3.1. Analysis of the Products of Thermolysis Reaction
The successful detection of CIDNP requires high NMR sensitivity. In particular the concentration of polarized molecule [M*] should not be less than [Mmin]—the minimum concentration of diamagnetic molecules which can be detected using NMR [Mmin] ~ 10−4 M. The concentration of polarized molecule is determined by the rate of its formation described by ξkd[A] (with ξ the coefficients describing the efficiency of CIDNP formation of geminate radical pair formed from thermally excited singlet molecule [A], kd the decomposition rate constant, and [A] the alkoxyamine concentration) and the rate of polarization decay due to nuclear spin relaxation with the rate 1/T1 (T1 the nuclear relaxation time). At initial time of thermolysis ([A] = [A]0 ) we can roughly evaluate [M*] ~ kd[A]0T1ξ. Thus for CIDNP observation the rate of alkoxyamine decay should be higher than kd[A]0 > [Mmin]/T1ξ. Taking into account nuclear relaxation time of CH3 group protons of alkoxyamine as T1 ~ 1 s and typical CIDNP enhancement factor as 102–103, we can conclude that the rate of alkoxyamine decay should be higher than 10−6M·s−1, that is, kd > 5·10−5 s−1 for initial alkoxyamine concentration [A]0 = 20 mM (our typical experimental conditions). Taking into account that the typical life time of alkyl radicals during thermolysis (1/kd) is shorter than nuclear relaxation time of alkyl radical 10−4–10−5 s−1, relaxation processes could be a factor decreasing polarization of escape radicals.
The CIDNP pattern strongly depends on the time of observation, the temperature of thermolysis and thiophenol concentration. The CIDNP intensity was very weak during homolysis at temperatures below 70 °C when the experiment was performed in C
6D
6, but was nicely observed at higher temperatures during experiments in FSol (
Figure 1). Note that at temperatures above 70 °C, the amount of generated radicals was high, thus, the requirement for the minimum concentration of polarized molecules was fulfilled and CIDNP was detected. In the absence of scavenger, CIDNP was not observed at all (temperatures 110–70 °C) due to the reversibility of alkoxyamine decay (about 97% for
1a taking into account H-atom transfer) [
9,
24] leading to cancellation of in-cage and escape products. Since a reversible reaction has no net chemical change, no polarization would be expected to be observed since geminate recombination of the radical pair leads to one polarization and the escaping radicals carry the opposite polarization, canceling out the whole effect. Indeed, as one can see from
Scheme 2, CIDNP on alkoxyamine is formed via two ways—geminate recombination of the radical pair leads to positive polarization (Reaction 1) and the escaping radicals carry the negative polarization (Reaction 3), canceling out CIDNP effect. Side reaction or nuclear relaxation time in the intermediate radicals in the absence of scavenger could play the same role and make CIDNP visible. Evaluation shows that the lifetime of alkyl radical is much less than nuclear spin relaxation time and could not explain CIDNP formation. Due to persistent radical effect [
5] in the absence of scavenger the concentration of nitroxide is more than several orders of magnitude higher than the same of free radicals. The rate of Reactions 6 and 7 is much smaller in comparison with Reaction 3 and CIDNP formation due to these reactions is negligible.
Scheme 2.
The kinetic scheme of decomposition of alkoxyamine in the presence of thiophenol. Letters in brackets indicate the sign of CIDNP: A—absorption, E—emission.
Scheme 2.
The kinetic scheme of decomposition of alkoxyamine in the presence of thiophenol. Letters in brackets indicate the sign of CIDNP: A—absorption, E—emission.
Figure 1.
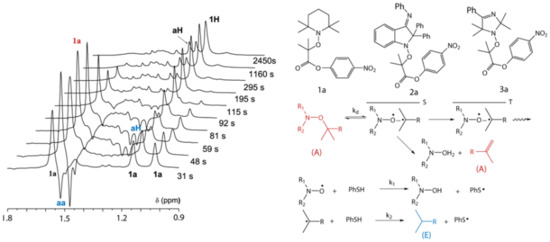
1H NMR spectra recorded at room temperature before (1) and after (3) thermolysis of 1a (a), 2a (c) in FSol and 3a (e) in m-C6D4Cl2 and spectra with polarized signals obtained during the thermolysis (2). 1H NMR spectra recorded at different time intervals from the beginning of the reaction during thermolysis of 1a (b) at 398 K, 0.02 M solution of alkoxyamine in FSol, 5.5 eq of thiophenol, 2a (d) at 386 K, 0.02 M solution of alkoxyamine in FSol, 3 eq of thiophenol, 3a (f) at 386 K, 0.02 M solution of alkoxyamine in m-C6D4Cl2, 6 eq of thiophenol. Signs of polarization: E—aa, a(-H), aH; A—1a–3a. Inset: enlargement of the chemical shift zone for a(-H) of 3a.
Figure 1.
1H NMR spectra recorded at room temperature before (1) and after (3) thermolysis of 1a (a), 2a (c) in FSol and 3a (e) in m-C6D4Cl2 and spectra with polarized signals obtained during the thermolysis (2). 1H NMR spectra recorded at different time intervals from the beginning of the reaction during thermolysis of 1a (b) at 398 K, 0.02 M solution of alkoxyamine in FSol, 5.5 eq of thiophenol, 2a (d) at 386 K, 0.02 M solution of alkoxyamine in FSol, 3 eq of thiophenol, 3a (f) at 386 K, 0.02 M solution of alkoxyamine in m-C6D4Cl2, 6 eq of thiophenol. Signs of polarization: E—aa, a(-H), aH; A—1a–3a. Inset: enlargement of the chemical shift zone for a(-H) of 3a.
In the presence of radical scavenger, alkoxyamine is formed only as in-cage product of geminate radical pair RP, whereas escape radicals react with PhSH with the formation of alkane aH, hydroxylamine and other compounds. Thus, in this case CIDNP intensity of alkoxyamine is equal to the overall intensity of CIDNP of escape products, but with the opposite sign.
According to Kaptein's rules [
25], the sign of CIDNP net effect (
) is determined by the initial spin multiplicity of intermediate RP (
µ), the sign of HFI constants (
AHFI), the sign of the difference in
g-factors of radical partners, and the way of product formation (ε) (in-cage or out-cage).
These parameters for the radical pair under investigation are the following:
a(CH
3)>0,
g-factor of intermediate radicals are
g = 2.0027 for tertiary alkyl radical [
26] and
g = 2.0059 for nitroxyl radical [
27,
28], thus Δ
g < 0, and ε = 1 for alkoxyamine which is in-cage product, or ε = −1 for other escape products (
aa,
aH,
etc.). Thus, as it follows from Equation (1), CIDNP is formed in the geminate
singlet RP (
µ = −1) but not in diffusion RP (
µ = 1). The kinetic scheme of decomposition of alkoxyamine in the presence of thiophenol is shown in
Scheme 2. The multiplicity of radical pairs in
Scheme 2 is denoted as S- for singlet RPs, T- for triplet RPs and F- for diffusion RPs. The expected signs of CIDNP of reaction products emission (E) or absorption (A) are indicated in
Scheme 2. It should be noted that CIDNP effect on the protons of
aa is originated from geminate RP only but not from diffusion RPs, because in high magnetic fields CIDNP in radical pairs of two similar radicals is equal to zero [
11].
NMR spectra before, during and after thermolysis of alkoxyamine
1a–
3a in the presence of PhSH are shown in
Figure 1. CIDNP is observed on NMR lines of alkoxyamines (
1a,
2a,
3a) and several reaction products of alkoxyamine decomposition in the presence of thiophenol,
i.e. dimer of two alkyl radicals (
aa), product of reaction of alkyl radicals with PhSH (
aH), and alkene product
a(-H).
The compound
a(-H) can be formed in three different reactions (
i) as a disproportionation product of two alkyl radicals (emissive CIDNP signal, Equation 7,
Scheme 2), (
ii) as a product of hydrogen transfer from alkyl to nitroxide radical in the geminate RP (absorption CIDNP signal, Equation 2,
Scheme 2) and in the diffusion RP (at the very early stage without CIDNP signal, Equation 9,
Scheme 2), and (
iii) as a product of intramolecular proton transfer (no CIDNP signal,
kdD in
Scheme 1). In the case of
1a thermolysis the signal of
a(-H) overlaps with much higher intensity signal of alkoxyamine
1a thus no conclusion about its CIDNP sign can be made. The observed CIDNP intensity of
a(-H) protons was very weak and was negative in the case of
2a thermolysis, and positive in the case of
3a thermolysis. CIDNP on the protons of
a(-H) can be formed in two ways: negative polarization is formed after recombination of two escape alkyl radicals (Equation 7,
Scheme 2), or positive polarization is formed in the case of hydrogen atom transfer in geminate RP. The negative CIDNP signal for
a(-H) during decomposition of
2a in the presence of scavenger was detected pointing at the reaction of two alkyl radicals and confirming the non-occurrence of intermolecular H-transfer for
2a. For
3a the intermolecular H-transfer reaction has a large contribution to the decomposition kinetics, so that the sign of polarization on
a(-H) is positive. Note that CIDNP intensity of NMR line of CH
3 group of
a(-H) is substantially lower than the same for
aa. Fischer and co-authors [
29] have studied coupling (Reaction 6,
Scheme 2) and disproportionnation (Reaction 7,
Scheme 2) and showed that the ratio between recombination and disproportionation products yields depends on temperature. For
T = 100 °C this ratio is ~1/5. In addition, the number of protons contributing to NMR lines of CH
3 groups of
aa is equal to 12 and while that for
a(-H) it is equal to 3. Thus, the expected difference in CIDNP intensity of CH
3 groups of
aa and
a(-H) is about 20-times and is in a good agreement with the experimental ratio of 23.
Consequently, the sign of polarization on
a(-H) provides valuable information on the main process that contributes to its formation: for experiment with
2a alkene
a(-H) is issued from Reaction 7 (
Scheme 2), whereas for
3a decomposition alkene is the product of H-atom transfer reaction (Equations 2 and 9 at the very early stages,
Scheme 2).
3.2. Kinetics of CIDNP
Figure 2 shows CIDNP kinetics obtained using NMR lines of different products during thermolysis of
2a. As expected, CIDNP intensity of
2a slightly exceeds the CIDNP intensity of escape products. Typical CIDNP kinetics consists of two parts (
Figure 1 and
Figure 2), rise and decay, and can be described by two exponent functions.
Figure 2.
Kinetics of polarized signals (normalized) during thermolysis of 0.02 M solution of alkoxyamines in FSol 2a (a) at 386 K in the presence of 3 eq. of thiophenol and 3a (a) at 386 K in m-C6D4Cl2 in the presence of 6 eq of thiophenol: (■)—alkoxyamine 2a, (●)—alkane aH, (∆)—dimer aa, (▽)—alkene a(-H). (b): (■)—alkoxyamine 3a, (●)—alkane aH, (∆)—dimer aa. Inset—the kinetics of CIDNP on a(-H) for 2a. Inset: enlargement of evolution of CIDNP signal for a(-H)—(▽) of 2a. Solid line—calculated dependence of CIDNP vs. time.
Figure 2.
Kinetics of polarized signals (normalized) during thermolysis of 0.02 M solution of alkoxyamines in FSol 2a (a) at 386 K in the presence of 3 eq. of thiophenol and 3a (a) at 386 K in m-C6D4Cl2 in the presence of 6 eq of thiophenol: (■)—alkoxyamine 2a, (●)—alkane aH, (∆)—dimer aa, (▽)—alkene a(-H). (b): (■)—alkoxyamine 3a, (●)—alkane aH, (∆)—dimer aa. Inset—the kinetics of CIDNP on a(-H) for 2a. Inset: enlargement of evolution of CIDNP signal for a(-H)—(▽) of 2a. Solid line—calculated dependence of CIDNP vs. time.
An increase of CIDNP intensity on alkoxyamine is observed at time intervals of less than 200 seconds. The emission signal on
aa is observed at even shorter time intervals (typically 100 s) in
Figure 1b and d. During this time the concentration of alkyl radicals is high enough to favor the reactions 6 and 7 (
Scheme 2) and recombination of alkyl radical with nitroxide,
i.e.Time evolution of CIDNP on the protons of
aa is much faster than the same for alkoxyamine and alkane
aH. The kinetic profile of CIDNP on
aH is the same as for alkoxyamines
1a, and
2a which highlights that alkane
aH is mainly issued from the reaction of alkyl radicals with thiophenol. CIDNP kinetics of
a(-H) and
aa (
Figure 2) during the decomposition of
2a exhibits the same time profile confirming that these two species are formed in the same reaction of alkyl radicals which is important at the earliest stage of thermolysis. The emissive polarization on
a(-H) (
Figure 2a inset) indicates that alkene is mainly formed in alkyl-alkyl radicals reaction confirming the very low level of H-transfer reaction in nitroxyl-alkyl geminate RP of
2a as concentrations as low as 10
−6 M of species can be detected. Meaning that fraction of ca. 0.01% of intermolecular H-transfer in alkoxyamine might be detected in our experimental conditions. Consequently, as H-transfer reactions exhibit detrimental effect for fraction larger than
ca. 0.5%, sensitivity of CIDNP is a valuable tool for screening the occurrence of H-transfer reaction in alkoxyamine.
Figure 3.
Kinetics of polarized NMR line of alkene a(-H) during thermolysis of 0.02 M solution of 3a (b) in m-C6D4Cl2 at 386 K in the presence of 6 eq of thiophenol: symbols—experimental data points, solid lines—calculated dependence vs. time.
Figure 3.
Kinetics of polarized NMR line of alkene a(-H) during thermolysis of 0.02 M solution of 3a (b) in m-C6D4Cl2 at 386 K in the presence of 6 eq of thiophenol: symbols—experimental data points, solid lines—calculated dependence vs. time.
Contrary to
2a, alkoxyamine
3a exhibits high fraction of H-atom transfer (at 95 °C, 30% and 15% for intramolecular proton transfer and intermolecular H-transfer reactions, respectively) [
10]. Thus, as mentioned above, one would expect positive polarization on
a(-H). Unfortunately there is no unpolarized line for
a(-H) product, so that the CIDNP kinetics cannot be extracted and, thus, the evolution of NMR signal of polarized CH
3 protons of alkene which is the sum of CIDNP and steady state NMR signal of alkene is displayed in
Figure 3. However, the growth of
a(-H) is well described by the proposed model (
vide infra) as well as the break in the growth of signal which is due to the overlapping between the CIDNP signal and the steady state NMR signal of alkene. This indicates that the main pathway of alkene formation is H-atom transfer reaction in geminate RPs.
The kinetics of the concentration and CIDNP of alkoxyamine are described by the following equations:
where [
A], [
N], [
R] are the concentrations of alkoxyamine, nitroxide and alkyl radical, respectively, and
PA and
PR are the nuclear polarization of alkoxyamine and alkyl radical; ξ and χ are the coefficients describing the efficiency of CIDNP formation of geminate radical pair formed from singlet precursor and diffusion radical pair of nitroxide and alkyl radical and
1/T1 is nuclear relaxation time. ξ and χ depend on hyperfine constants of radical partners, difference in g-factors, and radical pair lifetime, which is determined by mutual diffusion coefficient, reaction radius and recombination rate constant [
11].
Let us consider the case of high PhSH concentration. In this case the recombination rate of radicals in the bulk (Reactions (3), (6), (7) and (9),
Scheme 2) is negligible in comparison with the reaction rate of radicals with PhSH (Reaction (5),
Scheme 2). When condition
is valid, alkoxyamine is formed predominately as in-cage product of geminate radical pair while alkyl radical escaping from geminate radical pair into the bulk are scavenged by PhSH giving alkane RH. The concentration of alkoxyamine under this condition is described by monoexponential decay with the rate constant equal to
kd (Equation 14). The formation of alkoxyamine polarization in diffusion radical pairs of alkyl and nitroxyl radicals (Reaction 3,
Scheme 2) in the bulk (second term in Equation 12) can be neglected and the polarization of alkoxyamine
PA is described by Equation 13. With taking into account initial condition (polarization at
t = 0 is equal to zero), it is easy to obtain the analytical expression for the polarization of alkoxyamine
PA Equation (15) combining the Equations (13) and (14):
Thus, the concentration of polarized molecules is determined by two exponents with parameters
kd,
kdD and
1/T1. At short times
t << 1/
kd it grows with typical rise time
T1 and at longer times
t > 1/
T1 it decays with parameter
kd +
kdD. The intensity of polarization is determined by CIDNP enhancement factor, nuclear relaxation time and decay rate constant. The expected time of CIDNP maximum obtained from expressions (13–15) is equal to:
Substitution of
kd and
T1 gives much shorter time (several seconds) than experimentally observed. Although the NMR tube was placed into preheated NMR probe, the heating of the sample to the aimed temperature took 1–2 minutes and was different for different temperatures [
30].
In order to confirm the mechanism of formation of polarization the kinetic scheme of alkoxyamine thermolysis in the presence of scavenger was solved numerically (for the reactions see
Scheme 2, the equations are presented as SI). The resulting polarization dependences are presented in
Figure 2a and b (solid lines). The values of rate constants are the following
: kd = 0.003 s
−1 for
2a and
kd = 0.007 s
−1 for
3a, [
9]
kc = 10
7 M
−1 s
−1 for
2a, [
31]
kc = 6.3 × 10
8 M
−1 s
−1 for
3a, [
19,
32]
kt = 10
9 M
−1 s
−1,
k1 = 100 M
−1 s
−1, [
23]
k2 = 2.5 × 10
8 M
−1 s
−1, [
22], [
A]
0 = 0.02 M, [PhSH]
0 = 0.06 M,
T1 = 1 s,
P = 100. The values of nuclear spin relaxation time of products were measured for every NMR line in the spectrum. The numerical decay of CIDNP polarization agrees with experimental data (
Figure 2). As follows from the numerical calculations, the second part of the CIDNP kinetics of alkane
aH is fully governed by the decomposition of alkoxyamine. The kinetics of
aa formation are much faster as two processes contribute to the formation of polarization on
aa: (
i) polarization formed in bulk singlet radical pair which is emissive and (
ii) polarization in the triplet radical pair which gives absorption. The resulting evolution of CIDNP on
aa exhibits very sharp decay and growth. In the case of
2a the alkene
a(–H) is formed in the same reactions as dimer
aa the kinetics of CIDNP is due to similar processes, although due to low signal intensity not all peculiarities can be seen on it (
Figure 2a inset). The main contribution to the polarization on alkene during decomposition of
3a is H-atom transfer reaction in geminate RP. Thus the calculation confirmed that positive polarization should be observed in this case. As mentioned above there is no non-polarized lines for alkene in the spectra. The non-polarized signals of the protons of the ring of
p-Ph-NO
2 group overlap with the signals of PhSH. Thus to achieve agreement, the experimental data were simulated with the sum of concentration and polarization functions. The resulting calculated curve is in a good agreement with the experimental dependence of polarization on alkene.
The increase of polarization is fully governed by the value of nuclear spin relaxation time. For the values measured for alkoxyamine 1a and alkane aH (T1 = 1 s) the maximum of the polarization kinetics on alkoxyamine should be observed at t < 10 s. When the calculated function of alkoxyamine CIDNP kinetics is multiplied by the experimental curve of sample heating up in the probe head of NMR spectrometer, good enough agreement with the experimental data is observed. Thus, in our case the growth of the alkoxyamine CIDNP kinetics is governed by the heating process assuming the heating-up function is exponential. The parameter of exponent was determined experimentally in each case.
Figure 4.
Kinetics of CIDNP on alkoxyamine 1a during thermolysis of 0.02 M solution of alkoxyamine in FSol in the presence of thiophenol and its fit (line) with monoexponent in semi-logarithmic scale: 361 K, [PhSH] = 0.1 M (■), 373 K, [PhSH] = 0.136 M (●), 398 K, [PhSH] = 0.13 M (▼). The values of kobs obtained from the fit: 361 K—kobs = 2.0 × 10−3 s−1, 373 K—kobs = 5.0 × 10−3 s−1, 398 K—kobs = 1.5 × 10−2 s−1.
Figure 4.
Kinetics of CIDNP on alkoxyamine 1a during thermolysis of 0.02 M solution of alkoxyamine in FSol in the presence of thiophenol and its fit (line) with monoexponent in semi-logarithmic scale: 361 K, [PhSH] = 0.1 M (■), 373 K, [PhSH] = 0.136 M (●), 398 K, [PhSH] = 0.13 M (▼). The values of kobs obtained from the fit: 361 K—kobs = 2.0 × 10−3 s−1, 373 K—kobs = 5.0 × 10−3 s−1, 398 K—kobs = 1.5 × 10−2 s−1.
At time longer than 200 seconds, CIDNP kinetics are detected only on the protons of alkoxyamines and
aH. The CIDNP kinetics decays of
1a–
3a at
t > 200 s were described by monoexponential function with the decay parameter equal to
kobs(T). As an example
Figure 4 shows CIDNP kinetics in logarithmic scale for
1a providing
kobs of 2.0 × 10
−3 s
−1, 5.0 × 10
−3 s
−1, and 1.5 × 10
−2 s
−1 at 361 K, 373 K, and 398 K, respectively. Within experimental error
kobs coincide with
kd reported using unpolarized NMR signals of alkoxyamines or EPR [
9,
10]. Consequently, the presence of CIDNP effect does not impede the determination of
kd.