Calcite Biohybrids as Microenvironment for Stem Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Experiment Design
2.2. Scaffold Preparation
2.3. Cell Culturing
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. Cell Proliferation Assay
2.6. GAG Quantification
2.7. In Vitro Chondrogenic Differentiation Assay
2.8. Immunofluorescence Analysis
2.9. Gene Expression
| Gene | Sequence | Reference Sequence | |
|---|---|---|---|
| GAPDH | F | 5'-CCTGGAGAAACCTGCCAAGTAT-3' | NM_008084 |
| R | 5'-GATGCCTGCTTCACCACCTT-3' | ||
| Col1a2 | F | 5'-CCAAGAAGACATCCCTGAAGTCA-3' | NM_000089 |
| R | 5'-TGCACGTCATCGCACACA-3' | ||
| Col2a1 | F | 5'-CGAGATCCCCTTCGGAGAGT-3' | NM_001844 |
| R | 5'-CTGCCCCTTTGGCCCTAAT-3' | ||
| Sox9 | F | 5'-ACCAGTACCCGCATCTGCAC-3' | NM_011448 |
| R | 5'-CTCGTTCAGCAGCCTCCAG-3' | ||
| Runx2 | F | 5'-GAGTCATTTAAGGCTGCAAGCA-3' | NM_001145920 |
| R | 5'-CGGTGTCACTGCGCTGAA-3' | ||
| CD44 | F | 5'-CCTCAGCCCCTCCTGAAGA-3' | NM_000610 |
| R | 5'-CGAGTACCATCACGGTTGACA-3' |
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of HA Concentration on MSC Proliferation

3.2. Cell Proliferation Kinetics in Calcite, Calcite-HA and Gold Calcite Groups
3.3. SEM Analysis

3.4. Sulfated GAGs Quantification

3.5. In Vitro Chondrogenic Differentiation Assay

3.6. Gene Expression

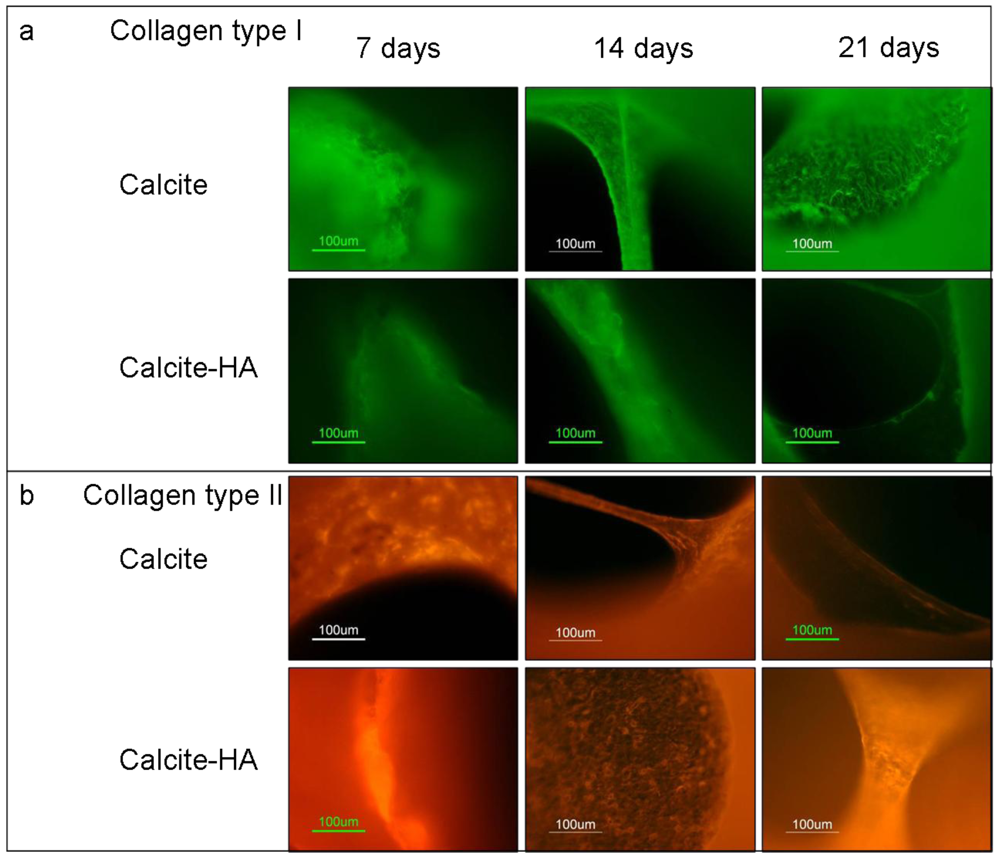
3.7. Immunohistochemical Staining

4. Conclusions
References
- Woodfield, T.B.; van Blitterswijk, C.A.; De Wijn, J.; Sims, T.J.; Hollander, A.P.; Riesle, J. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng. 2005, 11, 1297–1311. [Google Scholar]
- Raghunath, J.; Rollo, J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. Biomaterials and scaffold design: key to tissue-engineering cartilage. Appl. Biochem. Biotechnol. 2007, 46, 73–84. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar]
- Lange, C.; Schroeder, J.; Stute, N.; Lioznov, M.V.; Zander, A.R. High-potential human mesenchymal stem cells. Stem Cells Dev. 2005, 1, 70–80. [Google Scholar]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar]
- Butler, D.L.; Goldstein, S.A.; Guilak, F. Functional tissue engineering: The role of biomechanics. J. Biomech. Eng. 2000, 122, 570–575. [Google Scholar]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mat. Sci. Mat. Med. 2003, 14, 195–200. [Google Scholar]
- Lakes, R. Composite biomaterials. In The Biomedical Engineering Handbook, 2nd; Bronzino, J.D., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 208–232. [Google Scholar]
- Demers, C.; Hamdy, C.R.; Corsi, K.; Chellat, F.; Tabrizian, M.; Yahia, L. Natural coral exoskeleton as a bone graft substitute: a review. Bio-Med. Mat. Eng. 2002, 12, 15–35. [Google Scholar]
- Vago, R. Cnidarians biomineral in tissue engineering: a review. Marine Biotechnol. 2008, 10, 343–349. [Google Scholar]
- Chen, F.; Chen, S.; Tao, K.; Feng, X.; Liu, Y.; Lei, D.; Mao, T. Marrow-derived osteoblasts seeded into porous natural coral to prefabricate a vascularised bone graft in the shape of a human mandibular ramus: Experimental study in rabbits. Br. J. Oral Maxillofac. Surg. 2004, 42, 532–537. [Google Scholar]
- Vago, R. Beyond the skeleton: Cnidarian biomaterials as bioactive extracellular microenvironments for tissue engineering. Organogenesis 2008, 4, 18–22. [Google Scholar]
- Abramovitch-Gottlib, L.; Geresh, S.; Vago, R. Biofabricated marine hydrozoan: A bioactive crystalline material promoting ossification of mesenchymal stem cells. Tissue Eng. 2006, 12, 729–739. [Google Scholar]
- Fujita, Y.; Yamamuro, T.; Nakamura, T.; Kotani, S.; Ohtsuki, C.; Kokubo, T. The bonding behavior of calcite to bone. J. Biomed. Mat. Res. 1991, 25, 991–1003. [Google Scholar]
- Fricain, J.C.; Bareille, R.; Ulysse, F.; Dupuy, B.; Amedee, J. Evaluation of proliferation and protein expression of human bone marrow cells cultured on coral crystallized in the aragonite of calcite form. J. Biomed. Mat. Res. 1998, 42, 96–102. [Google Scholar]
- Gross-Aviv, T.; Astachov, L.; Kantaroving, K.; Bar, I.; Vago, R. Skeleton of Tetraclita rufotincta: a novel biomaterial for tissue engineering applications. In Biomimetic and Supramolecular Systems Research, 1st; Lima, H.A., Ed.; Nova Science: Hauppauge, NY, USA, 2009; pp. 207–213. [Google Scholar]
- Astachov, L.; Nevo, Z.; Aviv, M.; Vago, R. Crystalline calcium carbonate and hydrogels as microenvironment for stem cells. Front. Biosci. 2011, 16, 458–471. [Google Scholar]
- Toole, B.P.; Okayama, M.; Orkin, R.W.; Yoshimura, M.; Muto, M.; Kaji, A. Developmental roles of hyaluronate and chondroitin sulfate proteoglycans. Soc. Gen. Physiol. Ser. 1977, 32, 139–154. [Google Scholar]
- Camenisch, T.D.; McDonald, J.A. Hyaluronan: Is bigger better? Am. J. Respir. Cell Mol. Biol. 2000, 23, 431–433. [Google Scholar]
- Knudson, C.B.; Toole, B.P. Changes in the pericellular matrix during differentiation of limb bud mesoderm. Dev. Biol. 1985, 112, 308–318. [Google Scholar]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nature Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar]
- Toole, B.P. Hyaluronan: from extracellular glue to pericellular cue. Nature Rev. Cancer 2004, 4, 528–539. [Google Scholar]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar]
- Astachov, L.; Vago, R.; Aviv, M.; Nevo, Z. Hyaluronan and mesenchymal stem cells: From germ layer to cartilage and bone. Front. Biosci. 2011, 16, 261–276. [Google Scholar]
- Horn, E.M.; Beaumont, M.; Shu, X.Z.; Harvey, A.; Prestwich, G.D.; Horn, K.M.; Gibson, A.R.; Preul, M.C.; Panitch, A. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J. Neurosurg. Spine 2007, 6, 133–140. [Google Scholar]
- Meszar, Z.; Felszeghy, S.; Veress, G.; Matesz, K.; Szekely, G.; Modis, L. Hyaluronan accumulates around differentiating neurons in spinal cord of chicken embryos. Brain Res. Bull. 2000, 75, 414–418. [Google Scholar]
- Chan, C.K.; Wang, J.; Lin, L.; Hao, Y.; Chan, S.O. Enzymatic removal of hyaluronan affects routing of axons in the mouse optic chiasm. NeuroReport 2007, 18, 1533–1538. [Google Scholar]
- Zavan, B.; Abatangelo, G.; Mazzoleni, F.; Bassetto, F.; Cortivo, R.; Vindigni, V. New 3D hyaluronan-based scaffold for in vitro reconstruction of the rat sciatic nerve. Neurol. Res. 2008, 30, 190–196. [Google Scholar]
- Sakai, Y.; Matsuyama, Y.; Takahashi, K.; Sato, T.; Hattori, T.; Nakashima, S.; Ishiguro, N. New artificial nerve conduits made with photocrosslinked hyaluronic acid for peripheral nerve regeneration. Bio-Med. Mat. Eng. 2007, 17, 191–197. [Google Scholar]
- Angele, P.; Muller, R.; Schumann, D.; Englert, C.; Zellner, J.; Johnstone, B.; Yoo, J.; Hammer, J.; Fierlbeck, J.; Angele, M.K.; Nerlich, M.; Kujat, R. Characterization of esterified hyaluronan-gelatin polymer composites suitable for chondrogenic differentiation of mesenchymal stem cells. J. Biomed. Mat. Res. A 2009, 91, 416–427. [Google Scholar]
- Cristino, S.; Grassi, F.; Toneguzzi, S.; Piacentini, A.; Grigolo, B.; Santi, S.; Riccio, M.; Tognana, E.; Facchini, A.; Lisignoli, G. Analysis of mesenchymal stem cells grown on a three-dimensional HYAFF 11-based prototype ligament scaffold. J. Biomed. Mat. Res. A 2005, 73, 275–283. [Google Scholar]
- Lisignoli, G.; Cristino, S.; Piacentini, A.; Toneguzzi, S.; Grassi, F.; Cavallo, C.; Zini, N.; Solimando, L.; Maraldi, N.M.; Facchini, A. Cellular and molecular events during chondrogenesis of human mesenchymal stromal cells grown in a three-dimensional hyaluronan based scaffold. Biomaterials 2005, 26, 5677–5686. [Google Scholar]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar]
- Milella, E.; Brescia, E.; Massaro, C.; Ramires, P.A.; Miglietta, M.R.; Fiori, V.; Aversa, P. Physico-chemical properties and degradability of non-woven hyaluronan benzylic esters as tissue engineering scaffolds. Biomaterials 2002, 23, 1053–1063. [Google Scholar]
- Kito, H.; Matsuda, T. Biocompatible coatings for luminal and outer surfaces of small-caliber artificial grafts. J. Biomed. Mat. Res. 1996, 30, 321–330. [Google Scholar]
- Aigner, J.; Tegeler, J.; Hutzler, P.; Campoccia, D.; Pavesio, A.; Hammer, C.; Kastenbauer, E.; Naumann, A. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J. Biomed. Mat. Res. 1998, 42, 172–181. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nature Protoc. 2008, 3, 1101–1108. [Google Scholar]
- Stockwell, R. Biology of Cartilage Cells, 1st ed; Cambridge University Press: Cambridge, UK, 1979. [Google Scholar]
- Pilloni, A.; Bernard, G.W. The effect of hyaluronan on mouse intramembranous osteogenesis in vitro. Cell Tissue Res. 1998, 294, 323–333. [Google Scholar]
- Zou, X.; Li, H.; Chen, L.; Baatrup, A.; Bunger, C.; Lind, M. Stimulation of porcine bone marrow stromal cells by hyaluronan, dexamethasone and rhBMP-2. Biomaterials 2004, 25, 5375–5385. [Google Scholar]
- Huang, L.; Cheng, Y.Y.; Koo, P.L.; Lee, K.M.; Qin, L.; Cheng, J.C.; Kumta, S.M. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J. Biomed. Mat. Res. A 2003, 66, 880–884. [Google Scholar]
- Liu, C.M.; Yu, C.H.; Chang, C.H.; Hsu, C.C.; Huang, L.L. Hyaluronan substratum holds mesenchymal stem cells in slow-cycling mode by prolonging G1 phase. Cell Tissue Res. 2008, 334, 435–443. [Google Scholar]
- Pure, E.; Assoian, R.K. Rheostatic signaling by CD44 and hyaluronan. Cell. Signal. 2009, 21, 651–655. [Google Scholar]
- Lesley, J.; English, N.; Charles, C.; Hyman, R. The role of the CD44 cytoplasmic and transmembrane domains in constitutive and inducible hyaluronan binding. Eur. J. Immunol. 2000, 30, 245–253. [Google Scholar]
- Takeda, M.; Ogino, S.; Umemoto, R.; Sakakura, M.; Kajiwara, M.; Sugahara, K.N.; Hayasaka, H.; Miyasaka, M.; Terasawa, H.; Shimada, I. Ligand-induced structural changes of the CD44 hyaluronan-binding domain revealed by NMR. J. Biol. Chem. 2006, 281, 40089–40095. [Google Scholar]
- Lefebvre, V.; Behringer, R.R.; de Crombrugghe, B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthr. Cartil. 2001, 9, S69–S75. [Google Scholar]
- Ng, L.J.; Wheatley, S.; Muscat, G.E.; Conway-Campbell, J.; Bowles, J.; Wright, E.; Bell, D.M.; Tam, P.P.; Cheah, K.S.; Koopman, P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997, 183, 108–121. [Google Scholar]
- Wright, E.; Hargrave, M.R.; Christiansen, J.; Cooper, L.; Kun, J.; Evans, T.; Gangadharan, U.; Greenfield, A.; Koopman, P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat. Genet. 1995, 9, 15–20. [Google Scholar]
- Murakami, S.; Kan, M.; McKeehan, W.L.; de Crombrugghe, B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 1113–1118. [Google Scholar]
- Javed, A.; Afzal, F.; Bae, J.S.; Gutierrez, S.; Zaidi, K.; Pratap, J.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Specific residues of RUNX2 are obligatory for formation of BMP2-induced RUNX2-SMAD complex to promote osteoblast differentiation. Cell Tissue Organs 2009, 189, 133–137. [Google Scholar]
- Galle, J.; Bader, A.; Hepp, P.; Grill, W.; Fuchs, B.; Kas, J.A.; Krinner, A.; Marquass, B.; Müller, K.; Schiller, J.; et al. Mesenchymal stem cells in cartilage repair: state of the art and methods to monitor cell growth, differentiation and cartilage regeneration. Curr. Med. Chem. 2010, 17, 2274–2291. [Google Scholar]
- Palumbo, F.S.; Pitarresi, G.; Mandracchia, D.; Tripodo, G.; Giammona, G. New graft copolymers of hyaluronic acid and polylactic acid: synthesis and characterization. Carbohydr. Polym. 2006, 66, 379–385. [Google Scholar]
- Baumann, M.D.; Kang, C.E.; Tator, C.H.; Shoichet, M.S. Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury. Biomaterials 2010, 31, 7631–7639. [Google Scholar]
- Bakos, D.; Soldan, M.; Hernandez-Fuentes, I. Hydroxyapatite-collagen-hyaluronic acid composite. Biomaterials 1999, 20, 191–195. [Google Scholar]
- Gao, J.; Dennis, J.E.; Solchaga, L.A.; Awadallah, A.S.; Goldberg, V.M.; Caplan, A.I. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001, 7, 363–371. [Google Scholar]
- Gao, J.; Dennis, J.E.; Solchaga, L.A.; Goldberg, V.M.; Caplan, A.I. Repair of osteochondral defect with tissue-engineered two-phase composite material of injectable calcium phosphate and hyaluronan sponge. Tissue Eng. 2002, 8, 827–837. [Google Scholar]
- Radice, M.; Brun, P.; Cortivo, R.; Scapinelli, R.; Battaliard, C.; Abatangelo, G. Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J. Biomed. Mat. Res. 2000, 50, 101–109. [Google Scholar]
- Solchaga, L.A.; Yoo, J.U.; Lundberg, M.; Dennis, J.E.; Huibregtse, B.A.; Goldberg, V.M.; Caplan, A.I. Hyaluronan-based polymers in the treatment of osteochondral defects. J. Orthop. Res. 2000, 18, 773–780. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Astachov, L.; Nevo, Z.; Vago, R. Calcite Biohybrids as Microenvironment for Stem Cells. Polymers 2012, 4, 1065-1083. https://doi.org/10.3390/polym4021065
Astachov L, Nevo Z, Vago R. Calcite Biohybrids as Microenvironment for Stem Cells. Polymers. 2012; 4(2):1065-1083. https://doi.org/10.3390/polym4021065
Chicago/Turabian StyleAstachov, Liliana, Zvi Nevo, and Razi Vago. 2012. "Calcite Biohybrids as Microenvironment for Stem Cells" Polymers 4, no. 2: 1065-1083. https://doi.org/10.3390/polym4021065




