Facile Synthesis of Di-Mannitol Adipate Ester-Based Zinc Metal Alkoxide as a Bi-Functional Additive for Poly(Vinyl Chloride)
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation Process
2.2.1. Preparation of Di-Maltitol Adipate Ester
2.2.2. Preparation of Di-Mannitol Adipate Ester-Based Zinc Metal Alkoxide (DMAE-Zn)
2.3. Material Characterization
2.4. PVC Sample Preparation
2.5. Thermal Stability Test
2.5.1. Conductivity Measurement
2.5.2. Thermal Aging Test
2.5.3. UV-VIS Spectroscopy Test
2.5.4. Torque Rheometer Test
2.5.5. Capacity for Neutralizing HCl
3. Results and Discussion
3.1. Characterization of Metal Alkoxides
3.1.1. Fourier Transform Infrared Spectroscopy
3.1.2. Thermogravimetric Analysis
3.2. Thermal Stability Tests of DMAE-Zn on PVC
3.2.1. Conductivity Test
3.2.2. The Thermal Aging Test
3.2.3. UV-VIS Spectroscopy Test
3.2.4. Synergy between DMAE-Zn and CaSt2 or ZnSt2 on PVC Thermal Stability
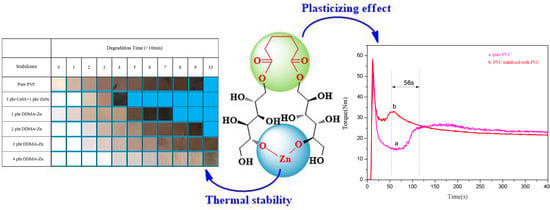
3.2.5. Torque Rheology Test of DMAE-Zn
3.3. The Thermal Stabilizing Mechanism of DMAE-Zn
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Avalos, A.S.; Hakkarainen, M.; Odelius, K. Superiorly plasticized PVC/PBSA blends through crotonic and acrylic acid functionalization of PVC. Polymers 2017, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Li, D.; Liu, H.; Zhang, J. Insights into the use of zinc–mannitol alkoxide as a novel thermal stabilizer for rigid poly(vinyl chloride). J. Appl. Polym. Sci. 2015, 132, 42038. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Wu, H.; Guo, S. Effect of pentaerythritol and organic tin with calcium/zinc stearates on the stabilization of poly(vinyl chloride). Polym. Degrad. Stab. 2006, 91, 2101–2109. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Wang, K.; Huang, J.; Li, K.; Nie, X.; Jiang, J. Epoxidized castor oil-based diglycidyl-phthalate plasticizer: Synthesis and thermal stabilizing effects on poly(vinyl chloride). J. Appl. Polym. Sci. 2018, 135, 47142. [Google Scholar] [CrossRef]
- Castañeda-Facio, A.; Benavides, R.; Martínez-Pardo, M.E. Thermal stability of PVC formulations gamma irradiated at different dose rates. Radiat. Phys. Chem. 2014, 97, 75–80. [Google Scholar] [CrossRef]
- Lu, W.; Yang, Q.; Yan, B.; Cheng, Y. Plasma-assisted synthesis of chlorinated polyvinyl chloride (CPVC) characterized by online UV–Vis analysis. Chem. Eng. 2012, 207–208, 923–930. [Google Scholar] [CrossRef]
- Meng, H.; Wang, S.; Chen, L.; Wu, Z.; Zhao, J. Investigation on Synergistic Effects and Char Morphology during Copyrolysis of Poly(vinyl chloride) Blended with Different Rank Coals from Northern China. Energy Fuels 2015, 29, 6645–6655. [Google Scholar]
- Xu, R.; Song, L.; Teng, Y.; Xia, J. Ferrous chloride-induced modification on thermal properties of polyvinyl chloride. Thermochim. Acta 2013, 565, 205–210. [Google Scholar] [CrossRef]
- Jeen, M.; Balanand, S.; Peer, M.; Ananthakumar, S. Design and fabrication of flexible polyvinyl chloride dielectric composite reinforced with ZnO microvaristors. J. Appl. Polym. Sci. 2017, 135, 46031. [Google Scholar]
- Li, M.; Zhang, J.; Xin, J.; Huang, K.; Li, S.; Wang, M.; Xia, J. Design of green zinc-based thermal stabilizers derived from tung oil fatty acid and study of thermal stabilization for PVC. J. Appl. Polym. Sci. 2016, 133, 44679. [Google Scholar] [CrossRef]
- Ye, F.; Ye, Q.F.; Zhan, H.H.; Ge, Y.Q.; Ma, X.T.; Xu, Y.Y.; Wang, X. Synthesis and Study of Zinc Orotate and Its Synergistic Effect with Commercial Stabilizers for Stabilizing Poly(Vinyl Chloride). Polymers 2019, 11, 194. [Google Scholar] [CrossRef]
- Reddeppa, N.; Ramamohan, K.; Ravi, M.; Guo, X. Effects of potassium iodide (KI) on crystallinity, thermal stability, and electrical properties of polymer blend electrolytes (PVC/PEO:KI). Solid State Ionics 2015, 278, 260–267. [Google Scholar]
- Ritu, S.; Deepak, P. Polyvinyl chloride degradation by Hybrid (Chemical and biological) Modification. Polym. Degrad. Stab. 2016, 123, 80–94. [Google Scholar]
- Emad, Y.; Jumat, S.; Nadia, S. New photostabilizers for PVC based on some diorganotin(IV) complexes. J. Saudi Chem. Soc. 2015, 19, 133–141. [Google Scholar]
- Fiaz, S.M.; Mark, C.; Amber, C.R.; Steven, R.S.; Jackson, S.; Esteban, U.; Rani, J.; Jeffrey, M.C.; Bharat, I.C.; Pamela, P.; et al. Enhanced thermal stabilization and reduced color formation of plasticized Poly(vinyl chloride) using zinc and calcium salts of 11-maleimideoundecanoic acid. Polym. Degrad. Stab. 2015, 111, 64–70. [Google Scholar]
- Wang, M.; Xia, J.; Jiang, J.; Li, S.; Huang, K.; Mao, W.; Li, M. A novel liquid Ca/Zn thermal stabilizer synthesized from tung-maleic anhydride and its effects on thermal stability and mechanical properties of PVC. Polym. Degrad. Stab. 2016, 133, 136–143. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Zhang, J.; Zhu, W.; Tian, T. Remarkably improved toughness and thermal stability of poly (vinyl chloride) (PVC)/poly (a-methylstyrene-acrylonitrile) (a-MSAN) blend with the assistance of two impact modifiers. Polym. Test. 2016, 51, 1–5. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Y.; Shentu, B.; Weng, Z. Preparation and characterization of zinc-mannitol complexes as PVC thermal stabilizers with high efficiency. Polym. Degrad. Stab. 2016, 133, 399–403. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, J.C.; Xia, J.L.; Li, S.H.; Li, M. Phosphate ester groups-containing ricinoleic acid-based Ca/Zn: Preparation and application as novel thermal stabilizer for PVC. J. Appl. Polym. Sci. 2018, 135, 45940. [Google Scholar] [CrossRef]
- Benavides, R.; Edge, M.; Allen, N.; Téllez, M. Stabilization of Poly(vinyl chloride) with Preheated Metal Stearates and Costabilizers. I. Use of a β-Diketone. J. Appl. Polym. Sci. 1998, 68, 1–10. [Google Scholar] [CrossRef]
- Johan, S.; Rik, L.; Daan, S.V.; Jacco, V.; John, W.G.; Leonardus, W.J. Long-term heat stabilisation by (natural) polyols in heavy metal- and zinc-free poly(vinyl chloride). Polym. Degrad. Stab. 2006, 91, 52–59. [Google Scholar]
- Daan, S.V.; Johan, S.; Guus, E.F.; Hans, C.V.; Jacco, V.; John, W.G.; Leonardus, W.J. The compatibility of (natural) polyols with heavy metal- and zinc-free poly(vinyl chloride): Their effect on rheology and implications for plate-out. Polym. Degrad. Stab. 2008, 93, 50–58. [Google Scholar]
- Xie, L.; Li, D.; Fu, M.; Zhang, J.; Zhang, L.; Zhang, Y.; Zhao, P. Study on Lanthanum-Pentaerythritol Alkoxide as a Thermal Stabilizer for Rigid Polyvinyl Chloride. J. Vinyl Addit. Technol. 2017, 23, 55–61. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Yu, X.; Zhang, Y.; Yu, Y.; Zhou, M.; Tang, S. Study on Pentaerythritol–Zinc as a Novel Thermal Stabilizer for Rigid Poly(Vinyl Chloride). J. Appl. Polym. Sci. 2012, 126, 569–574. [Google Scholar] [CrossRef]
- Li, D.; Xie, L.; Fu, M.; Zhang, J.; Indrawirawan, S.; Zhang, Y.; Tang, S. Synergistic effects of lanthanum-pentaerythritol alkoxide with zinc stearates and with β-diketone on the thermal stability of poly(vinyl chloride). Polym. Degrad. Stab. 2015, 114, 52–59. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Li, R.; Sun, H.; Zhang, Y.; Zhang, L.; Zhao, P. Synthesis of Pentaerythritol Stearate Ester-Based Zinc Alkoxide and Its Synergistic Effect with Calcium Stearate and Zinc Stearate on PVC Thermal Stability. J. Vinyl Addit. Technol. 2017, 24, 314–323. [Google Scholar] [CrossRef]
- Dong, T.; Li, D.; Li, Y.; Han, W.; Li, Z.; Xie, G.; Sunarsob, J.; Liu, S. Design and synthesis of polyol ester-based zinc metal alkoxides as a bi-functional thermal stabilizer for poly(vinyl chloride). Polym. Degrad. Stab. 2019, 159, 125–132. [Google Scholar] [CrossRef]
- Xie, L.; Li, D.; Zhang, J. The Effect of Pentaerythritol-Aluminum on the Thermal Stability of Rigid Poly(vinyl chloride). J. Appl. Polym. Sci. 2013, 130, 3704–3709. [Google Scholar] [CrossRef]
- Benaniba, M.T.; Massardier-Nageotte, V. Evaluation effects of biobased plasticizer on the thermal, mechanical, dynamical mechanical properties, and permanence of plasticized PVC. J. Appl. Polym. Sci. 2010, 118, 3499–3508. [Google Scholar] [CrossRef]
- Erdoğdu, C.A.; Atakul, S.; Balköse, D.; Ülkü, S. Development of Synergistic Heat Stabilizers for PVC from Zinc Borate-Zinc Phosphate. Chem. Eng. Commun. 2008, 196, 148–160. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.; Liu, H.; Ai, H.; Zhang, Y.; Zhang, L. Synergistic effects of zinc-mannitol alkoxide with calcium/zinc stearates and with β-diketone on thermal stability of rigid poly(vinyl chloride). J. Polym. Res. 2016, 23, 13–23. [Google Scholar] [CrossRef]
- Standard Practice for Fusion of Poly(vinyl chloride) (PVC) Compounds Using a Torque Rheometer; ASTM D2538-02(2010); ASTM International: West Conshohocken, PA, USA, 2010.
- Van Oosterhout, J.T.; Gilbert, M. Interactions between PVC and binary or ternary blends of plasticizers. Part I. PVC/plasticizer compatibility. Polymer 2003, 44, 8081–8094. [Google Scholar] [CrossRef]
- Li, D.; Zhou, M.; Xie, L.; Yu, X.; Yu, Y.; Ai, H.; Tang, S. Synergism of pentaerythritol-zinc with b-diketone and calcium stearate in poly(vinyl chloride) thermal stability. Polym. J. 2013, 45, 775–782. [Google Scholar] [CrossRef]












| Stabilizer | HCl Absorption Capacity, mg (HCl)/g (Stabilizer) |
|---|---|
| Lead salts | 280.1 |
| ZnSt2 | 98.2 |
| CaSt2 | 87.6 |
| DMAE-Zn | 131.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, D.; Han, W.; Zhang, M.; Ai, B.; Zhang, L.; Sun, H.; Cui, Z. Facile Synthesis of Di-Mannitol Adipate Ester-Based Zinc Metal Alkoxide as a Bi-Functional Additive for Poly(Vinyl Chloride). Polymers 2019, 11, 813. https://doi.org/10.3390/polym11050813
Li Y, Li D, Han W, Zhang M, Ai B, Zhang L, Sun H, Cui Z. Facile Synthesis of Di-Mannitol Adipate Ester-Based Zinc Metal Alkoxide as a Bi-Functional Additive for Poly(Vinyl Chloride). Polymers. 2019; 11(5):813. https://doi.org/10.3390/polym11050813
Chicago/Turabian StyleLi, Yuepeng, Degang Li, Wenyuan Han, Manqi Zhang, Bing Ai, Lipeng Zhang, Hongqi Sun, and Zhen Cui. 2019. "Facile Synthesis of Di-Mannitol Adipate Ester-Based Zinc Metal Alkoxide as a Bi-Functional Additive for Poly(Vinyl Chloride)" Polymers 11, no. 5: 813. https://doi.org/10.3390/polym11050813
APA StyleLi, Y., Li, D., Han, W., Zhang, M., Ai, B., Zhang, L., Sun, H., & Cui, Z. (2019). Facile Synthesis of Di-Mannitol Adipate Ester-Based Zinc Metal Alkoxide as a Bi-Functional Additive for Poly(Vinyl Chloride). Polymers, 11(5), 813. https://doi.org/10.3390/polym11050813