticles for thermoresponsive drug release. Colloid Polym. Sci. 2016, 294, 527–535. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Biodegradable and thermo-sensitive chitosan-g-poly(N-vinylcaprolactam) nanoparticles as a 5-fluorouracil carrier. Carbohydr. Polym. 2011, 83, 776–786. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Muthunarayanan, M.; Divyarani, V.V.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J. Colloid Interface Sci. 2011, 360, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Thomas, R.G.; Muthiah, M.; Chennazhi, K.P.; Park, I.-K.; Jeong, Y.Y.; Manzoor, K.; Jayakumar, R. Radio frequency triggered curcumin delivery from thermo and pH responsive nanoparticles containing gold nanoparticles and its in vivo localization studies in an orthotopic breast tumor model. RSC Adv. 2014, 4, 39408–39427. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Thomas, R.G.; Muthiah, M.; Chennazhi, K.P.; Manzoor, K.; Park, I.-K.; Jeong, Y.Y.; Jayakumar, R. Anti-cancer, pharmacokinetics and tumor localization studies of pH-, RF- and thermo-responsive nanoparticles. Int. J. Biol. Macromol. 2015, 74, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quiroz, D.; González-Gómez, Á.; Lizardi-Mendoza, J.; Vázquez-Lasa, B.; Goycoolea, F.M.; San Román, J.; Argüelles-Monal, W.M. Effect of the molecular architecture on the thermosensitive properties of chitosan-g-poly(N-vinylcaprolactam). Carbohydr. Polym. 2015, 134, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Thomas, R.G.; Muthiah, M.; Lee, H.J.; Jeong, Y.Y.; Park, I.-K.; Jayakumar, R. Breast Tumor Targetable Fe3O4 Embedded Thermo-Responsive Nanoparticles for Radiofrequency Assisted Drug Delivery. J. Biomed. Nanotechnol. 2016, 12, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Indulekha, S.; Arunkumar, P.; Bahadur, D.; Srivastava, R. Thermoresponsive polymeric gel as an on-demand transdermal drug delivery system for pain management. Mater. Sci. Eng. C 2016, 62, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quiroz, D.; González-Gómez, Á.; Lizardi-Mendoza, J.; Vázquez-Lasa, B.; Goycoolea, F.M.; Román, J.S.; Argüelles-Monal, W.M. Conformational study on the thermal transition of chitosan-g-poly(N-vinylcaprolactam) in aqueous solution. Colloid Polym. Sci. 2016, 294, 555–563. [Google Scholar] [CrossRef]
- Casettari, L.; Vllasaliu, D.; Castagnino, E.; Stolnik, S.; Howdle, S.; Illum, L. PEGylated chitosan derivatives: Synthesis, characterizations and pharmaceutical applications. Prog. Polym. Sci. 2012, 37, 659–685. [Google Scholar] [CrossRef]
- Wang, J.-P.; Chen, Y.-Z.; Ge, X.-W.; Yu, H.-Q. Gamma radiation-induced grafting of a cationic monomer onto chitosan as a flocculant. Chemosphere 2007, 66, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Chen, Y.-Z.; Zhang, S.-J.; Yu, H.-Q. A chitosan-based flocculant prepared with gamma-irradiation-induced grafting. Bioresour. Technol. 2008, 99, 3397–3402. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, Y.; Zhu, C.; Guo, J.; Zhao, C.; Liao, Y.; Guan, Q. UV-initiated polymerization of hydrophobically associating cationic flocculants: Synthesis, characterization, and dewatering properties. Chem. Eng. J. 2013, 234, 318–326. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, M.; Zhu, C.; Xu, Y.; Zheng, H.; Xiao, X.; Wu, H.; Xia, T.; You, Z. UV-Initiated Graft Copolymerization of Cationic Chitosan-Based Flocculants for Treatment of Zinc Phosphate-Contaminated Wastewater. Ind. Eng. Chem. Res. 2016, 55, 10025–10035. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, C.; Sun, W.; Xu, Y.; Xiao, X.; Zheng, H.; Wu, H.; Liu, C. Plasma-initiated polymerization of chitosan-based CS-g-P(AM-DMDAAC) flocculant for the enhanced flocculation of low-algal-turbidity water. Carbohydr. Polym. 2017, 164, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Yi, Y.; Wang, J. Removal of Cobalt Ions from Aqueous Solution Using Chitosan Grafted with Maleic Acid by Gamma Radiation. Nucl. Eng. Technol. 2018, 50, 211–215. [Google Scholar] [CrossRef]
- Saleh, A.S.; Ibrahim, A.G.; Elsharma, E.M.; Metwally, E.; Siyam, T. Radiation grafting of acrylamide and maleic acid on chitosan and effective application for removal of Co.(II) from aqueous solutions. Radiat. Phys. Chem. 2018, 144, 116–124. [Google Scholar] [CrossRef]
- Sosnik, A.; Imperiale, J.C.; Vázquez-González, B.; Raskin, M.M.; Muñoz-Muñoz, F.; Burillo, G.; Cedillo, G.; Bucio, E. Mucoadhesive thermo-responsive chitosan-g-poly(N-isopropylacrylamide) polymeric micelles via a one-pot gamma-radiation-assisted pathway. Colloids Surf. B Biointerfaces 2015, 136, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; García-Uriostegui, L.; Ortega, A.; Isoshima, T.; Burillo, G. Radiation grafting of N-vinylcaprolactam onto nano and macrogels of chitosan: Synthesis and characterization. Carbohydr. Polym. 2017, 155, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.Á.; Ortega, A.; Burillo, G. Dual-stimuli responsive copolymers based on N-vinylcaprolactam/chitosan. J. Radioanal. Nucl. Chem. 2014, 303, 2143–2150. [Google Scholar] [CrossRef]
- Pérez-Calixto, M.P.; Ortega, A.; Garcia-Uriostegui, L.; Burillo, G. Synthesis and characterization of N-vinylcaprolactam/N,N-dimethylacrylamide grafted onto chitosan networks by gamma radiation. Radiat. Phys. Chem. 2016, 119, 228–235. [Google Scholar] [CrossRef]
- Harada, A.; Hashidzume, A.; Takashima, Y. Cyclodextrin-Based Supramolecular Polymers. In Supramolecular Polymers Polymeric Betains Oligomers; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2001; Volume 201. [Google Scholar]
- Sajomsang, W.; Nuchuchua, O.; Gonil, P.; Saesoo, S.; Sramala, I.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. Water-soluble β-cyclodextrin grafted with chitosan and its inclusion complex as a mucoadhesive eugenol carrier. Carbohydr. Polym. 2012, 89, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Auzély-Velty, R.; Rinaudo, M. New Supramolecular Assemblies of a Cyclodextrin-Grafted Chitosan through Specific Complexation. Macromolecules 2002, 35, 7955–7962. [Google Scholar] [CrossRef]
- Yu, N.; Li, G.; Gao, Y.; Liu, X.; Ma, S. Stimuli-sensitive hollow spheres from chitosan-graft-β-cyclodextrin for controlled drug release. Int. J. Biol. Macromol. 2016, 93, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, B.-H.; Li, B.; Zhang, Y.-M.; Zhao, P.; Chen, Y.-T.; Wada, T.; Inoue, Y. Molecular Recognition Study on Supramolecular System. 14.1 Synthesis of Modified Cyclodextrins and Their Inclusion Complexation Thermodynamics with l-Tryptophan and Some Naphthalene Derivatives. J. Org. Chem. 1998, 63, 1444–1454. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Auzély, R.; Rinaudo, M. Controlled Chemical Modifications of Chitosan. Characterization and Investigation of Original Properties. Macromol. Biosci. 2003, 3, 562–565. [Google Scholar] [CrossRef]
- Daimon, Y.; Izawa, H.; Kawakami, K.; Żywicki, P.; Sakai, H.; Abe, M.; Hill, J.P.; Ariga, K. Media-dependent morphology of supramolecular aggregates of β-cyclodextrin-grafted chitosan and insulin through multivalent interactions. J. Mater. Chem. B 2014, 2, 1802–1812. [Google Scholar] [CrossRef]
- Daimon, Y.; Kamei, N.; Kawakami, K.; Takeda-Morishita, M.; Izawa, H.; Takechi-Haraya, Y.; Saito, H.; Sakai, H.; Abe, M.; Ariga, K. Dependence of Intestinal Absorption Profile of Insulin on Carrier Morphology Composed of β-Cyclodextrin-Grafted Chitosan. Mol. Pharm. 2016, 13, 4034–4042. [Google Scholar] [CrossRef] [PubMed]
- Tojima, T.; Katsura, H.; Han, S.-M.; Tanida, F.; Nishi, N.; Tokura, S.; Sakairi, N. Preparation of an α-cyclodextrin–linked chitosan derivative via reductive amination strategy. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 1965–1968. [Google Scholar] [CrossRef]
- Auzély-Velty, R.; Rinaudo, M. Chitosan Derivatives Bearing Pendant Cyclodextrin Cavities: Synthesis and Inclusion Performance. Macromolecules 2001, 34, 3574–3580. [Google Scholar] [CrossRef]
- Venter, J.P.; Kotzé, A.F.; Auzély-Velty, R.; Rinaudo, M. Synthesis and evaluation of the mucoadhesivity of a CD-chitosan derivative. Int. J. Pharm. 2006, 313, 36–42. [Google Scholar] [CrossRef] [PubMed]
- El-Tahlawy, K.; Gaffar, M.A.; El-Rafie, S. Novel method for preparation of β-cyclodextrin/grafted chitosan and it’s application. Carbohydr. Polym. 2006, 63, 385–392. [Google Scholar] [CrossRef]
- Chaleawlert-umpon, S.; Nuchuchua, O.; Saesoo, S.; Gonil, P.; Ruktanonchai, U.R.; Sajomsang, W.; Pimpha, N. Effect of citrate spacer on mucoadhesive properties of a novel water-soluble cationic β-cyclodextrin-conjugated chitosan. Carbohydr. Polym. 2011, 84, 186–194. [Google Scholar] [CrossRef]
- Furusaki, E.; Ueno, Y.; Sakairi, N.; Nishi, N.; Tokura, S. Facile preparation and inclusion ability of a chitosan derivative bearing carboxymethyl-β-cyclodextrin. Carbohydr. Polym. 1996, 29, 29–34. [Google Scholar] [CrossRef]
- Kono, H.; Teshirogi, T. Cyclodextrin-grafted chitosan hydrogels for controlled drug delivery. Int. J. Biol. Macromol. 2015, 72, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Takechi-Haraya, Y.; Tanaka, K.; Tsuji, K.; Asami, Y.; Izawa, H.; Shigenaga, A.; Otaka, A.; Saito, H.; Kawakami, K. Molecular Complex Composed of β-Cyclodextrin-Grafted Chitosan and pH-Sensitive Amphipathic Peptide for Enhancing Cellular Cholesterol Efflux under Acidic pH. Bioconjug. Chem. 2015, 26, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, L.; Zhang, Y.; Chen, K.; Wang, H.; Gong, R. Carboxymethyl-β-cyclodextrin grafted chitosan nanoparticles as oral delivery carrier of protein drugs. React. Funct. Polym. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Gao, X.; Shu, S.; Zhang, H.; Wang, Z.; Li, C. Chitosan bearing pendant cyclodextrin as a carrier for controlled protein release. Carbohydr. Polym. 2009, 77, 394–401. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y. Study on β-cyclodextrin grafting with chitosan and slow release of its inclusion complex with radioactive iodine. J. Appl. Polym. Sci. 2001, 82, 2414–2421. [Google Scholar] [CrossRef]
- Martel, B.; Devassine, M.; Crini, G.; Weltrowski, M.; Bourdonneau, M.; Morcellet, M. Preparation and sorption properties of a β-cyclodextrin-linked chitosan derivative. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 169–176. [Google Scholar] [CrossRef]
- Gonil, P.; Sajomsang, W.; Ruktanonchai, U.R.; Pimpha, N.; Sramala, I.; Nuchuchua, O.; Saesoo, S.; Chaleawlert-umpon, S.; Puttipipatkhachorn, S. Novel quaternized chitosan containing β-cyclodextrin moiety: Synthesis, characterization and antimicrobial activity. Carbohydr. Polym. 2011, 83, 905–913. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R.; Pimpha, N.; Sramala, I.; Nuchuchua, O.; Saesoo, S.; Chaleawlert-umpon, S.; Puttipipatkhachorn, S. Self-aggregates formation and mucoadhesive property of water-soluble β-cyclodextrin grafted with chitosan. Int. J. Biol. Macromol. 2011, 48, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ye, Y.; Gao, F.; Yuan, H.; Lan, M.; Lou, K.; Wang, W. Chitosan-graft-β-cyclodextrin nanoparticles as a carrier for controlled drug release. Int. J. Pharm. 2013, 446, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Phunpee, S.; Suktham, K.; Surassmo, S.; Jarussophon, S.; Rungnim, C.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. Controllable encapsulation of α-mangostin with quaternized β-cyclodextrin grafted chitosan using high shear mixing. Int. J. Pharm. 2018, 538, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Yi, Y. Synthesis and characterization of grafting β-cyclodextrin with chitosan. J. Appl. Polym. Sci. 2004, 94, 860–864. [Google Scholar] [CrossRef]
- Chiu, S.-H.; Chung, T.-W.; Giridhar, R.; Wu, W.-T. Immobilization of β-cyclodextrin in chitosan beads for separation of cholesterol from egg yolk. Food Res. Int. 2004, 37, 217–223. [Google Scholar] [CrossRef]
- Zha, F.; Li, S.; Chang, Y. Preparation and adsorption property of chitosan beads bearing β-cyclodextrin cross-linked by 1,6-hexamethylene diisocyanate. Carbohydr. Polym. 2008, 72, 456–461. [Google Scholar] [CrossRef]
- Sreenivasan, K. Synthesis and preliminary studies on a β-cyclodextrin-coupled chitosan as a novel adsorbent matrix. J. Appl. Polym. Sci. 1998, 69, 1051–1055. [Google Scholar] [CrossRef]
- Sajomsang, W.; Nuchuchua, O.; Saesoo, S.; Gonil, P.; Chaleawlert-umpon, S.; Pimpha, N.; Sramala, I.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. A comparison of spacer on water-soluble cyclodextrin grafted chitosan inclusion complex as carrier of eugenol to mucosae. Carbohydr. Polym. 2013, 92, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, X.; Qiu, F.; Zhao, H.; Yang, D.; Wang, J.; Wen, W. Synthesis of β-cyclodextrin–chitosan–graphene oxide composite and its application for adsorption of manganese ion (II). Mater. Technol. 2016, 31, 406–415. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Chen, L.; Kalliola, S.; Tang, J.; Iakovleva, E.; Tam, K.C.; Sillanpää, M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017, 7, 15811. [Google Scholar] [CrossRef] [PubMed]
- Krauland, A.; Alonso, M. Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int. J. Pharm. 2007, 340, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Thanh Nguyen, H.; Goycoolea, F.M. Chitosan/Cyclodextrin/TPP Nanoparticles Loaded with Quercetin as Novel Bacterial Quorum Sensing Inhibitors. Molecules 2017, 22, 1975. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Wang, Z.Y.; Duan, X.Y.; Jiang, L.J.; Cao, P.P.; Li, J.X.; Li, J.B. Design and evaluation of chitosan-β-cyclodextrin based thermosensitive hydrogel. Biochem. Eng. J. 2016, 111, 100–107. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Divya, P.L.; Nima, J. Synthesis and characterization of novel drug delivery system using modified chitosan based hydrogel grafted with cyclodextrin. Chem. Eng. J. 2016, 284, 1259–1269. [Google Scholar] [CrossRef]
- Sashiwa, H. Chemical Aspects of Chitin and Chitosan Derivatives. In Chitin and Chitosan Derivatives: Advances in Drug Discovery and Developments; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 93–111. ISBN 978-1-4665-6628-6. [Google Scholar]
- Sashiwa, H.; Shigemasa, Y.; Roy, R. Chemical Modification of Chitosan. 3. Hyperbranched Chitosan−Sialic Acid Dendrimer Hybrid with Tetraethylene Glycol Spacer. Macromolecules 2000, 33, 6913–6915. [Google Scholar] [CrossRef]
- Sashiwa, H.; Shigemasa, Y.; Roy, R. Chemical Modification of Chitosan. 10. Synthesis of Dendronized Chitosan−Sialic Acid Hybrid Using Convergent Grafting of Preassembled Dendrons Built on Gallic Acid and Tri(ethylene glycol) Backbone. Macromolecules 2001, 34, 3905–3909. [Google Scholar] [CrossRef]
- Sashiwa, H.; Shigemasa, Y.; Roy, R. Chemical Modification of Chitosan 11: Chitosan–Dendrimer Hybrid as a Tree Like Molecule. Carbohydr. Polym. 2002, 49, 195–205. [Google Scholar] [CrossRef]
- Sashiwa, H.; Yajima, H.; Aiba, S. Synthesis of a Chitosan−Dendrimer Hybrid and Its Biodegradation. Biomacromolecules 2003, 4, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, Z.; Akbari, A.; Latifi, A.M.; Amani, M.A. Design of a new integrated chitosan-PAMAM dendrimer biosorbent for heavy metals removing and study of its adsorption kinetics and thermodynamics. Bioresour. Technol. 2016, 205, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Tan, Z.; Sun, F.; Sheng, L.; Zhang, X.; Yao, F. Synthesis and characterization of quaternized carboxymethyl chitosan/poly(amidoamine) dendrimer core–shell nanoparticles. Mater. Sci. Eng. C 2012, 32, 2026–2036. [Google Scholar] [CrossRef]
- Wen, Y.; Yao, F.; Sun, F.; Tan, Z.; Tian, L.; Xie, L.; Song, Q. Antibacterial action mode of quaternized carboxymethyl chitosan/poly(amidoamine) dendrimer core–shell nanoparticles against Escherichia coli correlated with molecular chain conformation. Mater. Sci. Eng. C 2015, 48, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Jang, J.-W.; Park, J.-W. Carboxymethyl chitosan-modified magnetic-cored dendrimer as an amphoteric adsorbent. J. Hazard. Mater. 2016, 317, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Van Rantwijk, F.; Madeira Lau, R.; Sheldon, R.A. Biocatalytic transformations in ionic liquids. Trends Biotechnol. 2003, 21, 131–138. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Silva Pereira, C. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, S.; Li, S. Chitin and chitosan dissolved in ionic liquids as reversible sorbents of CO2. Green Chem. 2006, 8, 630–633. [Google Scholar] [CrossRef]
- Liebert, T.; Heinze, T. Interaction of Ionic Liquids with Polysaccharides 5. Solvents and Reaction Media for the Modification of Cellulose. Bioresources 2008, 3, 576–601. [Google Scholar]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of Carbohydrates in Ionic Liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xiao, W.; Zhou, L.; Wu, T.; Wu, Y. Hydrolysis of chitosan under microwave irradiation in ionic liquids promoted by sulfonic acid-functionalized ionic liquids. Polym. Degrad. Stab. 2012, 97, 49–53. [Google Scholar] [CrossRef]
- Tian, T.-C.; Xie, C.-X.; Li, L.; Wei, Q.-L.; Yu, S.-T.; Zhang, T. Research on the structure of amino acid ILs and its solubility for chitosan with chemical software. Polym. Degrad. Stab. 2016, 126, 17–21. [Google Scholar] [CrossRef]
- Yang, X.; Qiao, C.; Li, Y.; Li, T. Dissolution and resourcfulization of biopolymers in ionic liquids. React. Funct. Polym. 2016, 100, 181–190. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Mine, S.; Izawa, H.; Kaneko, Y.; Kadokawa, J. Acetylation of α-chitin in ionic liquids. Carbohydr. Res. 2009, 344, 2263–2265. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, S.; Wang, B.; Xu, F.; Sun, R. Homogeneous acetylation of chitosan in ionic liquids. J. Appl. Polym. Sci. 2013, 129, 28–35. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Boamah, P.-O.; Cao, L.; Zhang, Q.; Lu, Z.; Li, H. Homogeneous synthesis of linoleic acid-grafted chitosan oligosaccharide in ionic liquid and its self-assembly performance in aqueous solution. J. Appl. Polym. Sci. 2015, 132, 41727. [Google Scholar] [CrossRef]
- Li, L.; Yuan, B.; Liu, S.; Yu, S.; Xie, C.; Liu, F.; Zhang, C. N-acyl chitosan and its fiber with excellent moisture absorbability and retentivity: Preparation in a novel [Gly]Cl/water homogeneous system. J. Appl. Polym. Sci. 2013, 129, 3282–3289. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Li, C.; Zhang, D.; Xiao, Y.; Guan, G.; Zhu, W. Modification of chitosan with monomethyl fumaric acid in an ionic liquid solution. Carbohydr. Polym. 2015, 117, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, W.; Zhou, Y.; Zhang, S.; Hua, D.; Zhu, X. Modification of chitosan with carboxyl-functionalized ionic liquid for anion adsorption. Int. J. Biol. Macromol. 2013, 62, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; He, J.; Zhang, J.; Gan, Z. Homogeneous synthesis of amino-reserved chitosan-graft-polycaprolactone in an ionic liquid and the application in cell cultivation. Polym. Int. 2015, 64, 1045–1052. [Google Scholar] [CrossRef]
- Pei, L.; Cai, Z.; Shang, S.; Song, Z. Synthesis and antibacterial activity of alkylated chitosan under basic ionic liquid conditions. J. Appl. Polym. Sci. 2014, 131, 40052. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Qiao, C.; Mu, X.; Li, T.; Xu, J.; Shi, L.; Zhang, D. A simple and convenient method to synthesize N-[(2-hydroxyl)-propyl-3-trimethylammonium] chitosan chloride in an ionic liquid. Carbohydr. Polym. 2015, 130, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cui, S.; Zhao, Y.; Zhang, S.; Chen, H.; Peng, X.; Wang, B. O-Alkylation of Chitosan for Gene Delivery by Using Ionic Liquid in an in-situ Reactor. Engineering 2012, 4, 114–117. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yoshida, S.; Mine, S.; Kadokawa, J. Synthesis of chitin-graft-polystyrene via atom transfer radical polymerization initiated from a chitin macroinitiator. Polym. Chem. 2013, 4, 3384–3389. [Google Scholar] [CrossRef]
- Lin, C.; Liu, D.; Luo, W.; Liu, Y.; Zhu, M.; Li, X.; Liu, M. Functionalization of chitosan via single electron transfer living radical polymerization in an ionic liquid and its antimicrobial activity. J. Appl. Polym. Sci. 2015, 132, 42754. [Google Scholar] [CrossRef]
- Chen, H.; Cui, S.; Zhao, Y.; Zhang, C.; Zhang, S.; Peng, X. Grafting Chitosan with Polyethylenimine in an Ionic Liquid for Efficient Gene Delivery. PLoS ONE 2015, 10, e0121817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, L.; Li, C.; Zhang, D.; Xiao, Y.; Guan, G.; Zhu, W. A novel and simple procedure to synthesize chitosan-graft-polycaprolactone in an ionic liquid. Carbohydr. Polym. 2013, 94, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, M.; Luo, X.; Huang, L.; Chen, L. Acidic ionic liquid catalyzed crosslinking of oxycellulose with chitosan for advanced biocomposites. Carbohydr. Polym. 2014, 113, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ling, Y.; Wang, X.; Han, Y.; Zeng, X.; Sun, R. Maillard reaction products from chitosan–xylan ionic liquid solution. Carbohydr. Polym. 2013, 98, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Guyomard-Lack, A.; Buchtová, N.; Humbert, B.; Bideau, J.L. Ion segregation in an ionic liquid confined within chitosan based chemical ionogels. Phys. Chem. Chem. Phys. 2015, 17, 23947–23951. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Ghiaci, M.; Shchukarev, A. Cross-linked chitosan with a dicationic ionic liquid as a recyclable biopolymer-supported catalyst for cycloaddition of carbon dioxide with epoxides into cyclic carbonates. New J. Chem. 2018, 42, 587–597. [Google Scholar] [CrossRef]
- Hua, D.; Jiang, J.; Kuang, L.; Jiang, J.; Zheng, W.; Liang, H. Smart Chitosan-Based Stimuli-Responsive Nanocarriers for the Controlled Delivery of Hydrophobic Pharmaceuticals. Macromolecules 2011, 44, 1298–1302. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Li, C.; Wu, S.; Xiao, Y. Preparation and antimicrobial activity of sulfopropyl chitosan in an ionic liquid aqueous solution. J. Appl. Polym. Sci. 2017, 134, 44989. [Google Scholar] [CrossRef]
- Ishii, D.; Ohashi, C.; Hayashi, H. Facile enhancement of the deacetylation degree of chitosan by hydrothermal treatment in an imidazolium-based ionic liquid. Green Chem. 2014, 16, 1764–1767. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, C.; Wang, Q.; Zhao, Z.K. Efficient hydrolysis of chitosan in ionic liquids. Carbohydr. Polym. 2009, 78, 685–689. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, A.; Hou, Y.; Shan, C.; Ding, H.; Shan, Y. An innovative method for oxidative degradation of chitosan with molecular oxygen catalyzed by metal phthalocyanine in neutral ionic liquid. Carbohydr. Res. 2009, 344, 2010–2013. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Kerton, F.M.; Yan, N. Conversion of chitin and N-acetyl-d-glucosamine into a N-containing furan derivative in ionic liquids. RSC Adv. 2015, 5, 20073–20080. [Google Scholar] [CrossRef]
- Husson, E.; Hadad, C.; Huet, G.; Laclef, S.; Lesur, D.; Lambertyn, V.; Jamali, A.; Gottis, S.; Sarazin, C.; Nhien, A.N.V. The effect of room temperature ionic liquids on the selective biocatalytic hydrolysis of chitin via sequential or simultaneous strategies. Green Chem. 2017, 19, 4122–4131. [Google Scholar] [CrossRef]
- Yuan, B.; Li, L.; Xie, C.; Liu, K.; Yu, S. Preparation of oligochitosan via In situ enzymatic hydrolysis of chitosan by amylase in [Gly]BF4 ionic liquid/water homogeneous system. J. Appl. Polym. Sci. 2014, 131, 41152. [Google Scholar] [CrossRef]
- Zhao, G.; Lang, X.; Wang, F.; Li, J.; Li, X. A one-pot method for lipase-catalyzed synthesis of chitosan palmitate in mixed ionic liquids and its characterization. Biochem. Eng. J. 2017, 126, 24–29. [Google Scholar] [CrossRef]
















| Monomers | Applications | References |
|---|---|---|
| Chitin “grafting from” copolymers | ||
| Acrylamide | Water absorbents, chelating agents | [82] |
| Acrylic acid | Water absorbents, chelating agents. Wound dressing. Nanofibers | [82,85,88] |
| Methyl methacrylate | Gel-like mass for biomedicine | [84] |
| Itaconic acid | Waste-water treatment | [86] |
| Indole | Antimicrobial activity | [87] |
| -caprolactone | Biomedical field | [90] |
| Glycidytrimethylammonium chloride | Wound healing | [91] |
| Pyrrole | Electrically-conducting material | [94] |
| Chitosan “grafting from” copolymers | ||
| Acrylic acid | Controlled release devices, ion-exchange bioseparation, antibacterial activity, removal of heavy metal ions | [95,103,104,111] |
| N-butyl acrylate | Biodegradable packaging materials, recovery of heavy metals from waste waters | [105,109] |
| Iodine | Cervical antibacterial biomembrane | [110] |
| acrylamide-co-acrylic acid | Drug release hydrogels | [112] |
| Styrene | Recovery of heavy metals from waste waters | [105] |
| Aniline | Antibacterial activity | [121] |
| PNIPAm | Biomedical field: tissue engineering, drug delivery systems. | [96,97,98,99,100,101,102,106,107,108,113,123,145] |
| Lactide | Gene delivery, complex with DNA | [119] |
| -caprolactone | Nanoparticle stabilizer, drug delivery systems | [37,116,117,118] |
| N-vinyl-2-pyrrolidone | Antimicrobial activity, nanoparticle stabilizer | [115,116] |
| Carbamate (urethane) | Drug delivery systems | [120] |
| Indole | Antimicrobial activity | [87] |
| Chitosan “grafting onto” copolymers | ||
| Pluronic | Injectable cell delivery carrier, gene expression, controlled release | [140,141] |
| -caprolactone | Drug carriers, antimicrobial activity | [125,126,127] |
| Ethylene glycol | Bioactive molecules delivery, polymeric surfactants, gene delivery, apoptosis-inducing activity. | [128,131,133,135,137,138] |
| PNIPAm | Drug/gene delivery, | [57,58,98,142,143,144,145,146] |
| PVCL | Controlled drug delivery systems | [147,148,149,150,151,152,153,154] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argüelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342. https://doi.org/10.3390/polym10030342
Argüelles-Monal WM, Lizardi-Mendoza J, Fernández-Quiroz D, Recillas-Mota MT, Montiel-Herrera M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers. 2018; 10(3):342. https://doi.org/10.3390/polym10030342
Chicago/Turabian StyleArgüelles-Monal, Waldo M., Jaime Lizardi-Mendoza, Daniel Fernández-Quiroz, Maricarmen T. Recillas-Mota, and Marcelino Montiel-Herrera. 2018. "Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials" Polymers 10, no. 3: 342. https://doi.org/10.3390/polym10030342