Electrospinning Pullulan Fibers from Salt Solutions †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Electrospinning
2.2.2. Solution Analysis
2.2.3. Fiber Characterization
2.2.4. Statistical Analysis
3. Results and Discussion
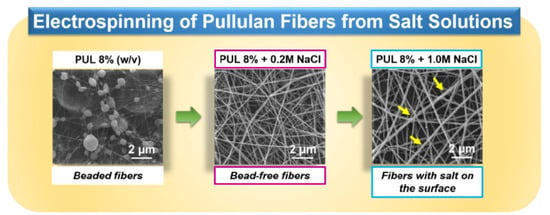
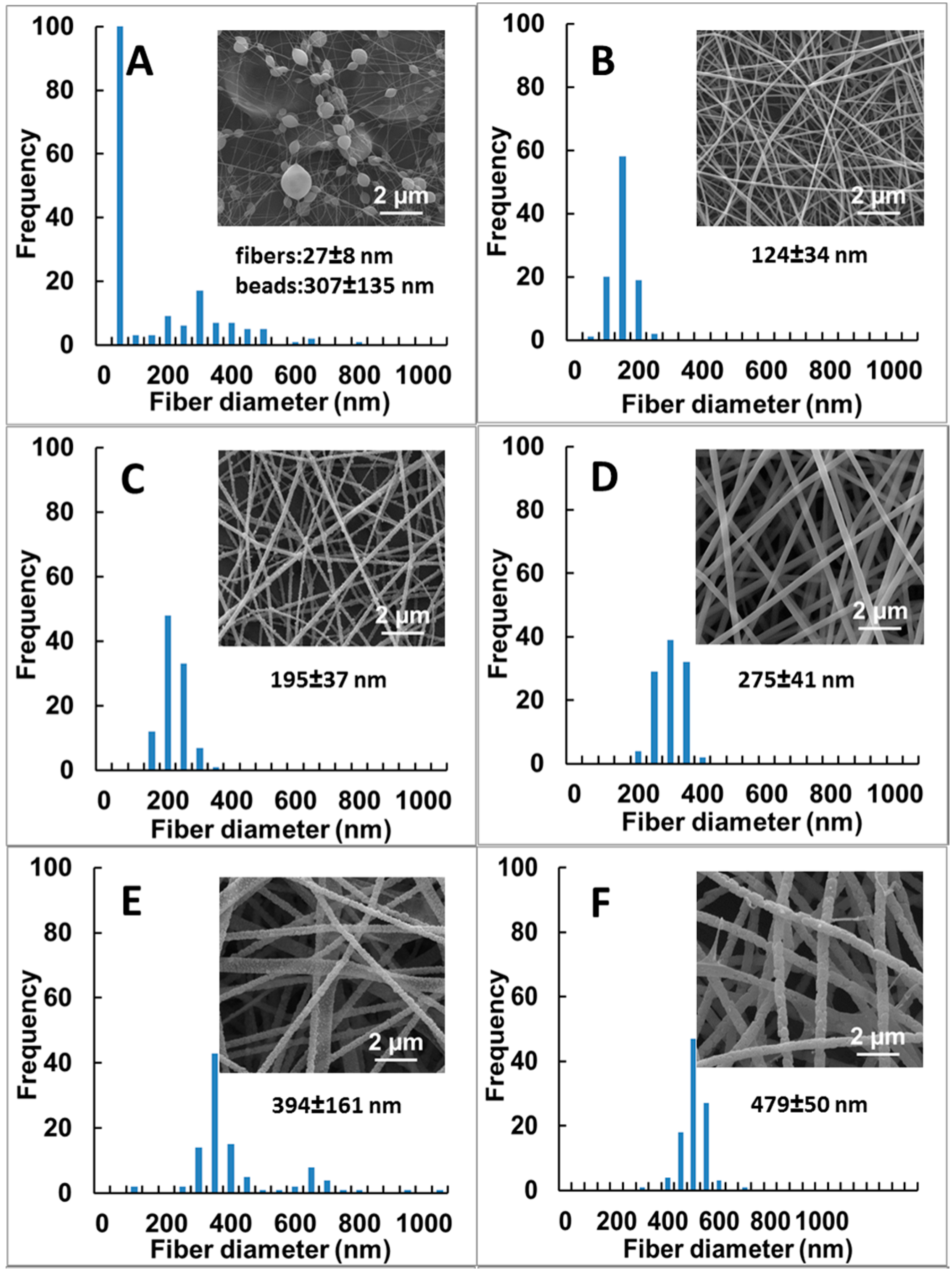
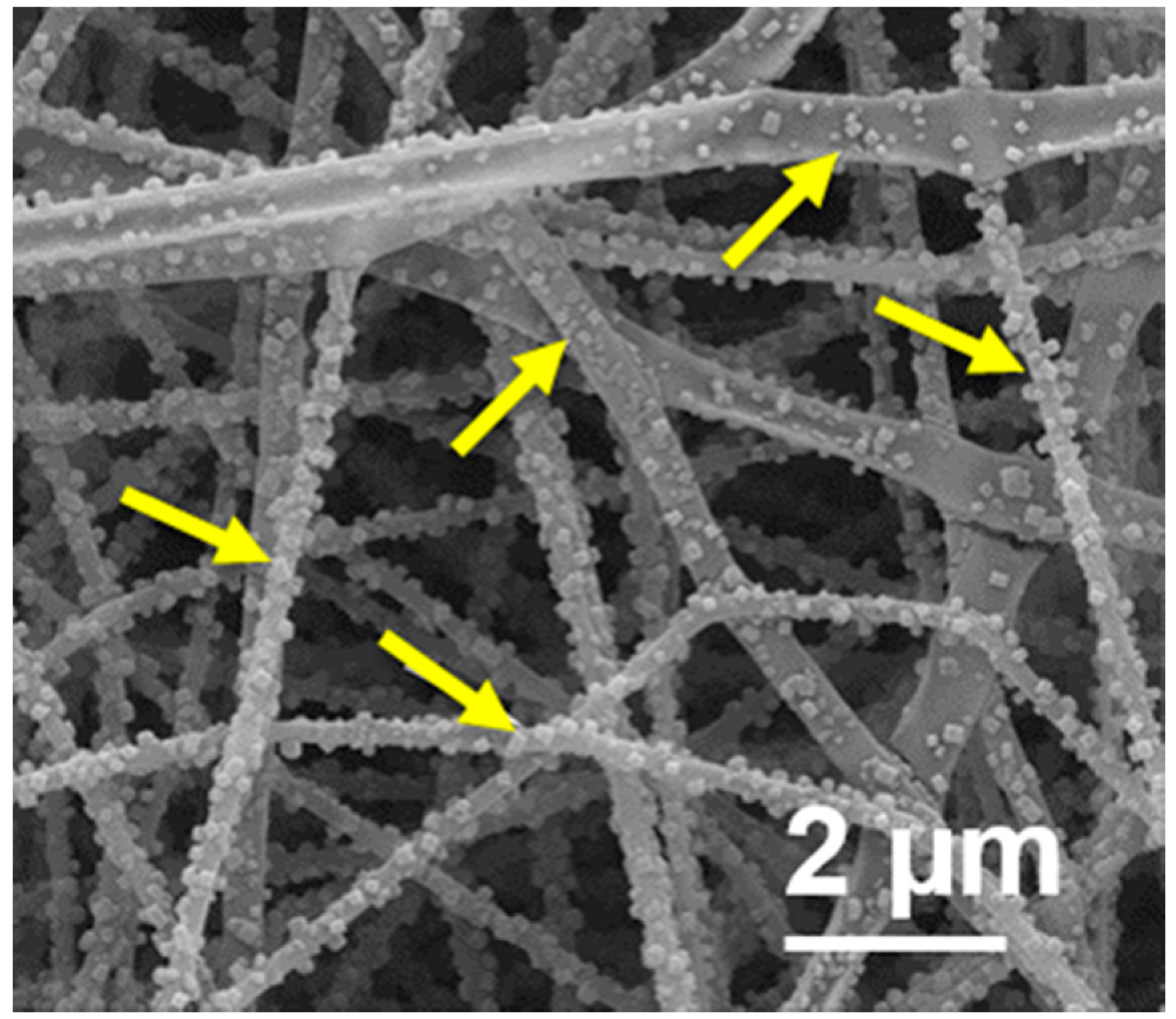
3.1. Effect of Sodium Salts on the Electrospinnability and Morphologies of PUL Fibers
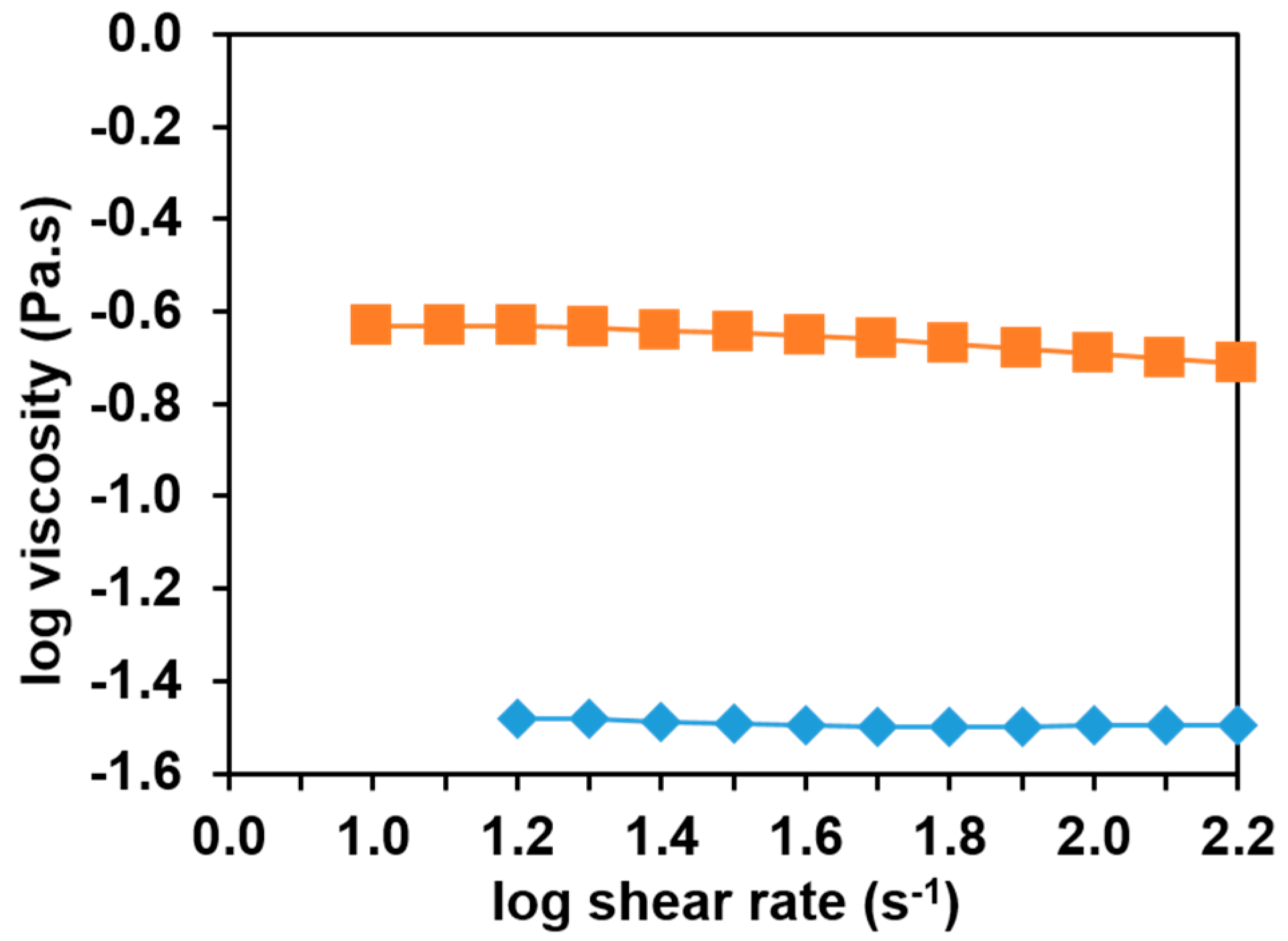
3.2. Effect of Sodium Salts on Solution Properties
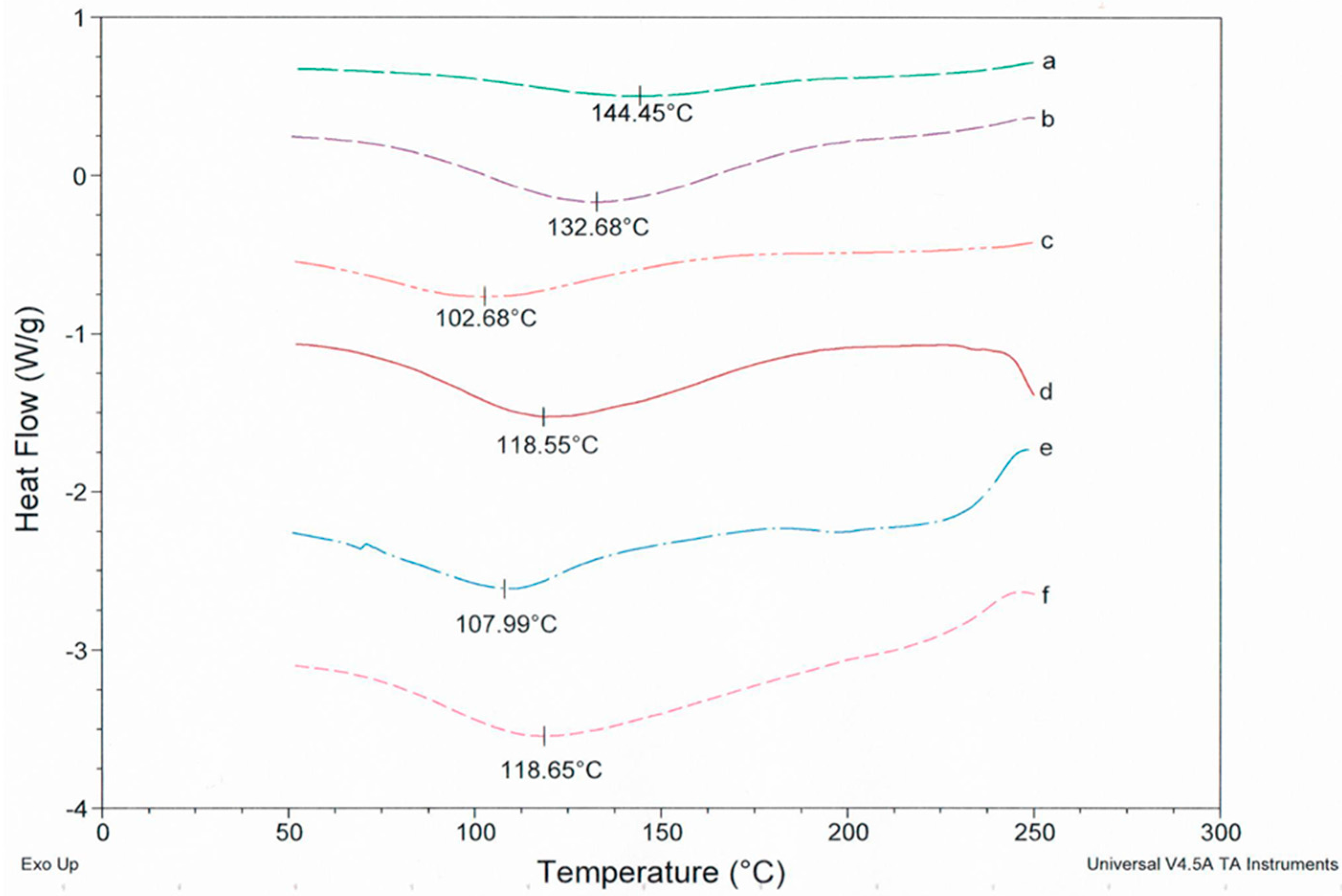
3.3. Effect of Sodium Salts on the Thermodynamic Property of Resultant Fiber
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Catley, B.J.; Ramsay, A.; Servis, C. Observations on the structure of the fungal extracellulose polysaccharide, pullulan. Carbohydr. Res. 1986, 153, 79–86. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Effect of plastic composite support and pH profiles on pullulan production in biofilm reactor. Appl. Microbiol. Biotechnolol. 2010, 86, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Damle, M.A.; Madhura, M.P.; Adivarekar, R.V. Bio-based products and their applications in textile. J. Text. Assoc. 2013, 83, 152–155. [Google Scholar]
- Farris, S.; Introzzi, L.; Fuentes-Alventosa, J.M.; Santo, N.; Rocca, R.; Piergiovanni, L. Self-assembled pullulan-silica oxygen barrier hybrid coatings for food packaging applications. J. Agric. Food Chem. 2012, 60, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Jeong, Y.I.; Kim, S.H. Characterization of hydrophobized pullulan with various hydrophobicities. Int. J. Pharm. 2003, 254, 109–121. [Google Scholar] [CrossRef]
- Lack, S.; Dulong, V.; Le Cerf, D.; Picton, L.; Argillier, J.F.; Muller, G. Hydrogels based on pullulan crosslinked with sodium trimetaphosphate (STMP): Rheological study. Polym. Bull. 2004, 52, 429–436. [Google Scholar] [CrossRef]
- Madi, N.S.; Harvey, L.M.; Mehlert, A.; McNeil, B. Synthesis of two distinct exopolysaccharide fractions by cultures of the polymorphic Fungus Aureobasidium pullulans. Carbohydr. Polym. 1997, 32, 307–314. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K. Pullulan-hyperproducing color variant strain of Aureobasidium pullulans FB-1 newly isolated from phylloplane of Ficus sp. Bioresour. Technol. 2008, 99, 3896–3899. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; Cutter, C.N.; Floros, D. Effects of ingredient composition on optical and mechanical properties of pullulan film for food-packaging applications. LWT-Food Sci. Technol. 2011, 44, 2296–3301. [Google Scholar] [CrossRef]
- Yuen, S. Pullulan and its applications. Process Biochem. 1974, 11, 7–9. [Google Scholar]
- Leathers, T.D. Pullulan. In Polysaccharides II: Polysaccharides from Eukaryotes; Vandamme, E.J., De Baets, S., Steinblichel, A., Eds.; Wiley-VCH: Weiheim, Germany, 2002; pp. 1–35. [Google Scholar]
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnolol. 2003, 62, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Shingel, K.I. Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide, pullulan. Carbohydr. Res. 2004, 339, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.W.; Garleb, K.A.; Choe, Y.S.; Humphrey, P.M.; Maki, K.C. Pullulan is a slowly digested carbohydrate in humans. J. Nutr. 2003, 133, 1051–1055. [Google Scholar] [PubMed]
- Mäkeläinen, H.; Saarinen, M.; Stowell, J.; Rautonen, N.; Ouwehand, A.C. Xylo-oligosaccharides and lactitol promote the growth of Bifidobacterium lactis and Lactobacillus species in pure cultures. Benef. Microbes 2010, 1, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tomasula, P.M.; Sousa, A.M.M.; Liu, S.-C.; Li, R.; Bonnaillie, L.M.; Liu, L.S. Electrospinning of casein/pullulan blends for food-grade applications. J. Dairy Sci. 2016, 99, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Li, R.; Tomasula, P.M.; Liu, L.S. Electrospinning of pectin with pullulan for food–grade application. J. Food Nutr. 2016, 7, 636–646. [Google Scholar]
- Stijnman, A.C.; Bodnar, I.; Hans, T.R. Electrospinning of food grade polysaccharides. Food Hydrocoll. 2011, 25, 1393–1398. [Google Scholar] [CrossRef]
- Sun, X.; Jia, D.; Kang, W.; Cheng, B.; Li, Y. Research on electrospinning process of pullulan nanofibers. Appl. Mech. Mater. 2013, 268, 198–201. [Google Scholar] [CrossRef]
- Kriegel, C.; Arrechi, A.; Kit, K.; McClements, D.J.; Weiss, J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit. Rev. Food Sci. Nutr. 2008, 48, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Tomasula, P.; Li, R. Fabrication of pullulan and pectin submicron fibers by electrospinning. In International Chemical Congress of Pacific Basin Society; ENVR: Honolulu, HI, USA, 2015; pp. 15–20. [Google Scholar]
- Kong, L.; Ziegler, G.R. Role of molecular entanglements in starch fiber formation by electrospinning. Biomacromolecules 2012, 13, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Ziegler, G.R. Rheological aspects in fabricating pullulan fibers by electro-wet-spinning. Food Hydrocoll. 2014, 38, 220–226. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Lim, T.-C.; Ma, Z. (Eds.) Chapter 3 Electrospinning Process. In An Introduction to Electrospinning and Nanofibers; World Scientific Publishing Co.: Singapore, 2005; pp. 90–153.
- Sousa, A.M.M.; Souza, H.K.S.; Uknalis, J.; Liu, S.C.; Conҫalves, M.P.; Liu, L.S. Electrospinning of agar/PVA aqueous solutions and its relation with rheological properties. Carbohydr. Polym. 2015, 115, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.L.; Chau, H.K.; Kolpak, F.; Brady, J. Solvent effects on molecular properties of pectin. J. Agric. Food Chem. 2001, 49, 4494–4501. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.H.; Norton, I.T. Gelation behavior of concentrated locust bean gum solutions. Macromolecules 1998, 31, 1575–1598. [Google Scholar] [CrossRef]
- Ogawa, E. Conformational transition of polysaccharide sodium-gellan gum in aqueous solutions. Macromolecules 1996, 29, 5178–5182. [Google Scholar] [CrossRef]
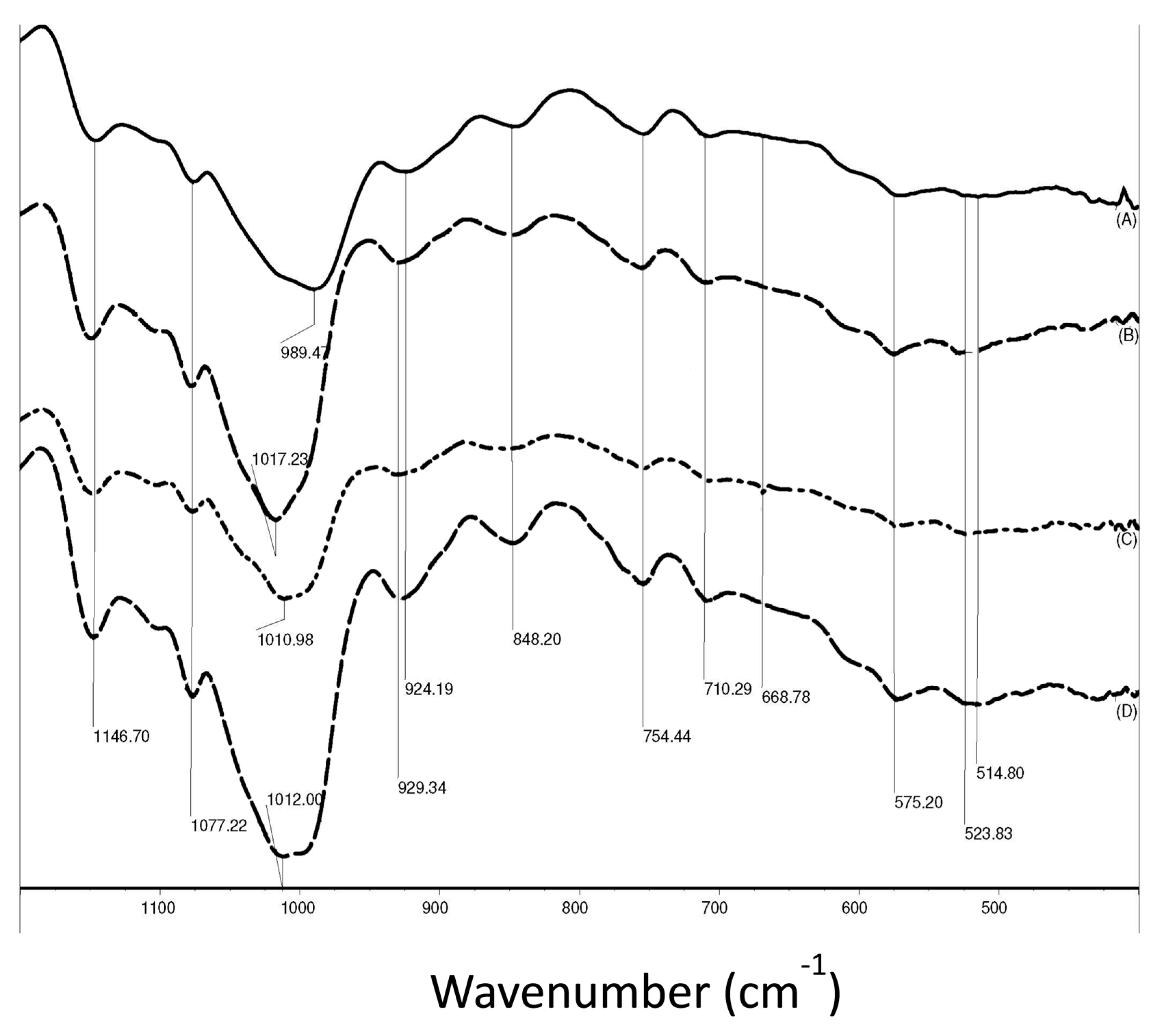
- Mitić, Z.; Cakić, M.; Nikolić, G.M.; Nikolić, R.; Nikolić, G.S.; Pavlović, R.; Santaniello, E. Synthesis, physicochemical and spectroscopic characterization of copper(II)-polysaccharide pullulan complexes by UV-vis, ATR-FTIR, and EPR. Carbohydr. Res. 2011, 346, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Rades, T.; Mueller-Goymann, C.C. Interaction between fenoprofen sodium and poly(ethylene oxide). Eur. J. Pharm. Biopharm. 1998, 46, 51–59. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Analogue calorimetric studies of blends of poly(vinyl ester)s and polyacrylates. Macromolecules 1996, 29, 1579–1583. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Analogue calorimetry of polymer blends: Poly(styrene-co-acrylonitrile) and poly(phenyl acrylate) or poly(vinyl benzoate). Polymer 1996, 37, 2439–2443. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Miscibility and phase diagrams of poly(phenyl acrylate) and poly(styrene-co-acrylonitrile) blends. Polymer 1993, 34, 1454–1459. [Google Scholar] [CrossRef]
- Ero-Phillips, O.; Jenkins, M.; Stamboulis, A. Tailoring crystallinity of electrospun pullulan fibres by control of electrospinning parameters. Polymers 2012, 4, 1331–1348. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Lee, D.S. Thermal properties of electrospun polyesters. Polym. J. 2000, 32, 616–618. [Google Scholar] [CrossRef]
- Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Effect of the electrospinning process on polymer crystallization chain conformation in nylon-6 and nylon-12. Macromolecules 2004, 37, 877–881. [Google Scholar] [CrossRef]



| PUL wt % | NaCl, M | Na3C6H5O7, M | Specific viscosity, Pa·s |
|---|---|---|---|
| 15 | 0 | 0 | 204 ± 16 |
| 15 | 0.20 | 0 | 256 ± 21 |
| 15 | 2.0 | 0 | 333 ± 14 |
| 15 | 5.0 | 0 | 157 ± 17 |
| 15 | 0 | 0.05 | 237 ± 25 |
| 15 | 0 | 0.50 | 274 ± 32 |
| 8 | 0 | 0 | 19 ± 7 |
| 8 | 0.20 | 0 | 27 ± 2 |
| 8 | 1.0 | 0 | 36 ± 3 |
| 8 | 2.0 | 0 | 43 ± 11 |
| 8 | 0 | 0.05 | 22 ± 9 |
| 8 | 0 | 0.50 | 29 ± 4 |
| Submission to electrospinning | Mw/Mn | Mw × 10−3 (g/mol) | Rgz (nm) | a (mol.L/g) |
|---|---|---|---|---|
| Before | 2.60 ± 0.03 | 360 ± 6 | 42.2 ± 1 | 0.672 ± 0.003 |
| After | 2.28 ± 0.02 | 330 ± 1 | 34.7 ± 1 | 0.663 ± 0.004 |
| With 1.0 M NaCl | 1.86 ± 0.02 | 226 ± 2 | 17.2 ± 1 | 0.668 ± 0.002 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Tomasula, P.; De Sousa, A.M.M.; Liu, S.-C.; Tunick, M.; Liu, K.; Liu, L. Electrospinning Pullulan Fibers from Salt Solutions. Polymers 2017, 9, 32. https://doi.org/10.3390/polym9010032
Li R, Tomasula P, De Sousa AMM, Liu S-C, Tunick M, Liu K, Liu L. Electrospinning Pullulan Fibers from Salt Solutions. Polymers. 2017; 9(1):32. https://doi.org/10.3390/polym9010032
Chicago/Turabian StyleLi, Ran, Peggy Tomasula, Ana Margarida Moreira De Sousa, Shih-Chuan Liu, Michael Tunick, Kevin Liu, and Linshu Liu. 2017. "Electrospinning Pullulan Fibers from Salt Solutions" Polymers 9, no. 1: 32. https://doi.org/10.3390/polym9010032