Ionic Liquids Incorporating Polyamide 6: Miscibility and Physical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the PA6/VBIM Blends
2.3. Characterization
3. Results
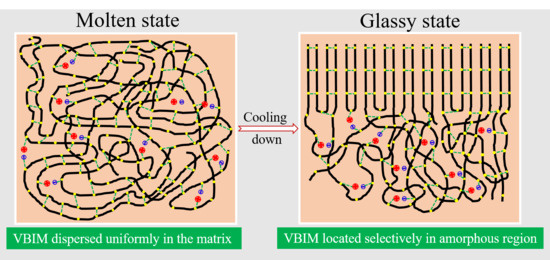
3.1. The Morphology of PA6 and the PA6/VBIM Blends
3.2. The Crystallization Behaviors of PA and the PA6/VBIM Blends
3.2.1. The Crystalline Form
3.2.2. The Crystallization and Melting Behaviors
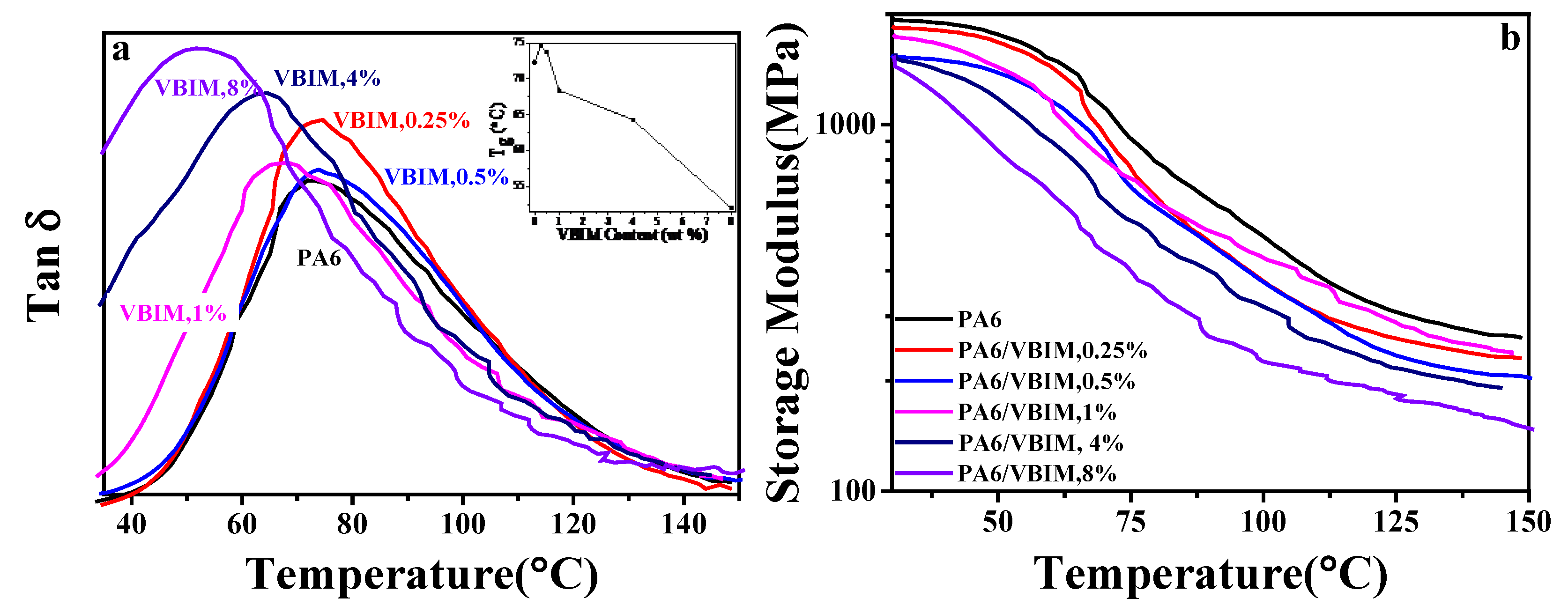
3.3. Thermal Behaviors of PA6 and the PA6/VBIM Blends
3.3.1. The Glass Transition Temperature
3.3.2. Thermal Stability of PA6 and PA6/VBIM Blends
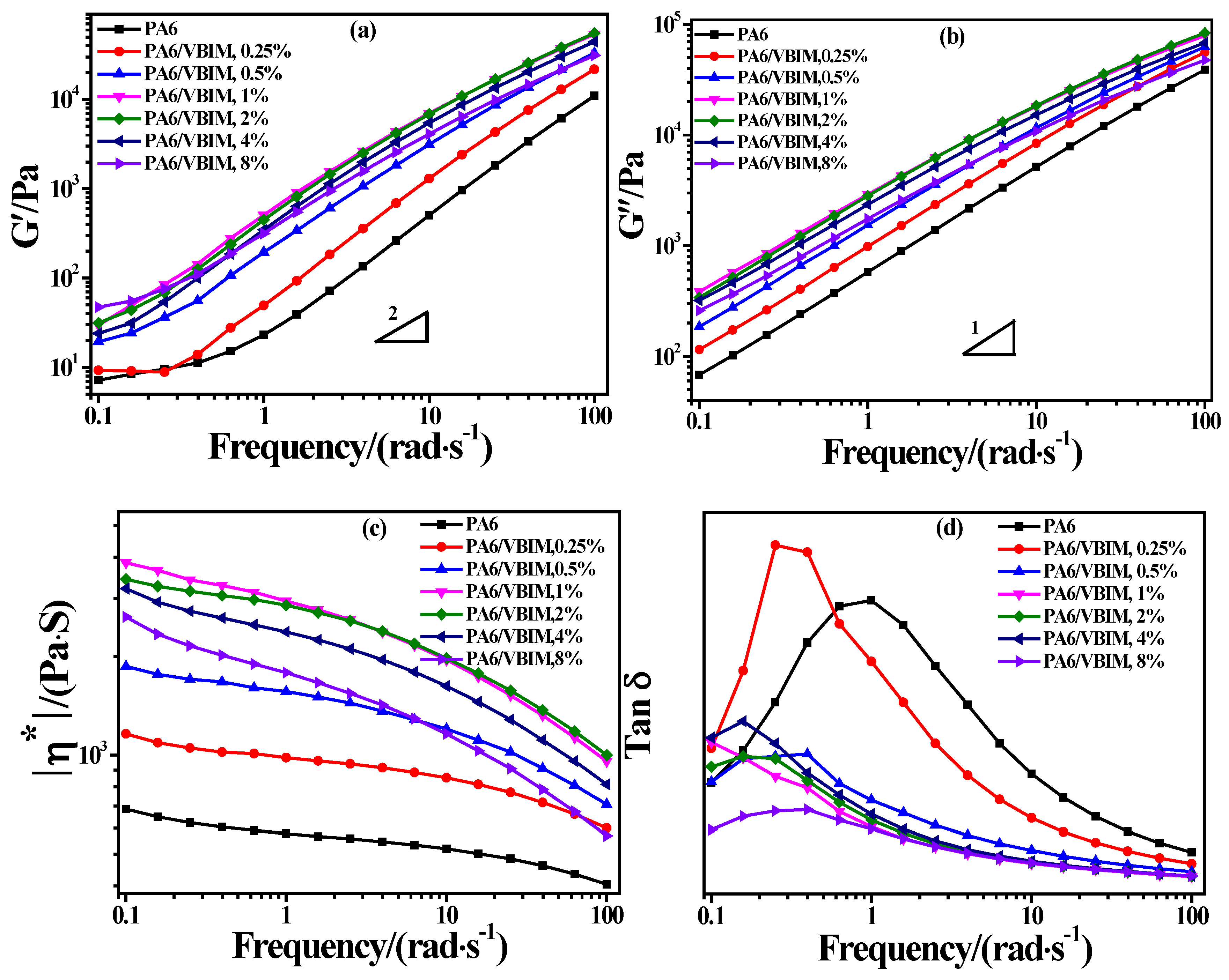
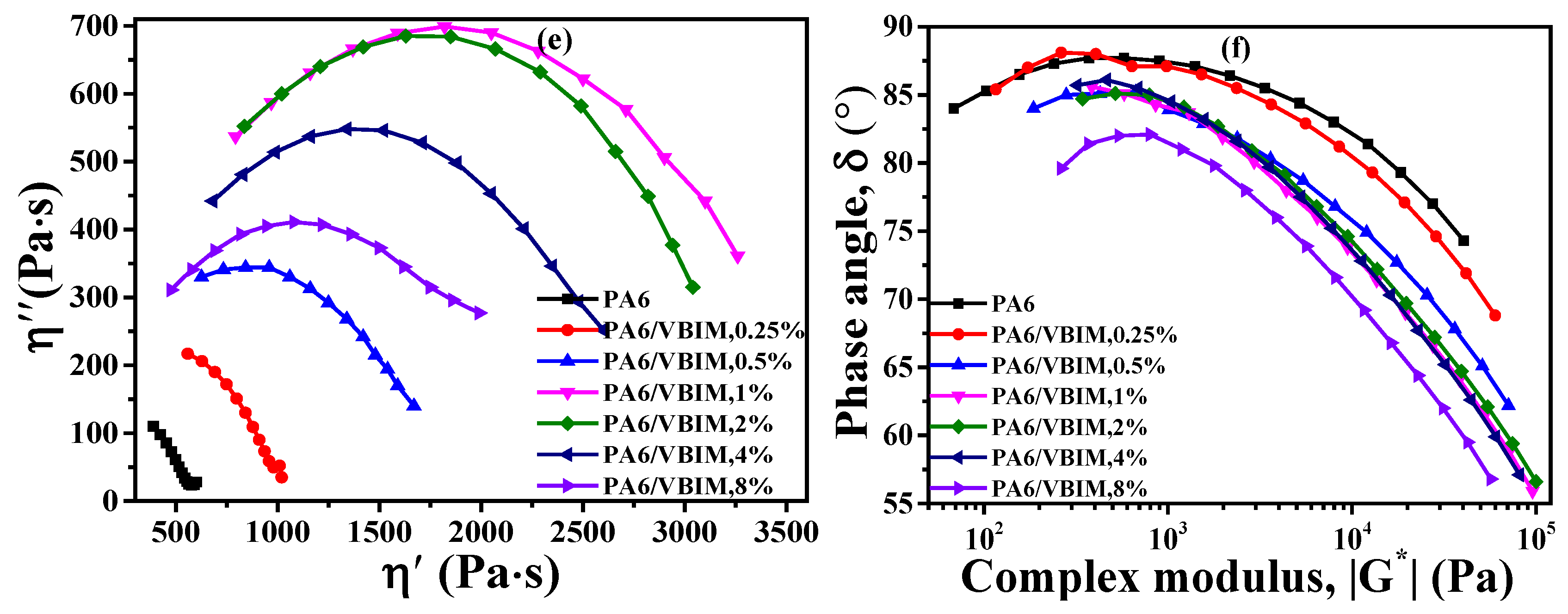
3.4. Rheological Properties of PA6 and the PA6/VBIM Blends
3.5. Physical Properties of PA6 and the PA6/VBIM Blends
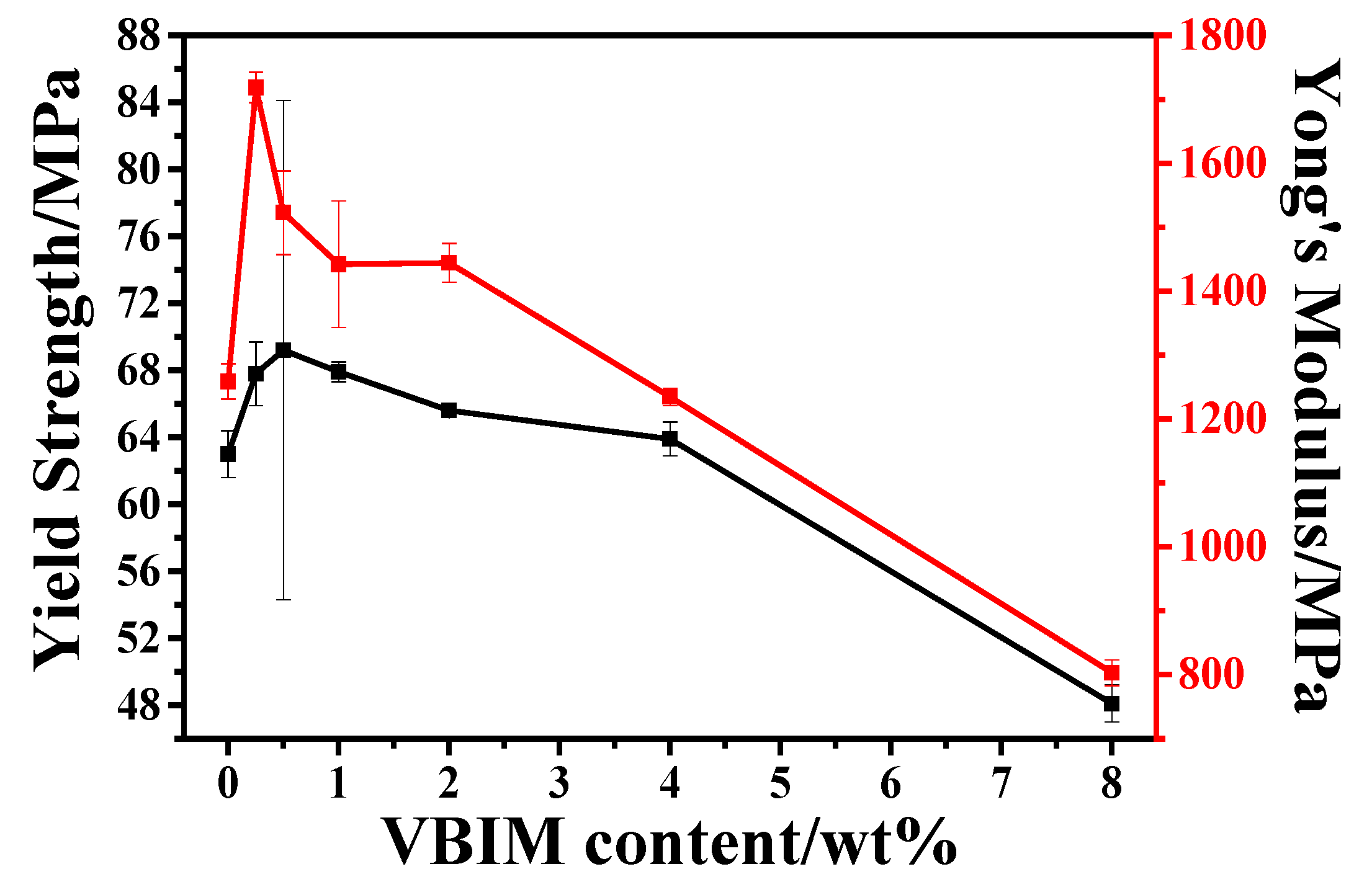
3.5.1. Mechanical Properties
3.5.2. The Antistatic Performance of PA6 and the PA6/VBIM Blends
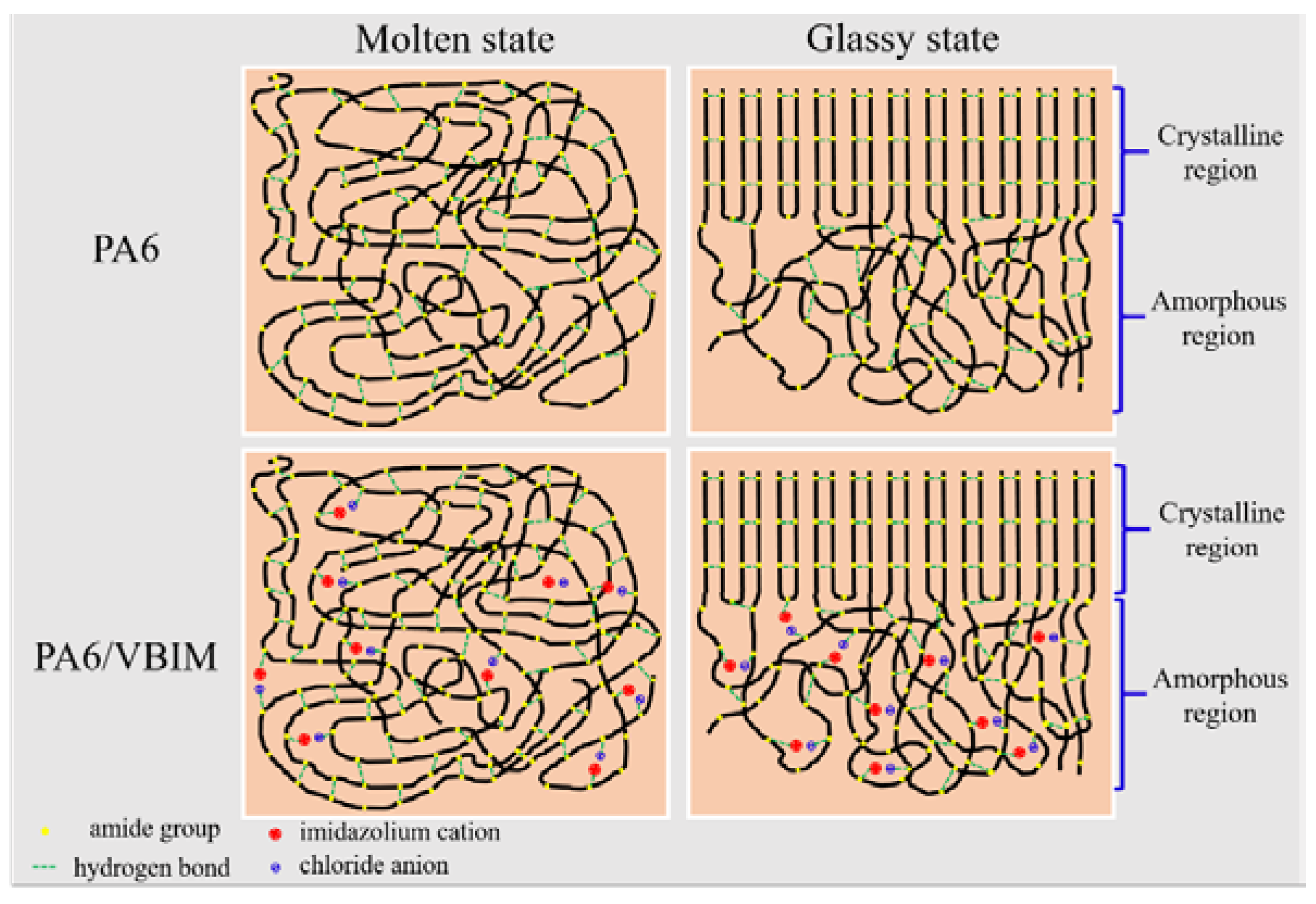
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casetta, M.; Michaux, G.; Bourbigot, S. Key role of magnesium hydroxide surface treatment in the flame retardancy of glass fiber reinforced polyamide 6. Polym. Degrad. Stab. 2018, 148, 95–103. [Google Scholar] [CrossRef]
- Li, Y.; Shimizu, H. Co-continuous polyamide 6 (PA6)/acrylonitrile-butadiene-styrene (ABS) nanocomposites. Macromol. Rapid Commun. 2005, 26, 710–715. [Google Scholar] [CrossRef]
- Chiu, F.C.; Lai, S.M.; Chen, Y.L. Investigation on the polyamide 6/organoclay nanocomposites with or without a maleated polyolefin elastomer as a toughener. Polymer 2005, 46, 11600–11609. [Google Scholar] [CrossRef]
- Murthy, N.S. Hydrogen bonding, mobility, and structural transitions in aliphatic polyamides. J. Polym. Sci. Polym. Phys. 2006, 44, 1763–1782. [Google Scholar] [CrossRef]
- Fernandes, F.C.; Gadioli, R.; Yassitepe, E.; De Paoli, M.A. Polyamide-6 composites reinforced with cellulose fibers and fabricated by extrusion: Effect of fiber bleaching on mechanical properties and stability. Polym. Compos. 2017, 38, 299–308. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Liew, K.M. Functionalization of multi-walled carbon nanotubes grafted with self-generated functional groups and their polyamide 6 composites. Carbon 2010, 48, 721–729. [Google Scholar] [CrossRef]
- García-López, D.; Gobernado-Mitre, I.; Pastor, J.M. Influence of clay modification process in PA6-layered silicate nanocomposite properties. Polymer 2005, 46, 2758–2765. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloid Surf. B 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Damm, C.; Münstedt, H.; Rösch, A. Long-term antimicrobial polyamide 6/silver-nanocomposites. J. Mater. Sci. 2007, 42, 6067–6073. [Google Scholar] [CrossRef]
- Pang, Z.; Fu, J.; Wei, Q. Fabrication of PA6/TiO2/PANI composite nanofibers by electrospinning–electrospraying for ammonia sensor. Colloid Surf. A 2014, 461, 113–118. [Google Scholar] [CrossRef]
- Kubisa, P. Ionic liquids in the synthesis and modification of polymers. J. Polym. Sci. Polym. Chem. 2005, 43, 4675–4683. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.; Bugatti, V.; Livi, S. Ionic liquid as surfactant agent of hydrotalcite: Influence on the final properties of polycaprolactone matrix. Polymers 2018, 10, 44. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Seddon, K.R. Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Cevasco, G.; Chiappe, C. Are ionic liquids a proper solution to current environmental challenges? Green Chem. 2014, 16, 2375–2385. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Livi, S.; Soares, B.G. Development of sustainable thermosets from cardanol-based epoxy prepolymer and ionic liquids. ACS Sustain. Chem. Eng. 2017, 5, 8429–8438. [Google Scholar] [CrossRef]
- Lu, W.; Fadeev, A.G.; Qi, B. Use of ionic liquids for π-conjugated polymer electrochemical devices. Science 2002, 297, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Masuda, G.; Takagi, K. Electrochemical properties of novel ionic liquids for electric double layer capacitor applications. Electrochim. Acta 2004, 49, 3603–3611. [Google Scholar] [CrossRef]
- Waliszewski, D.; Stępniak, I.; Piekarski, H. Heat capacities of ionic liquids and their heats of solution in molecular liquids. Thermochim. Acta 2005, 433, 149–152. [Google Scholar] [CrossRef]
- Fröba, A.P.; Rausch, M.H.; Krzeminski, K. Thermal conductivity of ionic liquids: Measurement and prediction. Int. J. Thermophys. 2010, 31, 2059–2077. [Google Scholar] [CrossRef]
- Lins, L.C.; Bugatti, V.; Livi, S. Phosphonium ionic liquid as interfacial agent of layered double hydroxide: Application to a pectin matrix. Carbohydr. Polym. 2018, 182, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Trewyn, B.G.; Whitman, C.M.; Lin, V.S.Y. Morphological control of room-temperature ionic liquid templated mesoporous silica nanoparticles for controlled release of antibacterial agents. Nano Lett. 2004, 4, 2139–2143. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Lv, M. Antisuperbug cotton fabric with excellent laundering durability. ACS Appl. Mater. Interfaces 2016, 8, 19866–19871. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.; Špulák, M.; Garcia, M.T. Antimicrobial toxicity studies of ionic liquids leading to a ‘hit’ mrsa selective antibacterial imidazolium salt. Green Chem. 2012, 14, 1350–1356. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Liu, W.; Chen, Y. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar] [CrossRef]
- Gu, S.; Zhu, L.; Mercier, C. Glass fiber networks as an orbit for ions: Fabrication of excellent antistatic pp/gf composites with extremely low organic salts loadings. ACS Appl. Mater. Interfaces 2017, 9, 18305–18313. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Guan, J.; Li, Y. Effect of a room-temperature ionic liquid on the structure and properties of electrospun poly (vinylidene fluoride) nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 4447–4457. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qin, H.; Yang, X. Influence of ionic liquids on the structure of polyamide 6. Mater. Lett. 2016, 180, 200–202. [Google Scholar] [CrossRef]
- Li, D.; Pang, Z.; Wang, Q. Fabrication and characterization of polyamide6-room temperature ionic liquid (PA6-RTIL) composite nanofibers by electrospinning. Fibers Polym. 2013, 14, 1614–1619. [Google Scholar] [CrossRef]
- Rafique, F.Z.; Vasanthan, N. Crystallization, crystal structure, and isothermal melt crystallization kinetics of novel Polyamide 6/SiO2 nanocomposites prepared using the sol-gel technique. J. Phys. Chem. B 2014, 118, 9486–9495. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Wei, K.H. Induced γ → α crystal transformation in blends of polyamide 6 and liquid crystalline copolyester. Macromolecules 2000, 33, 5181–5186. [Google Scholar] [CrossRef]
- Fereydoon, M.; Tabatabaei, S.H.; Ajji, A. X-ray and trichroic infrared orientation analyses of uniaxially stretched PA6 and MXD6 nanoclay composite films. Macromolecules 2014, 47, 2384–2395. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Z.; Zhu, X. Studies on nylon 6/clay nanocomposites by melt-intercalation process. J. Appl. Polym. Sci. 1999, 71, 1133–1138. [Google Scholar] [CrossRef]
- Wang, K.; Wang, C.; Fu, Q. Effects of clay on phase morphology and mechanical properties in polyamide 6/EPDM-g-MA/organoclay ternary nanocomposites. Polymer 2007, 48, 2144–2154. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1952, 1, 123–125. [Google Scholar]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers. I. Non-crystalline copolymers. J. Chem. Technol. Biotechnol. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Fu, Z. Rheology of nanosilica-compatibilized immiscible polymer blends: Formation of a “heterogeneous network” facilitated by interfacially anchored hybrid nanosilica. Macromolecules 2017, 50, 9494–9506. [Google Scholar] [CrossRef]
- Miao, D.; Qiang, Z.; Mei, Y.H. Dynamic rheological behavior for polymer composites filled with particles. Nihon Reoroji Gakkaishi 2003, 31, 305–311. [Google Scholar] [CrossRef]
- Huang, S.J.; Bai, L.; Macosko, C.W. Controlling the morphology of immiscible cocontinuous polymer blends via silica nanoparticles jammed at the interface. Macromolecules 2016, 49, 3911–3918. [Google Scholar] [CrossRef]
- Svoboda, P.; Kressler, J.; Ougizawa, T. FTIR and calorimetric analyses of the specific interactions in poly (ε-caprolactone)/poly (styrene-co-acrylonitrile) blends using low molecular weight analogues. Macromolecules 1997, 30, 1973–1979. [Google Scholar] [CrossRef]
- Onogi, S.; Masuda, T.; Kitagawa, K. Rheological properties of anionic polystyrenes. I. Dynamic viscoelasticity of narrow-distribution polystyrenes. Macromolecules 1970, 3, 109–116. [Google Scholar] [CrossRef]
- Bates, F.S. Block copolymers near the microphase separation transition. 2. Linear dynamic mechanical properties. Macromolecules 1984, 17, 2607–2613. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef] [PubMed]
- Carrot, C.; Guillet, J. From dynamic moduli to molecular weight distribution: A study of various polydisperse linear polymers. J. Rheol. 1997, 41, 1203–1220. [Google Scholar] [CrossRef]
- Li, S.; Xiao, M.; Wei, D. The melt grafting preparation and rheological characterization of long chain branching polypropylene. Polymer 2009, 50, 6121–6128. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zhang, Y. Rheological behavior of polypropylene/octavinyl polyhedral oligomeric silsesquioxane composites. J. Polym. Sci. Polym. Phys. 2008, 46, 526–533. [Google Scholar] [CrossRef]
- Malmberg, A.; Gabriel, C.; Steffl, T. Long-chain branching in metallocene-catalyzed polyethylenes investigated by low oscillatory shear and uniaxial extensional rheometry. Macromolecules 2002, 35, 1038–1048. [Google Scholar] [CrossRef]
- Song, Y.H.; Zheng, Q. Linear rheology of nanofilled polymers. J. Rheol. 2015, 59, 155–191. [Google Scholar] [CrossRef]
- Van Gurp, M. Palmen, J. Time-temperature superposition for polymeric blends. Rheol. Bull. 1998, 67, 5–8. [Google Scholar]
- Trinkle, S.; Walter, P.; Friedrich, C. Van Gurp-Palmen plot II–classification of long chain branched polymers by their topology. Rheol. Acta 2002, 41, 103–113. [Google Scholar] [CrossRef]
- Trinkle, S.; Friedrich, C. Van Gurp-Palmen-plot: A way to characterize polydispersity of linear polymers. Rheol. Acta 2001, 40, 322–328. [Google Scholar] [CrossRef]
- Yeganeh, J.K.; Goharpey, F.; Foudazi, R. Can only rheology be used to determine the phase separation mechanism in dynamically asymmetric polymer blends (PS/PVME)? RSC Adv. 2012, 2, 8116–8127. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Sun, Y. Rheological properties and crystallization behavior of multi-walled carbon nanotube/poly (ε-caprolactone) composites. J. Polym. Sci. Polym. Phys. 2007, 45, 3137–3147. [Google Scholar] [CrossRef]
- White, K.L.; Li, P.; Sumi, Y. Rheology of disentangled multiwalled carbon nanotubes dispersed in uncured epoxy fluid. J. Phys. Chem. B 2013, 118, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.P.; Rahman, M.; Brazel, C.S. Application of ionic liquids as low-volatility plasticizers for PMMA. Eur. Polym. J. 2003, 39, 1947–1953. [Google Scholar] [CrossRef]
- Skrovanek, D.J.; Painter, P.C.; Coleman, M.M. Hydrogen bonding in polymers. 2. Infrared temperature studies of Nylon 11. Macromolecules 1986, 19, 699–705. [Google Scholar] [CrossRef]


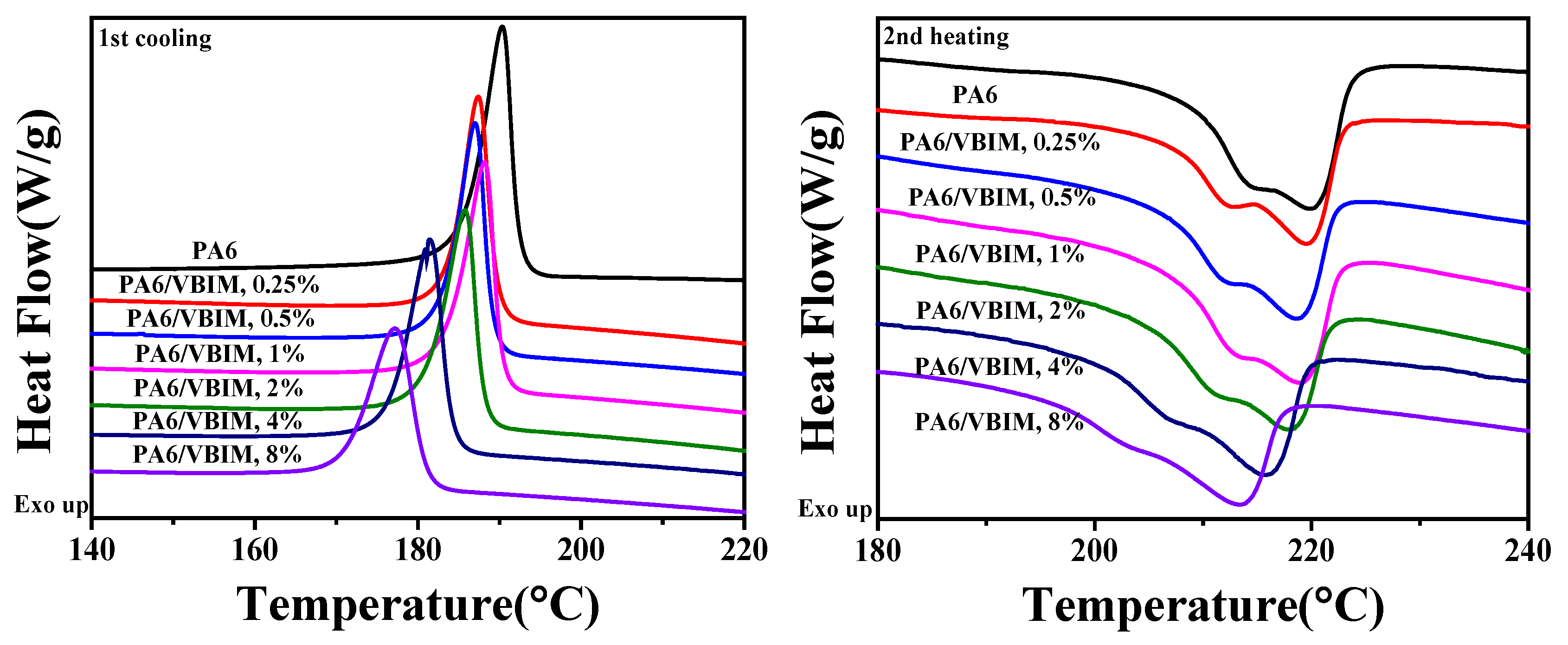





| Sample | Tm/°C | ∆Hm/J·g−1 | Tc/°C | ∆Hc/J·g−1 | /% |
|---|---|---|---|---|---|
| Neat PA6 | 219.8 | 48.6 | 190.4 | 53.1 | 25.9 |
| PA6/VBIM, 0.25% | 219.4 | 49.0 | 187.4 | 48.0 | 26.1 |
| PA6/VBIM, 0.5% | 218.3 | 49.4 | 187.1 | 48.6 | 26.1 |
| PA6/VBIM, 1% | 218.5 | 49.1 | 188.2 | 47.6 | 25.9 |
| PA6/VBIM, 2% | 217.5 | 44.3 | 185.8 | 47.1 | 23.1 |
| PA6/VBIM, 4% | 215.2 | 44.0 | 181.5 | 51.4 | 22.5 |
| PA6/VBIM, 8% | 212.9 | 37.6 | 177.3 | 50.1 | 18.5 |
| Sample | T5%/°C | T50%/°C | Tmax/°C |
|---|---|---|---|
| PA6 | 398.0 | 448.3 | 454.2 |
| PA6/VBIM, 0.25% | 393.3 | 448.3 | 455.8 |
| PA6/VBIM, 0.5% | 392.5 | 447.0 | 453.4 |
| PA6/VBIM, 1% | 384.1 | 448.7 | 456.4 |
| PA6/VBIM, 2% | 366.1 | 445.4 | 456.0 |
| PA6/VBIM, 4% | 341.0 | 444.2 | 457.6 |
| PA6/VBIM, 8% | 317.4 | 441.0 | 452.4 |
| VBIM | 231.7 | 286.0 | 282.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Lin, Q.; Jiang, P.; Li, Y.; Li, J. Ionic Liquids Incorporating Polyamide 6: Miscibility and Physical Properties. Polymers 2018, 10, 562. https://doi.org/10.3390/polym10050562
Zheng X, Lin Q, Jiang P, Li Y, Li J. Ionic Liquids Incorporating Polyamide 6: Miscibility and Physical Properties. Polymers. 2018; 10(5):562. https://doi.org/10.3390/polym10050562
Chicago/Turabian StyleZheng, Xin, Qingqing Lin, Pan Jiang, Yongjin Li, and Jingye Li. 2018. "Ionic Liquids Incorporating Polyamide 6: Miscibility and Physical Properties" Polymers 10, no. 5: 562. https://doi.org/10.3390/polym10050562