Comparative Study of Diethylaminoethyl-Chitosan and Methylglycol-Chitosan as Potential Non-Viral Vectors for Gene Therapy
Abstract
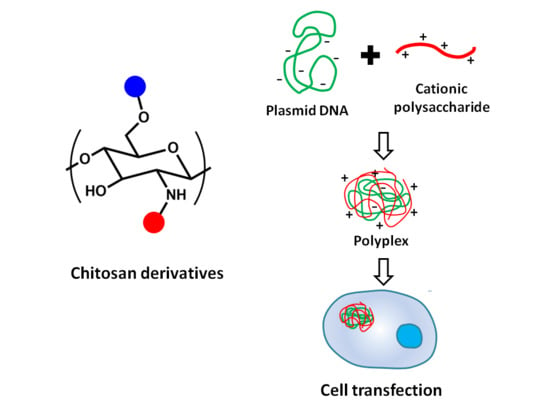
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of DEAE-CSI under Heterogeneous Conditions
2.3. Synthesis of DEAE-CSII under Homogeneous Conditions
2.4. Characterization Methods
2.5. Size Exclusion Chromatography Coupled to Multi-Angle Light Scattering (SEC-MALS)
2.6. Dynamic Light Scattering (DLS)
2.7. Gel-Retardation Assay
2.8. Ethidium Bromide Displacement Assay
2.9. Cell Culture
2.10. DNA Transfection
2.11. Bradford Protein Quantification
2.12. Assessing the Toxicity In Vitro
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of MG-CS
3.2. Synthesis and Characterization of DEAE-CS
3.3. Assessing the Toxicity In Vitro
3.4. Evaluation of DNA Binding Affinity
3.5. DLS Measurements
3.6. In Vitro DNA Transfection Efficiency
4. Conclusions
- The interaction of DEAE-Cl with chitosan at pH 5–6 furnishes N-substituted DEAE-CSII with a moderate DS, whereas the reaction in alkaline medium leads to a highly substituted product with a more uncertain pattern of substitution.
- Both DEAE-CS and MG-CS efficiently bind DNA to form stable self-assembled polyplexes at polysaccharide:DNA ratios greater than 2:1. The stoichiometry of the polyplexes was about 1:1.
- In Calu-3 cells, the DNA transfection activity of MG-CS improves with increasing polysaccharide:DNA ratios. By contrast, the transfection efficiency of DEAE-CS is lower and has no expressed dependency on the DS or the polysaccharide:DNA ratio. In JAWSII cells, both Lipofectamine and MG-CS show significantly less DNA transfection activity, while the efficiency of DEAE-CS remains at the same level as seen in Calu-3 experiments (or even slightly increases).
- The toxicity of DEAE-CS rises with increasing concentrations of the polymer and its DS, whereas MG-CS demonstrates only moderate cell toxicity, even at the highest concentration studied.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Pair vs. Naked DNA | Calu-3 Cells | JAWSII Cells | |||
|---|---|---|---|---|---|
| Bonferroni p-Value | Significance | Bonferroni p-Value | Significance | ||
| MG-CS | 2:1 1 | 0.85 | ns | 2.8 | ns |
| 5:1 | 0.005 | ** | 0.22 | ns | |
| 10:1 | 5 × 10−5 | ** | 0.026 | * | |
| DEAE-CSI | 2:1 | 0.025 | * | 1.8 | ns |
| 5:1 | 0.002 | ** | 0.14 | ns | |
| 10:1 | 0.005 | ** | 0.28 | ns | |
| DEAE-CSII | 2:1 | 0.21 | ns | 2.1 | ns |
| 5:1 | 0.061 | ns | 0.14 | ns | |
| 10:1 | 0.025 | * | 0.025 | * | |
References
- Keeler, A.M.; ElMallah, M.K.; Flotte, T.R. Gene therapy 2017: Progress and future directions. CTS Clin. Transl. Sci. 2017, 10, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, W.; Xiao, Z.L.; Ye, S.; Ding, C.D.; Fu, J.J. Smart drug and gene co-delivery system for cancer therapy. Progress Chem. 2017, 29, 502–512. [Google Scholar]
- Duzgunes, N.; de Ilarduya, C.T. Genetic nanomedicine: Gene delivery by targeted lipoplexes. Methods Enzymol. 2012, 509, 355–367. [Google Scholar] [PubMed]
- Simcikova, M.; Prather, K.L.J.; Prazeres, D.M.F.; Monteiro, G.A. Towards effective non-viral gene delivery vector. Biotechnol. Genet. Eng. Rev. 2015, 31, 82–107. [Google Scholar] [CrossRef] [PubMed]
- Cevher, E.; Sezer, A.D.; Caglar, E.S. Gene delivery systems: Recent progress in viral and non-viral therapy. In Recent Advances in Novel Drug Carrier Systems; Intech: London, UK, 2012; pp. 437–470. [Google Scholar]
- Candiani, G. Non-viral gene delivery vectors methods and protocols preface. In Non-Viral Gene Delivery Vectors: Methods and Protocols; Candiani, G., Ed.; Springer: Berlin, Germany, 2016; Volume 1445, pp. V–VII. [Google Scholar]
- Borchard, G. Chitosans for gene delivery. Adv. Drug Deliv. Rev. 2001, 52, 145–150. [Google Scholar] [CrossRef]
- Erbacher, P.; Zou, S.; Bettinger, T.; Steffan, A.-M.; Remy, J.-S. Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability. Pharm. Res. 1998, 15, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Kwon, I.C.; Kim, Y.H.; Jo, W.H.; Jeong, S.Y. Preparation of chitosan self-aggregates as a gene delivery system. J. Control Release 1998, 51, 213–220. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Andranovits, S.; Skorik, Y.A. Chitosan and its derivatives: Vectors in gene therapy. Rus. Chem. Rev. 2017, 86, 231–239. [Google Scholar] [CrossRef]
- Raik, S.V.; Poshina, D.N.; Lyalina, T.A.; Polyakov, D.S.; Vasilyev, V.B.; Kritchenkov, A.S.; Skorik, Y.A. N-[4-(N,N,N-trimethylammonium)benzyl]chitosan chloride: Synthesis, interaction with DNA and evaluation of transfection efficiency. Carbohydr. Polym. 2018, 181, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Aied, A.; Greiser, U.; Pandit, A.; Wang, W.X. Polymer gene delivery: Overcoming the obstacles. Drug Discov. Today 2013, 18, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.; Kim, T.I. Therapeutic gene delivery using bioreducible polymers. Arch. Pharm. Res. 2014, 37, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, V.A. DNA condensation by multivalent cations. Biopolymers 1997, 44, 269–282. [Google Scholar] [CrossRef]
- Abdelhady, H.G.; Allen, S.; Davies, M.C.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M. Direct real-time molecular scale visualisation of the degradation of condensed DNA complexes exposed to DNase I. Nucleic Acids Res. 2003, 31, 4001–4005. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, D.V.; Lauffenburger, D.A. Optimization of cell surface binding enhances efficiency and specificity of molecular conjugate gene delivery. J. Biol. Chem. 1998, 273, 28004–28009. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Pelet, J.M.; Putnam, D. Polymer systems for gene delivery-past, present, and future. Progress Polym. Sci. 2007, 32, 799–837. [Google Scholar] [CrossRef]
- Oliveira, F.D.P.; Dalla Picola, I.P.; Shi, Q.; Barbosa, H.F.G.; Tiera, V.A.D.; Fernandes, J.C.; Tiera, M.J. Synthesis and evaluation of diethylethylamine-chitosan for gene delivery: Composition effects on the in vitro transfection efficiency. Nanotechnology 2013, 24. [Google Scholar]
- Wyatt, P.J. Combined differential light scattering with various liquid chromatography separation techniques. In Laser Light Scattering in Biochemistry; Harding, S.E., Sattelle, D.B., Bloomfield, V.A., Eds.; Royal Society of Chemistry: Cambridge, UK, 1992; pp. 35–58. [Google Scholar]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. Macromolecular conformation of chitosan in dilute solution: A new global hydrodynamic approach. Carbohydr. Polym. 2009, 76, 616–621. [Google Scholar] [CrossRef]
- Rinaudo, M.; Milas, M.; Dung, P.L. Characterization of chitosan. Influence of ionic strength and degree of acetylation on chain expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Weinhold, M.X.; Sauvageau, J.C.M.; Keddig, N.; Matzke, M.; Tartsch, B.; Grunwald, I.; Kubel, C.; Jastorff, B.; Thoming, J. Strategy to improve the characterization of chitosan for sustainable biomedical applications: Sar guided multi-dimensional analysis. Green Chem. 2009, 11, 498–509. [Google Scholar] [CrossRef]
- Corsi, K.; Chellat, F.; Yahia, L.; Fernandes, J.C. Mesenchymal stem cells, mg63 and hek293 transfection using chitosan-DNA nanoparticles. Biomaterials 2003, 24, 1255–1264. [Google Scholar] [CrossRef]
- Koping-Hoggard, M.; Tubulekas, I.; Guan, H.; Edwards, K.; Nilsson, M.; Varum, K.M.; Artursson, P. Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther. 2001, 8, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Dai, Y.; Lv, L.; Zhao, H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE 2014, 9, e84703. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Cox, R.A. Transfection of murine dendritic cell line (jaws ii) by a nonviral transfection reagent. Biotechniques 2003, 35, 600–605. [Google Scholar] [PubMed]
- Verma, I.M.; Weitzman, M.D. Gene therapy: Twenty-first century medicine. Annu. Rev. Biochem. 2005, 74, 711–738. [Google Scholar] [CrossRef] [PubMed]







| Sample | DS 1 | DA 1 | Mw 2 (g/mol) | PDI = Mw/Mn 2 | Elemental Composition (%) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| MG-CS | 1.5 (DG) | 0.13 | 114,000 ± 3000 | 1.36 ± 0.07 | 30.8 | 4.95 | 2.91 |
| 0.87 (DQ) | |||||||
| Chitosan | - | 0.15 | 81,400 ± 1600 | 2.10 ± 0.40 | 36.6 | 8.21 | 6.54 |
| (0.17) 3 | |||||||
| DEAE-CSI | 1.2 | 0.17 | 134,000 ± 1000 | 1.68 ± 0.08 | 45.1 | 7.61 | 7.51 |
| DEAE-CSII | 0.57 | 0.18 | 84,500 ± 1700 | 1.64 ± 0.07 | 39.8 | 8.08 | 7.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raik, S.V.; Andranovitš, S.; Petrova, V.A.; Xu, Y.; Lam, J.K.-W.; Morris, G.A.; Brodskaia, A.V.; Casettari, L.; Kritchenkov, A.S.; Skorik, Y.A. Comparative Study of Diethylaminoethyl-Chitosan and Methylglycol-Chitosan as Potential Non-Viral Vectors for Gene Therapy. Polymers 2018, 10, 442. https://doi.org/10.3390/polym10040442
Raik SV, Andranovitš S, Petrova VA, Xu Y, Lam JK-W, Morris GA, Brodskaia AV, Casettari L, Kritchenkov AS, Skorik YA. Comparative Study of Diethylaminoethyl-Chitosan and Methylglycol-Chitosan as Potential Non-Viral Vectors for Gene Therapy. Polymers. 2018; 10(4):442. https://doi.org/10.3390/polym10040442
Chicago/Turabian StyleRaik, Sergei V., Stanislav Andranovitš, Valentina A. Petrova, Yingying Xu, Jenny Ka-Wing Lam, Gordon A. Morris, Alexandra V. Brodskaia, Luca Casettari, Andreii S. Kritchenkov, and Yury A. Skorik. 2018. "Comparative Study of Diethylaminoethyl-Chitosan and Methylglycol-Chitosan as Potential Non-Viral Vectors for Gene Therapy" Polymers 10, no. 4: 442. https://doi.org/10.3390/polym10040442