Block Copolymers: Synthesis, Self-Assembly, and Applications
Abstract
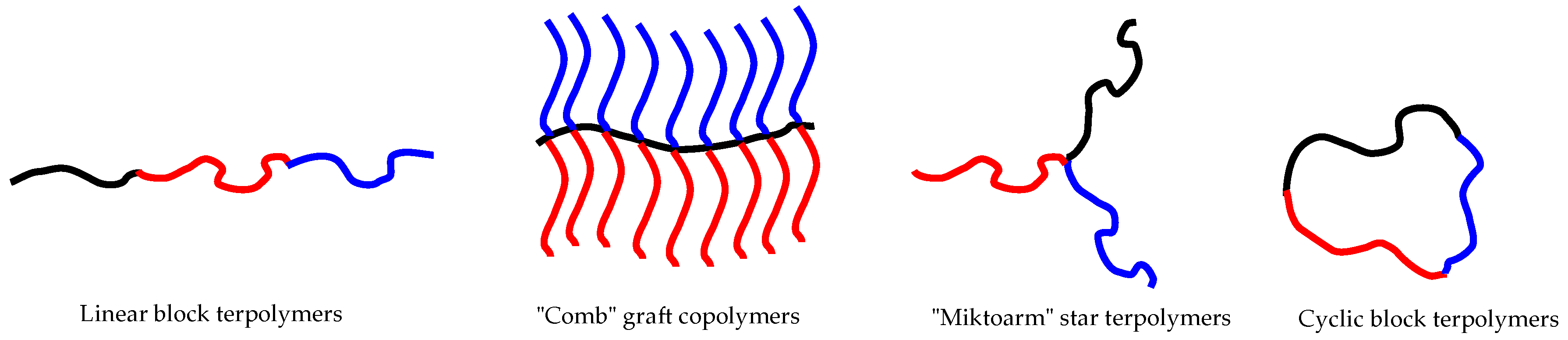
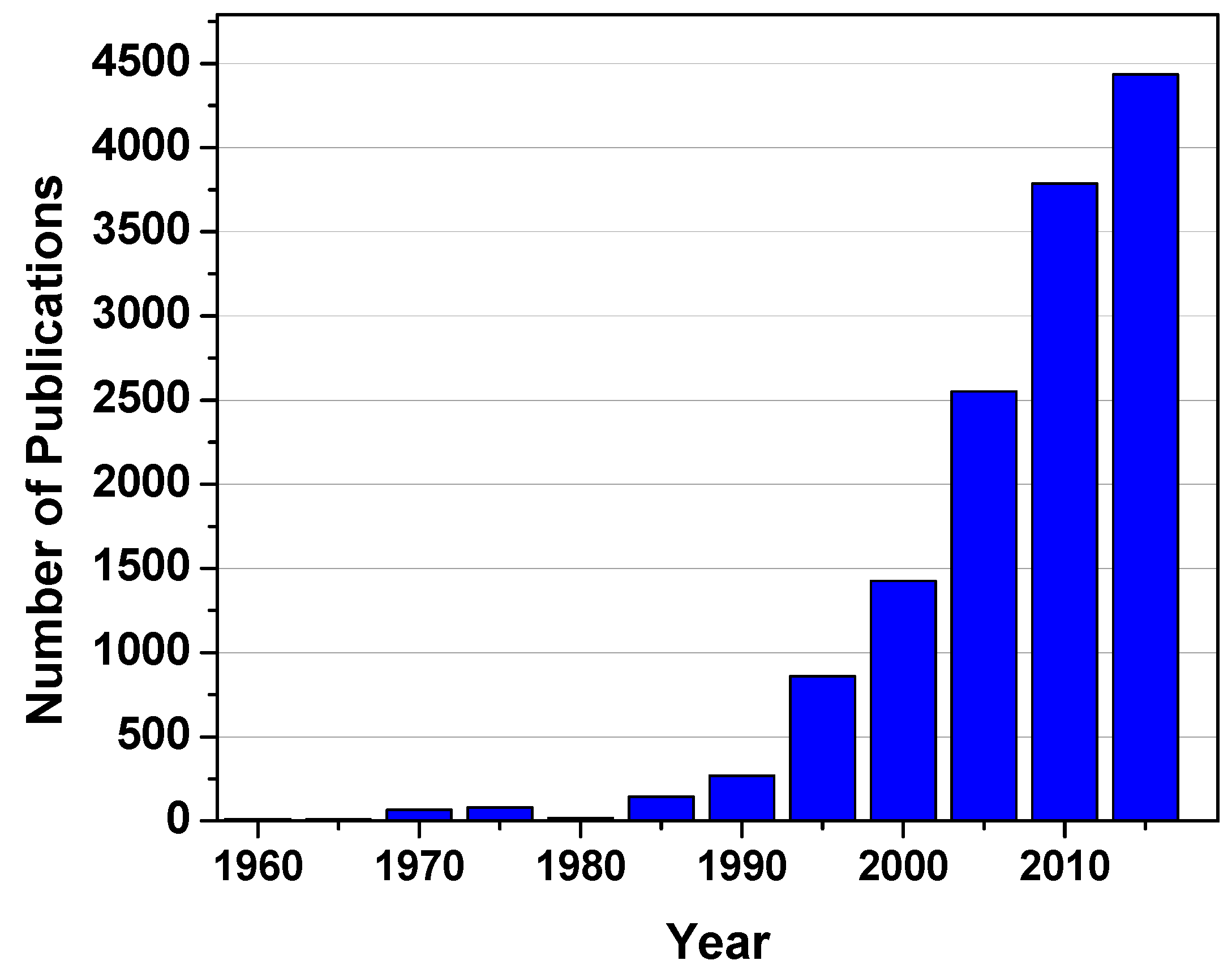
:1. Introduction
2. Synthesis of BCPs
2.1. Sequential Addtion Polymerization
2.1.1. Controlled Polymerization
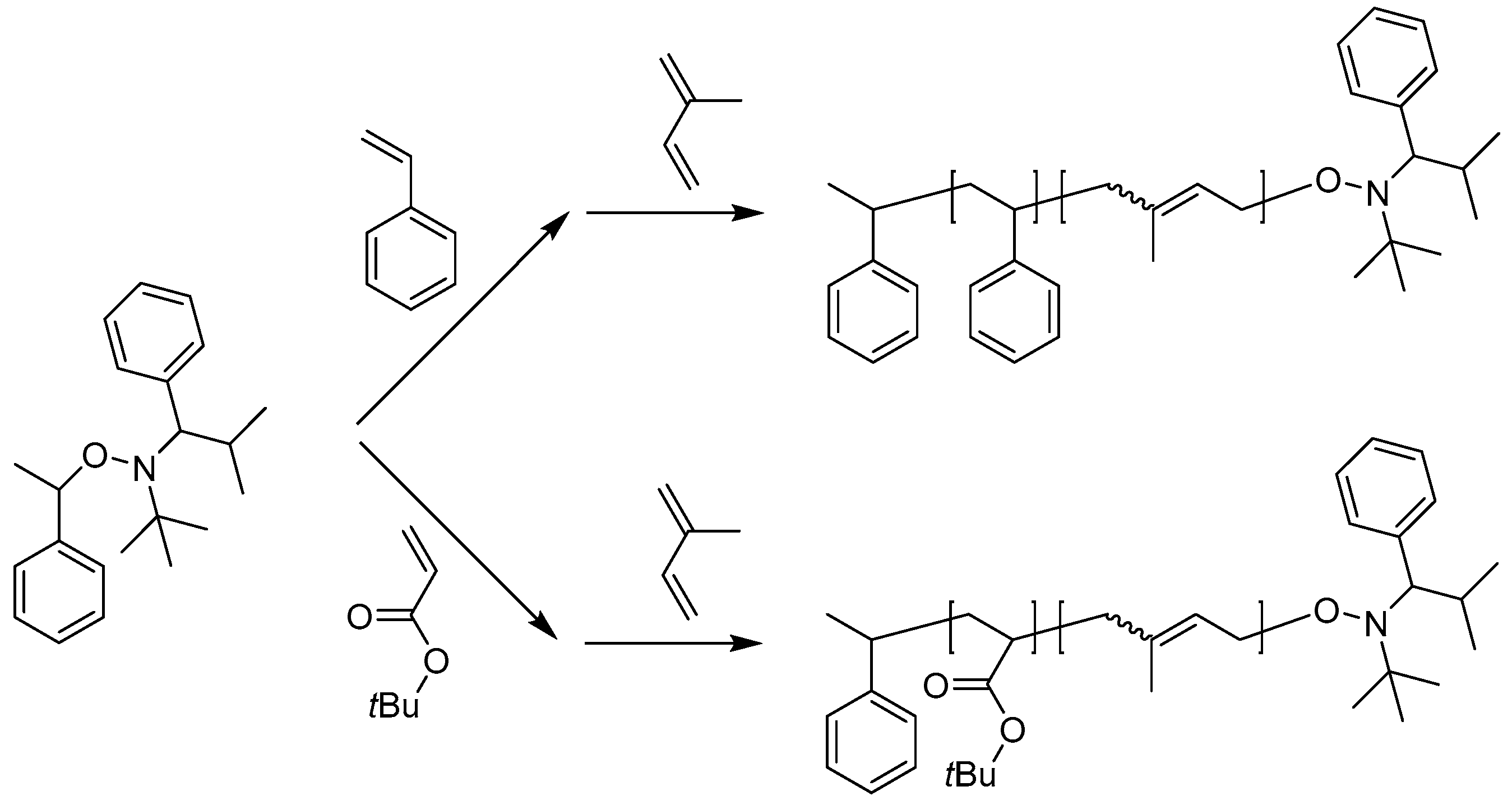
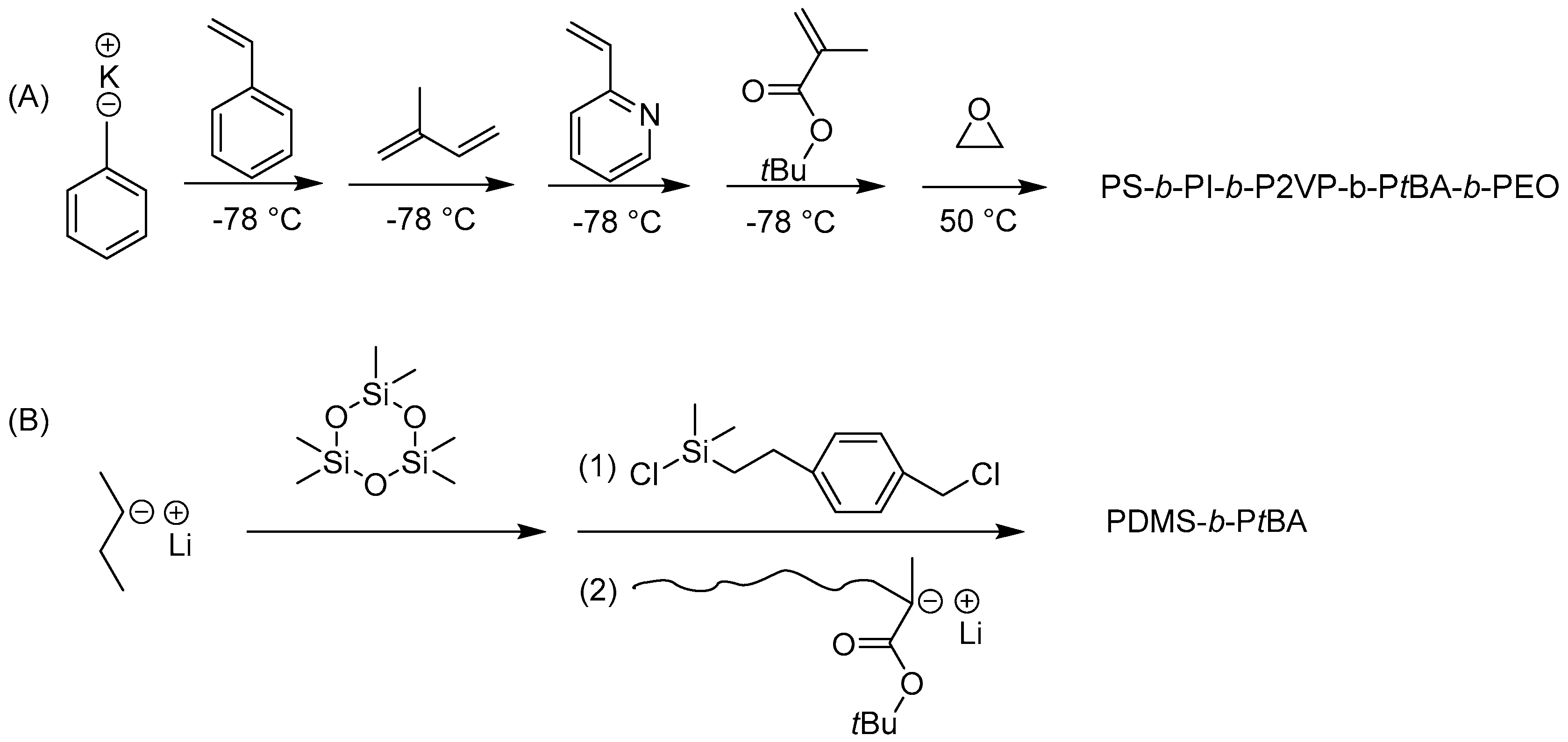
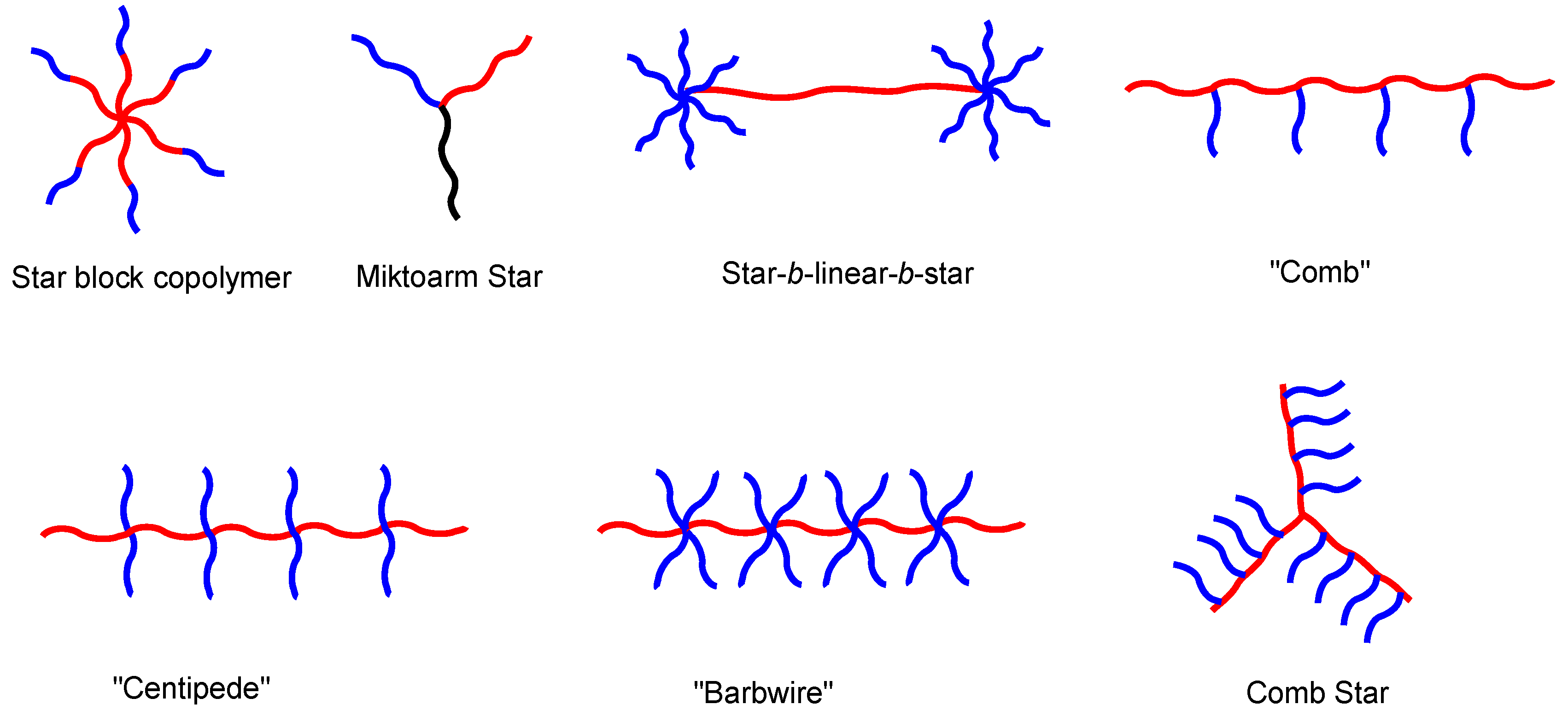
2.1.2. Living Anionic Polymerization
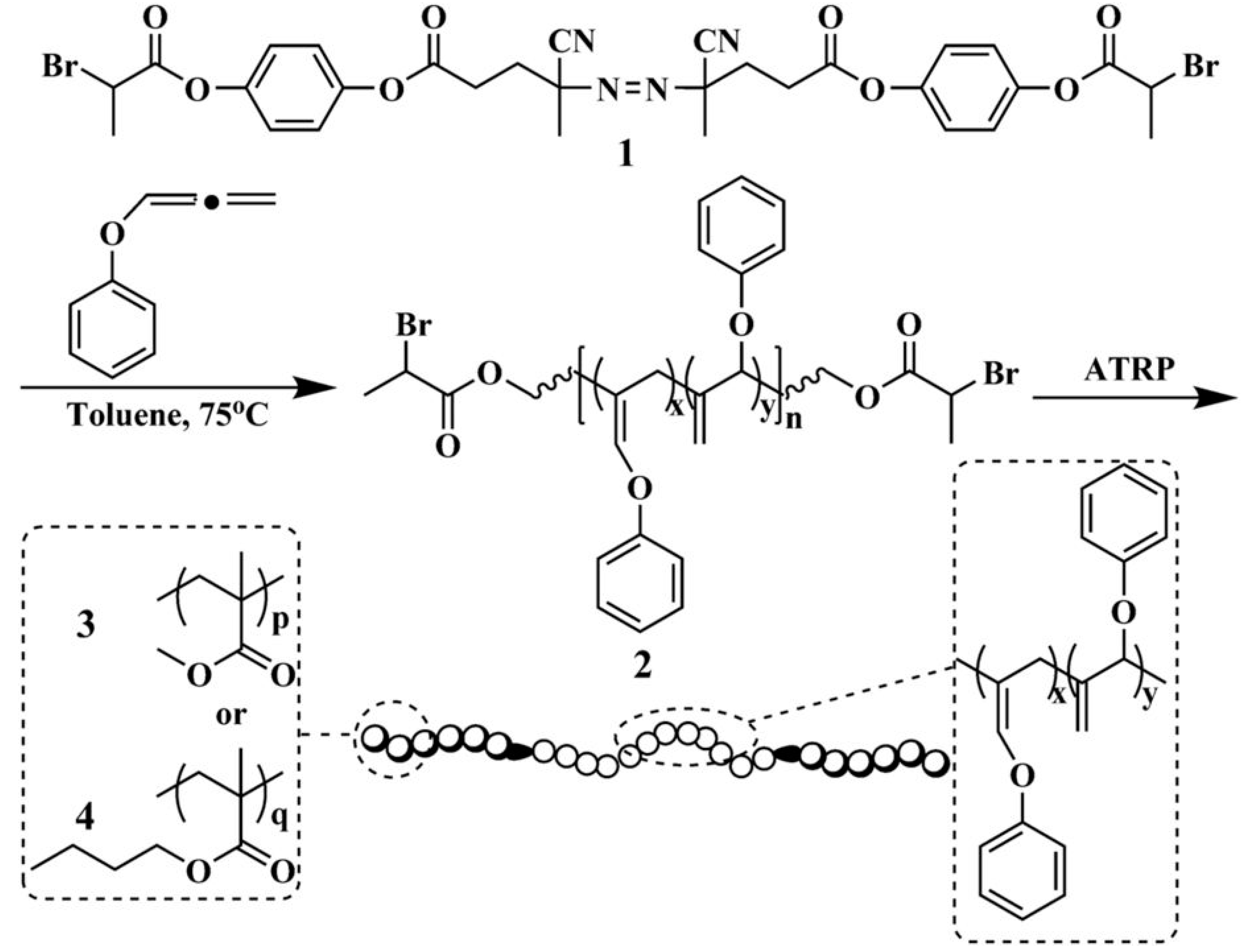
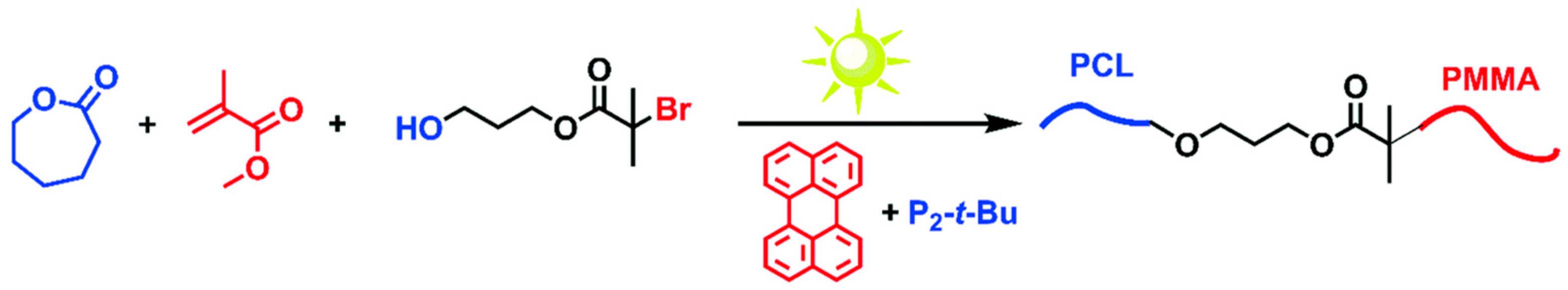
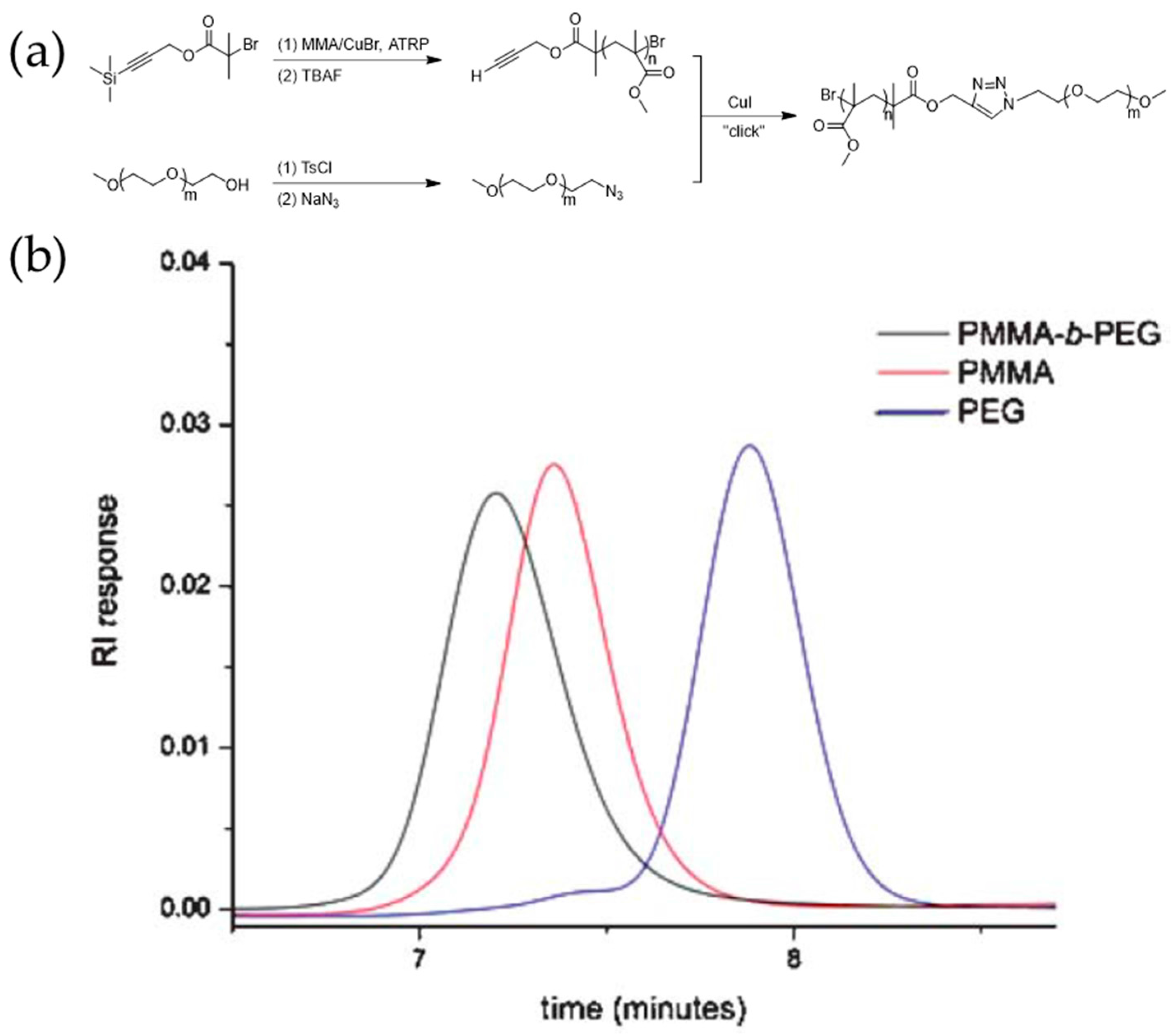
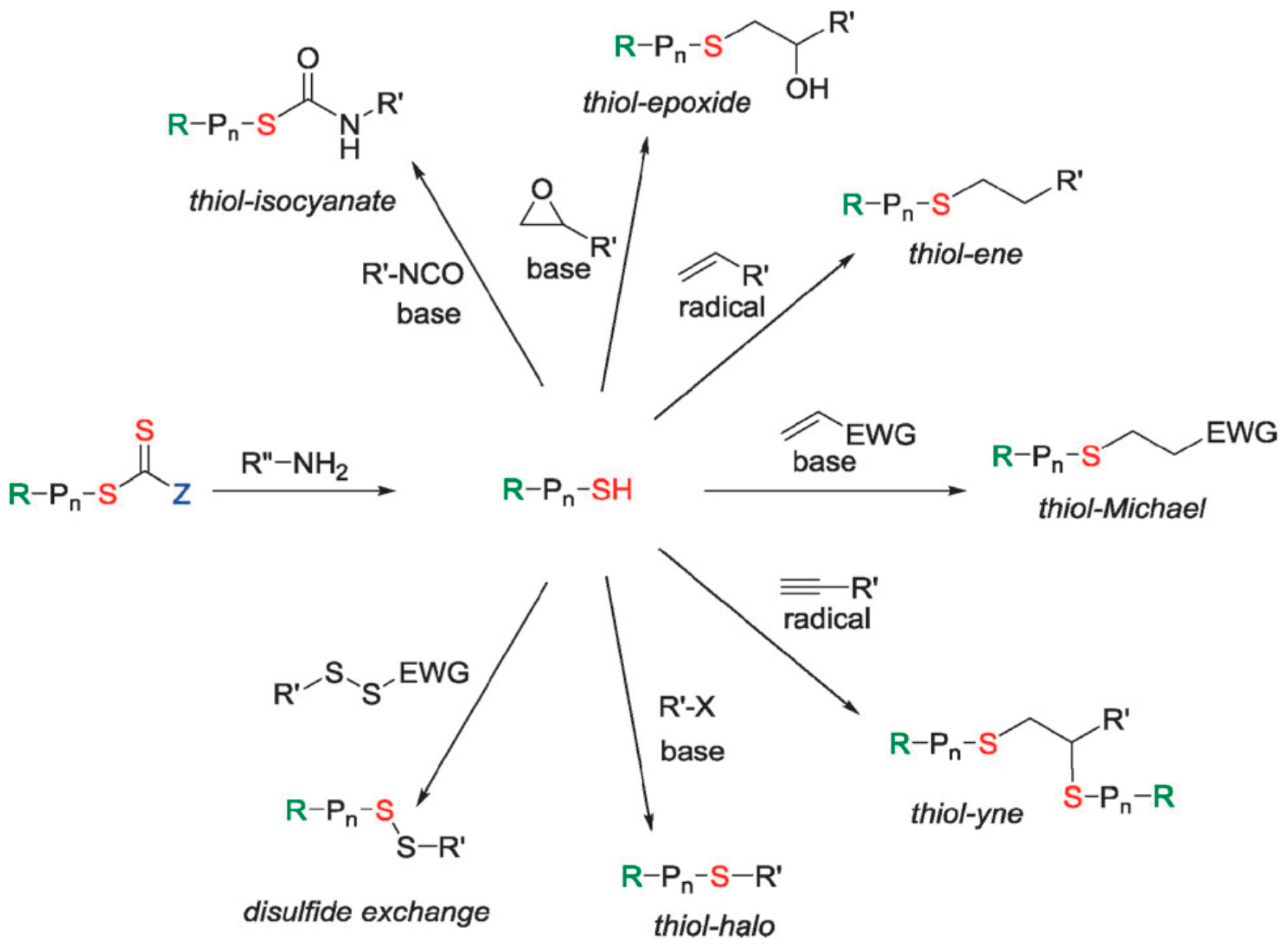
2.2. Combination of Different Polymerization Techniques
3. Self-Assembly of BCPs
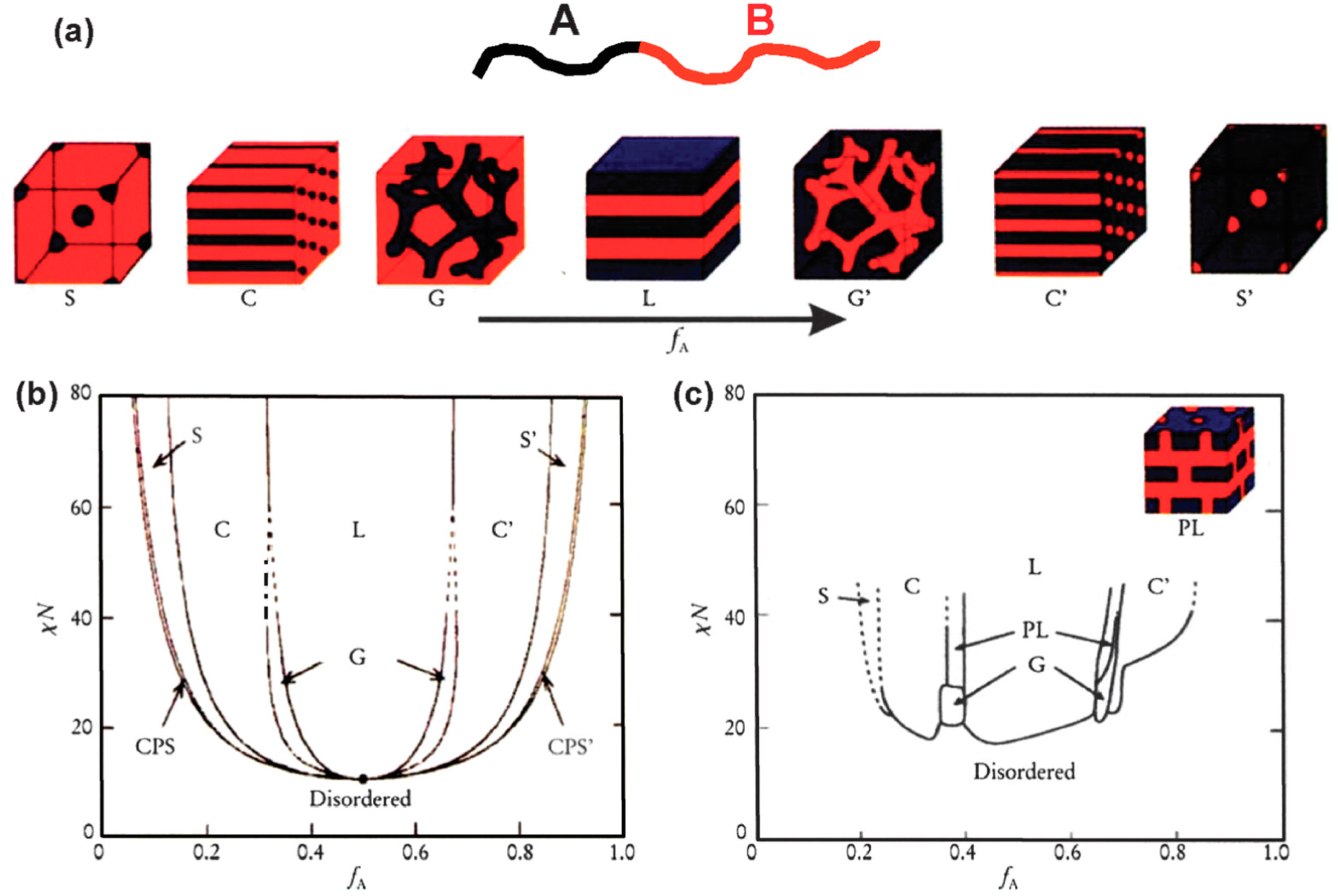
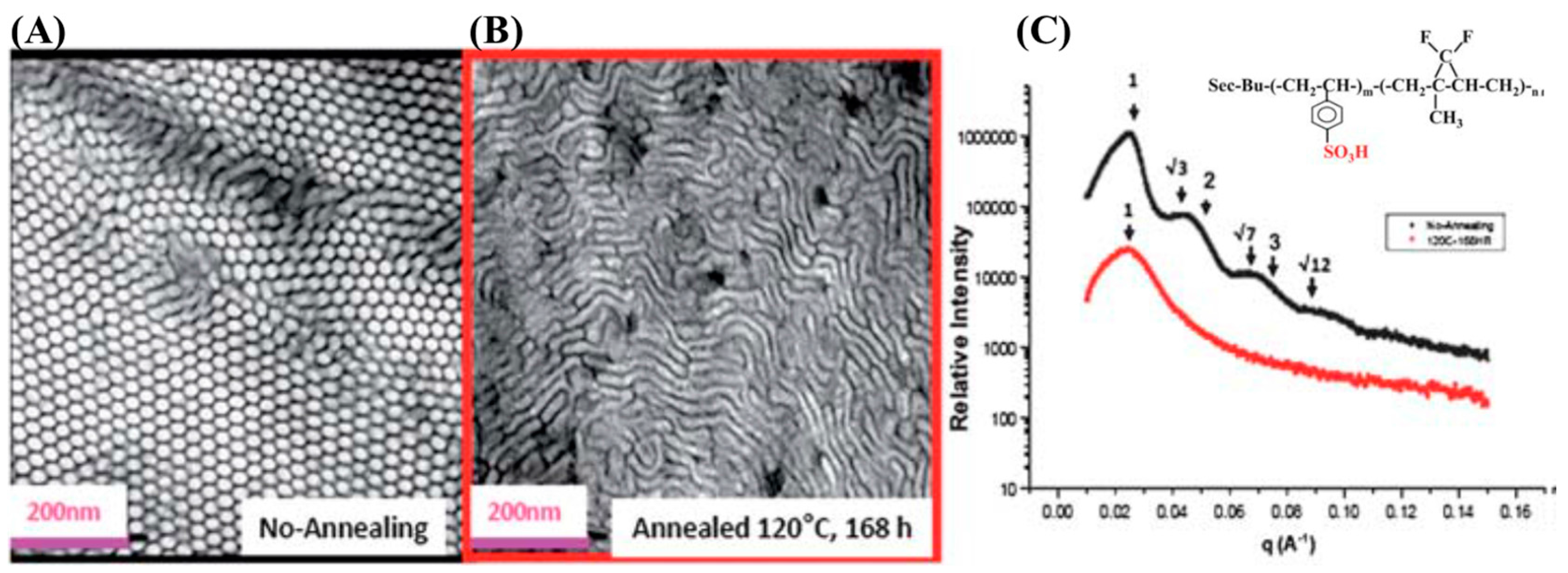
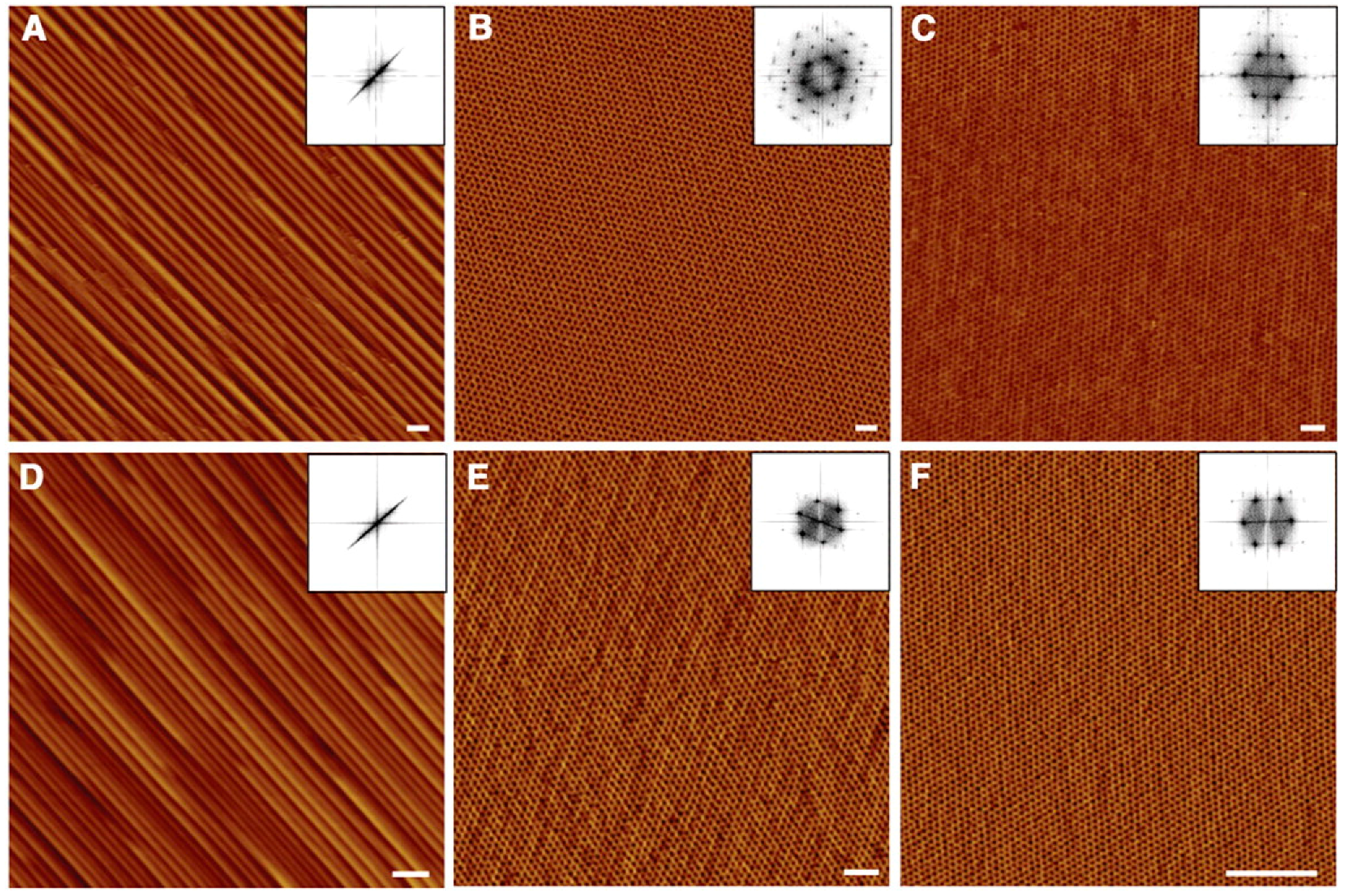
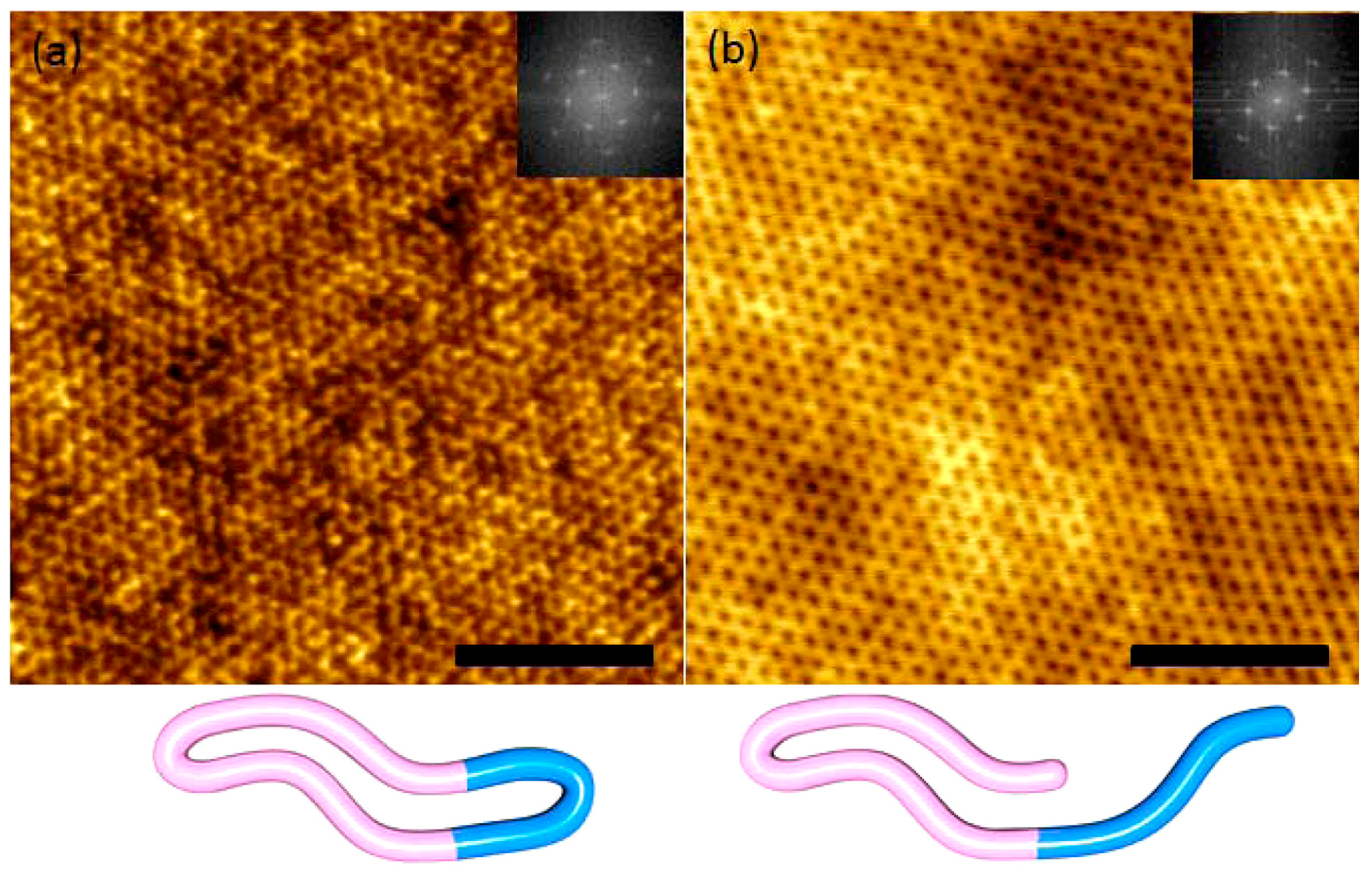
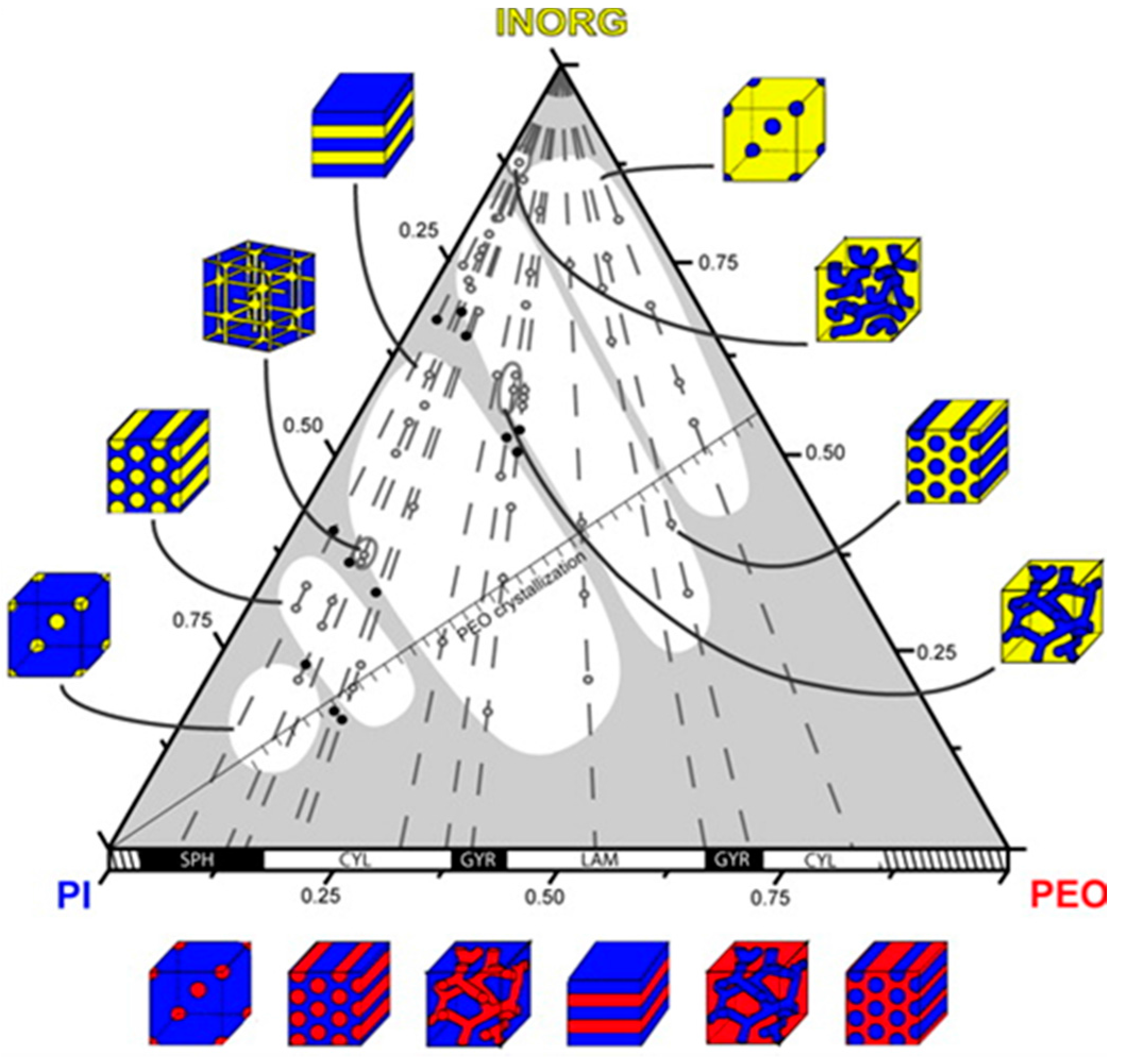
3.1. Self-Assembly in Bulk
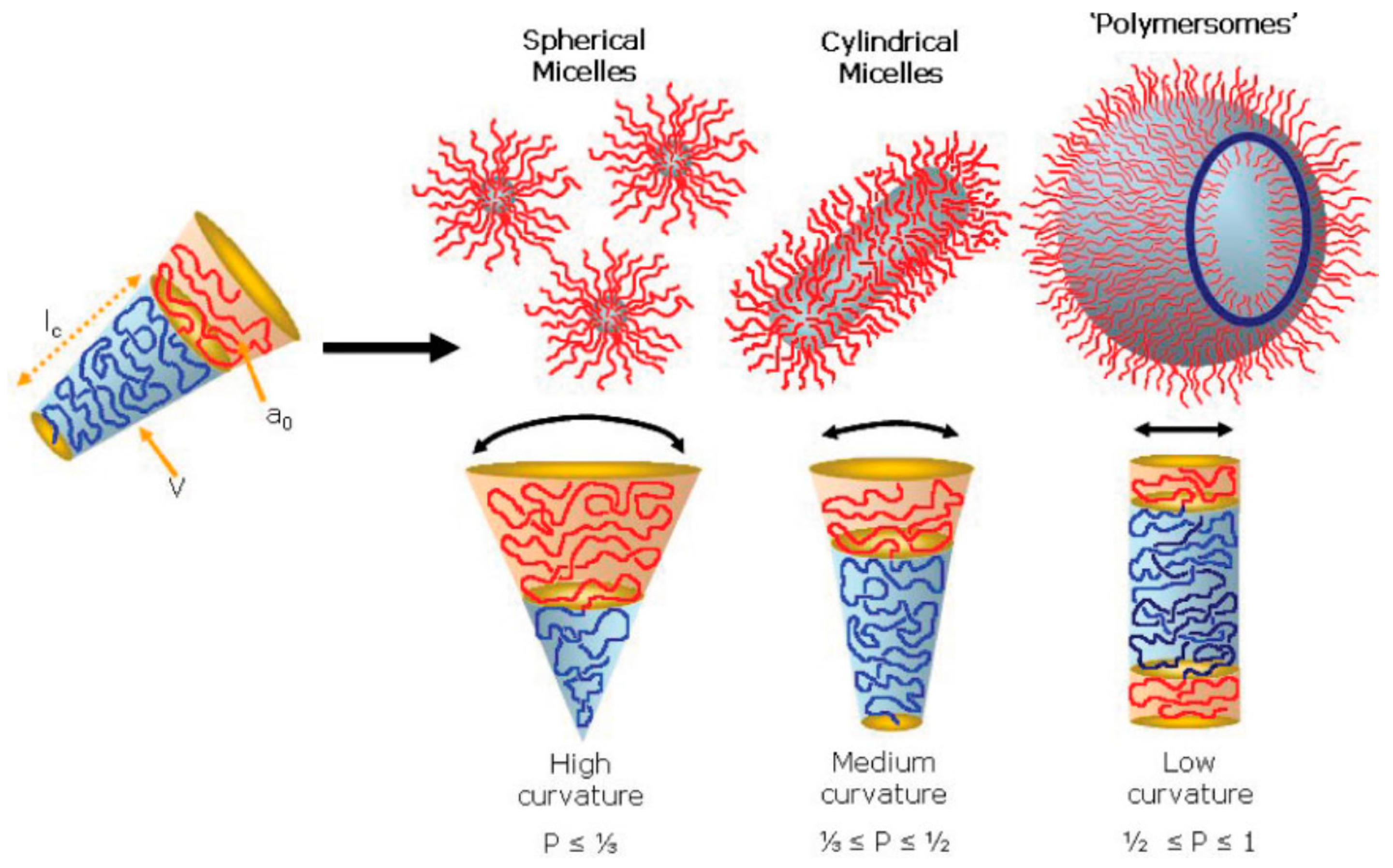
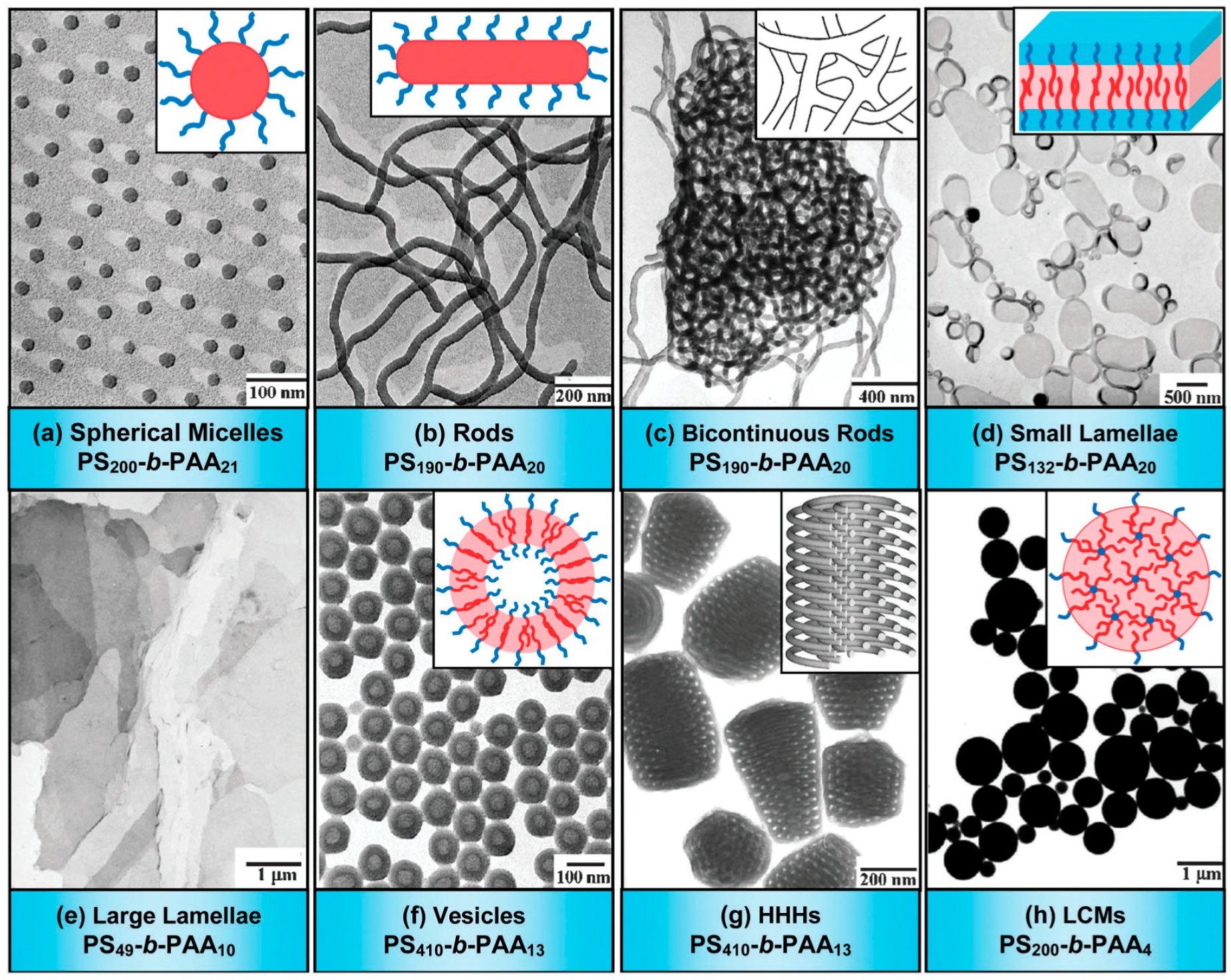
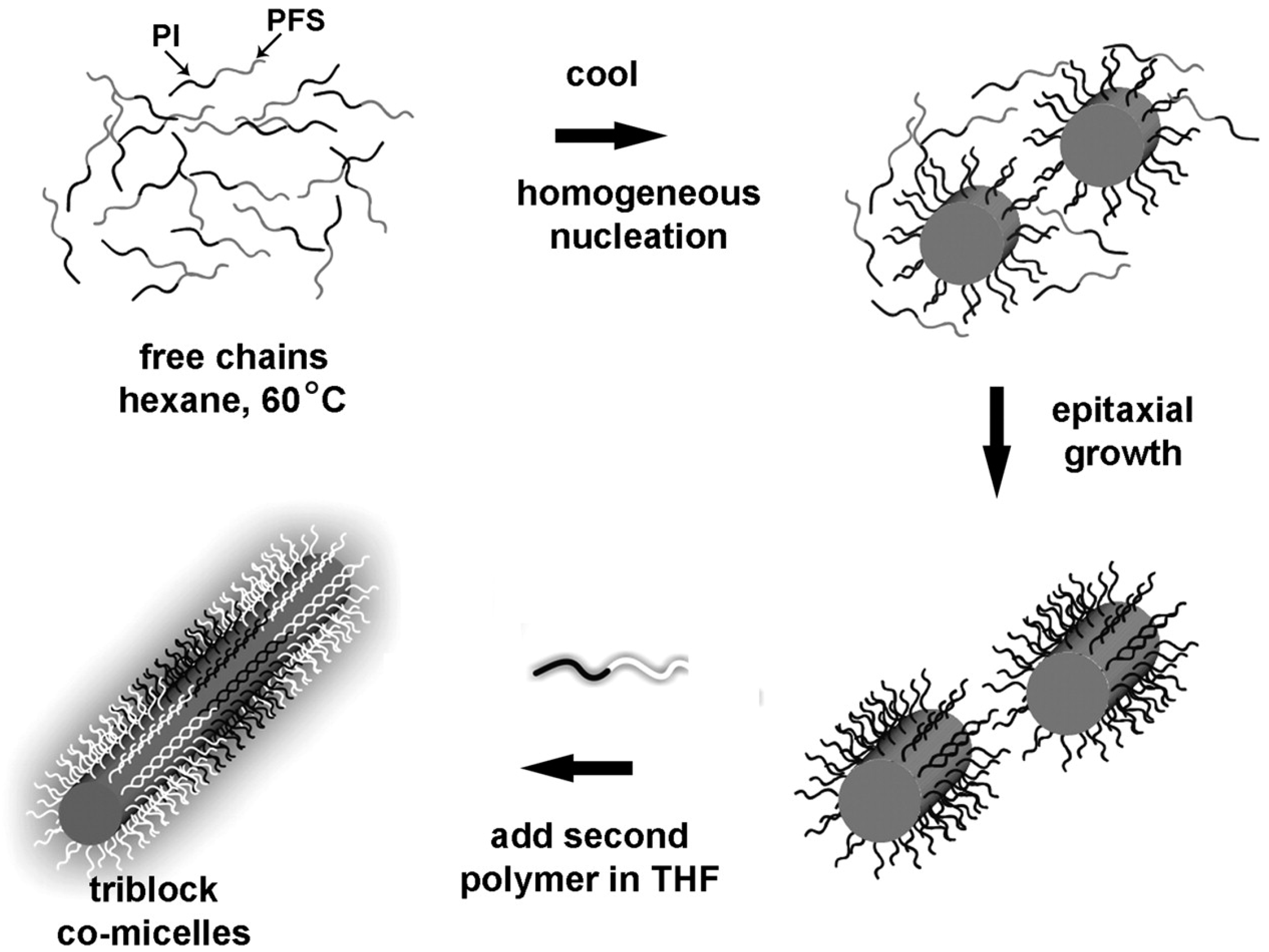
3.2. Self-Assembly in Solution
4. Applications
4.1. Applications as Thermoplastic Elastomers
4.2. Applications in Drug Delivery and Release
4.3. Applications in Soft Lithography
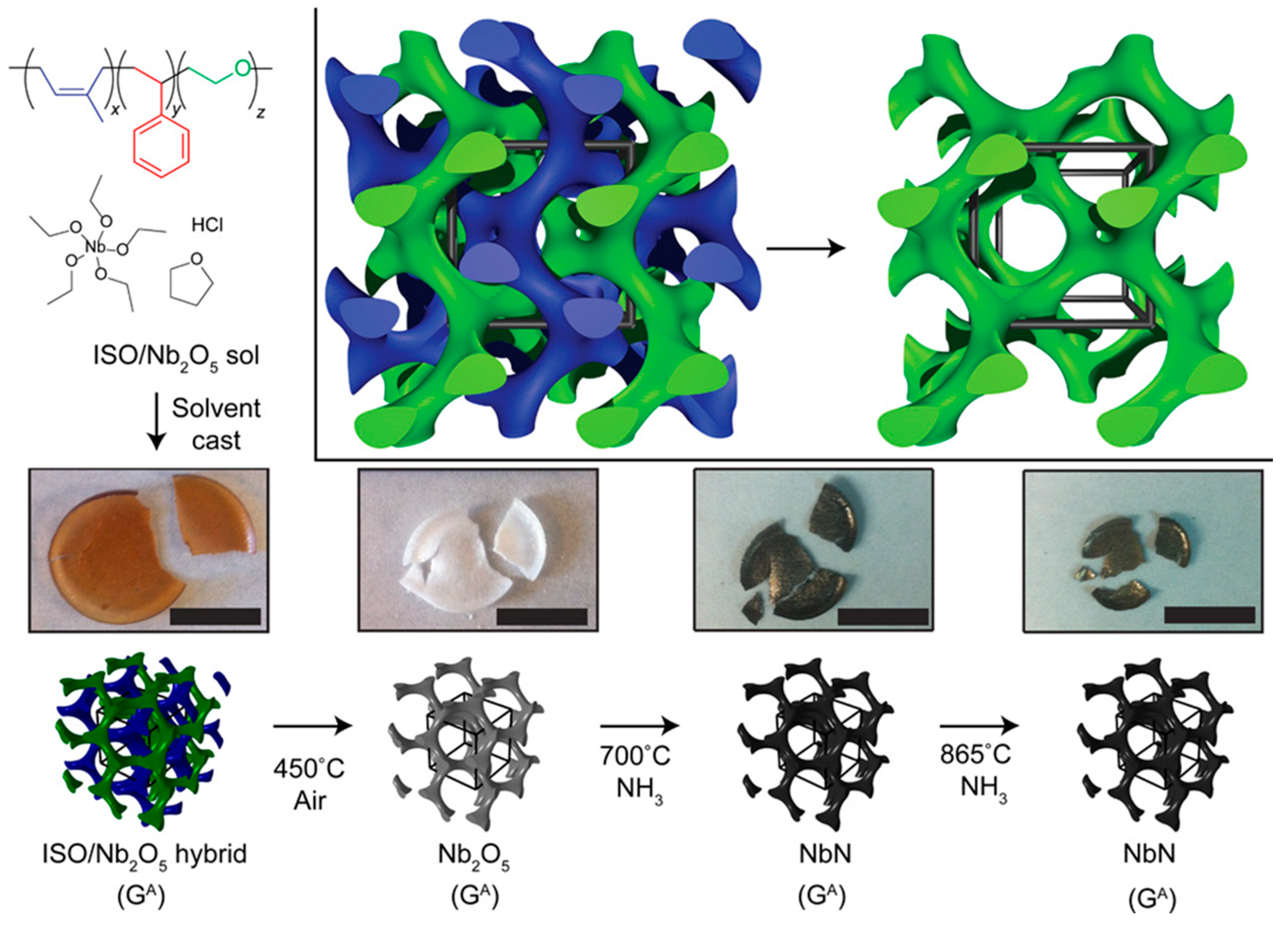
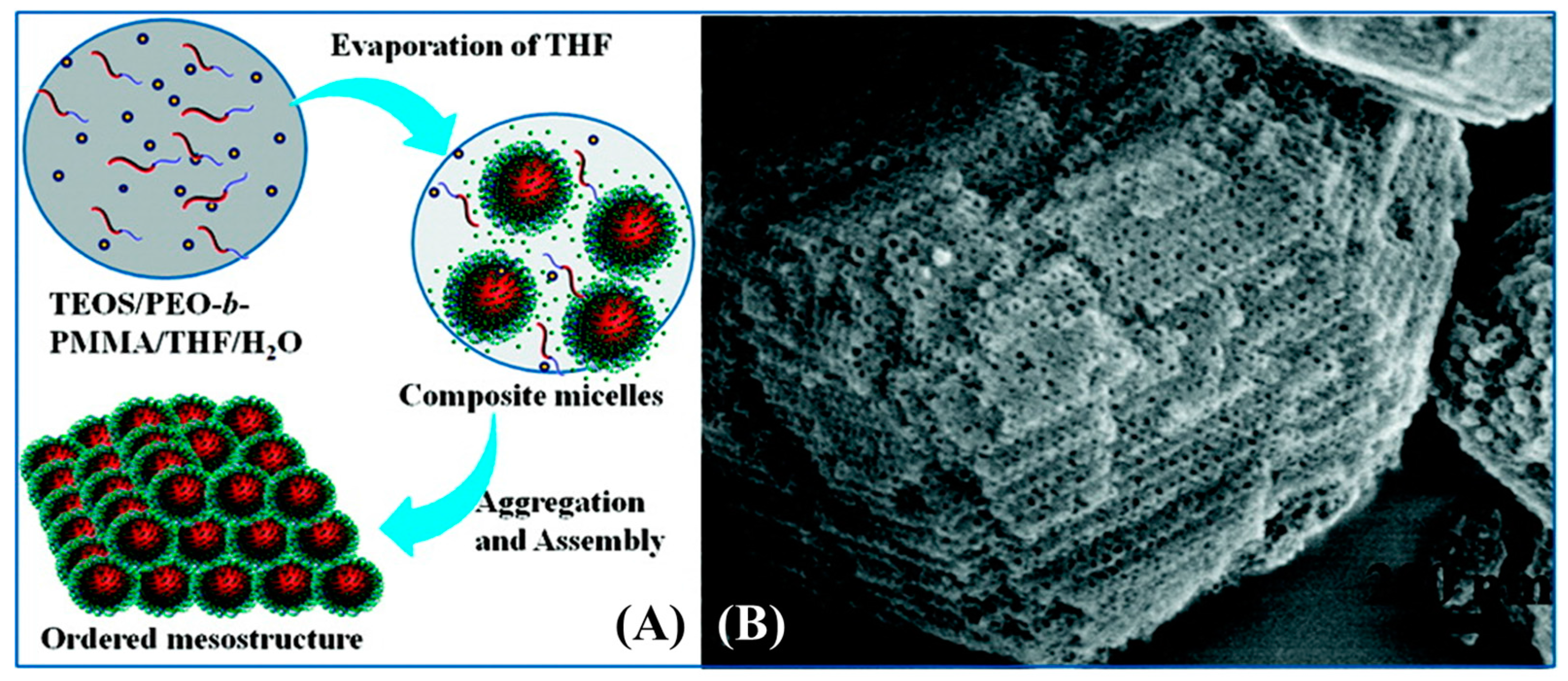
4.4. Applications in Mesoporous Materials
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hamley, I.W. The Physics of Block Copolymers; Oxford University Press: New York, NY, USA, 1998; Volume 19. [Google Scholar]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J. Macromolecular architectures by living and controlled/living polymerizations. Prog. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J.W. Experimental techniques in high-vacuum anionic polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6179–6222. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, K.; Mays, J.W. Synthesis of block copolymers of styrene and methyl methacrylate by conventional free radical polymerization in room temperature ionic liquids. Macromolecules 2002, 35, 5738–5741. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J.W. Synthesis of combs, centipedes, and barbwires: Poly (isoprene-graft-styrene) regular multigraft copolymers with trifunctional, tetrafunctional, and hexafunctional branch points. Macromolecules 2002, 35, 7182–7190. [Google Scholar] [CrossRef]
- Hong, K.; Uhrig, D.; Mays, J.W. Living anionic polymerization. Curr. Opin. Solid State Mater. Sci. 1999, 4, 531–538. [Google Scholar] [CrossRef]
- Pochan, D.J.; Gido, S.P.; Pispas, S.; Mays, J.W.; Ryan, A.J.; Fairclough, J.P.A.; Hamley, I.W.; Terrill, N.J. Morphologies of microphase-separated A2B simple graft copolymers. Macromolecules 1996, 29, 5091–5098. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymers—Designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Lennon, E.M.; Katsov, K.; Fredrickson, G.H. Free energy evaluation in field-theoretic polymer simulations. Phys. Rev. Lett. 2008, 101, 138302. [Google Scholar] [CrossRef] [PubMed]
- Matsen, M.W.; Bates, F.S. Origins of complex self-assembly in block copolymers. Macromolecules 1996, 29, 7641–7644. [Google Scholar] [CrossRef]
- Poelma, J.E.; Ono, K.; Miyajima, D.; Aida, T.; Satoh, K.; Hawker, C.J. Cyclic block copolymers for controlling feature sizes in block copolymer lithography. ACS Nano 2012, 6, 10845–10854. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Park, S.-M.; Hinsberg, W.D. Block copolymer based nanostructures: Materials, processes, and applications to electronics. Chem. Rev. 2009, 110, 146–177. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Wooley, K.L. The convergence of synthetic organic and polymer chemistries. Science 2005, 309, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Wang, W.Y.; Holt, A.P.; Feng, H.B.; Uhrig, D.; Lu, X.Y.; Hong, T.; Wang, Y.Y.; Kang, N.G.; Mays, J.; et al. Effect of molecular weight on the ion transport mechanism in polymerized ionic liquids. Macromolecules 2016, 49, 4557–4570. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Sumerlin, B.S.; Tsarevsky, N.V. Progress in Controlled Radical Polymerization: Mechanisms and Techniques; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012. [Google Scholar]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Gody, G.; Zetterlund, P.B.; Perrier, S.; Harrisson, S. The limits of precision monomer placement in chain growth polymerization. Nat. Commun. 2016, 7, 10514. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.-F.; Wojnarowska, Z.; Hong, T.; Carroll, B.; Li, B.; Feng, H.; Parsons, L.; Wang, W.; Lokitz, B.S.; Cheng, S.; et al. A star-shaped single lithium-ion conducting copolymer by grafting a poss nanoparticle. Polymer 2017, 124, 117–127. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. “Living”/controlled radical polymerization. Transition-metal-catalyzed atom transfer radical polymerization in the presence of a conventional radical initiator. Macromolecules 1995, 28, 7572–7573. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Orilall, M.C.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Y.; Armes, S.P.; Morris, C.J.; Rose, S.F.; Lloyd, A.W.; Lewis, A.L. Synthesis and characterization of biocompatible thermo-responsive gelators based on aba triblock copolymers. Biomacromolecules 2005, 6, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.J.; Park, S.; Zhong, M.J.; Pan, X.C.; Pietrasik, J.; Bettinger, C.J.; Matyjaszewski, K. Facile arm-first synthesis of star block copolymers via arget atrp with ppm amounts of catalyst. Macromolecules 2016, 49, 6752–6760. [Google Scholar] [CrossRef]
- Min, K.; Gao, H.; Matyjaszewski, K. Preparation of homopolymers and block copolymers in miniemulsion by atrp using activators generated by electron transfer (aget). J. Am. Chem. Soc. 2005, 127, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Gao, H.; Matyjaszewski, K. Use of ascorbic acid as reducing agent for synthesis of well-defined polymers by arget atrp. Macromolecules 2007, 40, 1789–1791. [Google Scholar] [CrossRef]
- Kwak, Y.; Matyjaszewski, K. Arget atrp of methyl methacrylate in the presence of nitrogen-based ligands as reducing agents. Polym. Int. 2009, 58, 242–247. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. Atrp in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugihara, S.; Sugihara, K.; Armes, S.P.; Ahmad, H.; Lewis, A.L. Synthesis of biomimetic poly (2-(methacryloyloxy) ethyl phosphorylcholine) nanolatexes via atom transfer radical dispersion polymerization in alcohol/water mixtures. Macromolecules 2010, 43, 6321–6329. [Google Scholar] [CrossRef]
- Ding, A.; Lu, G.; Guo, H.; Huang, X. Double-bond-containing polyallene-based triblock copolymers via phenoxyallene and (meth)acrylate. Sci. Rep. 2017, 7, 43706. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Le, T.P.; Moad, G.; Rizzardo, E.; Thang, S.H. A more versatile route to block copolymers and other polymers of complex architecture by living radical polymerization: The raft process. Macromolecules 1999, 32, 2071–2074. [Google Scholar] [CrossRef]
- You, Y.; Hong, C.; Wang, W.; Lu, W.; Pan, C. Preparation and characterization of thermally responsive and biodegradable block copolymer comprised of pnipaam and pla by combination of rop and raft methods. Macromolecules 2004, 37, 9761–9767. [Google Scholar] [CrossRef]
- Chaduc, I.; Zhang, W.; Rieger, J.; Lansalot, M.; D’Agosto, F.; Charleux, B. Amphiphilic block copolymers from a direct and one-pot raft synthesis in water. Macromol. Rapid Commun. 2011, 32, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Y.; Li, B.; Zhu, S. Ab initio batch emulsion raft polymerization of styrene mediated by poly(acrylic acid-b-styrene) trithiocarbonate. Macromolecules 2009, 42, 6414–6421. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Li, B.-G.; Zhu, S. Toward well-controlled ab initio raft emulsion polymerization of styrene mediated by 2-(((dodecylsulfanyl)carbonothioyl)sulfanyl)propanoic acid. Macromolecules 2011, 44, 221–229. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Zhu, Y.; Li, B.-G.; Zhu, S. Polystyrene-block-poly (n-butyl acrylate)-block-polystyrene triblock copolymer thermoplastic elastomer synthesized via raft emulsion polymerization. Macromolecules 2010, 43, 7472–7481. [Google Scholar] [CrossRef]
- Benoit, D.; Harth, E.; Fox, P.; Waymouth, R.M.; Hawker, C.J. Accurate structural control and block formation in the living polymerization of 1, 3-dienes by nitroxide-mediated procedures. Macromolecules 2000, 33, 363–370. [Google Scholar] [CrossRef]
- Szwarc, M.; Levy, M.; Milkovich, R. Polymerization initiated by electron transfer to monomer. A new method of formation of block polymers1. J. Am. Chem. Soc. 1956, 78, 2656–2657. [Google Scholar] [CrossRef]
- Szwarc, M. ‘Living’ polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Pitsikalis, M.; Pispas, S.; Mays, J.W.; Hadjichristidis, N. Nonlinear block copolymer architectures. In Blockcopolymers-Polyelectrolytes-Biodegradation; Springer: Berlin, Germany, 1998; 137p. [Google Scholar]
- Hirao, A.; Loykulnant, S.; Ishizone, T. Recent advance in living anionic polymerization of functionalized styrene derivatives. Prog. Polym. Sci. 2002, 27, 1399–1471. [Google Scholar] [CrossRef]
- Matsuo, A.; Watanabe, T.; Hirao, A. Synthesis of well-defined dendrimer-like branched polymers and block copolymer by the iterative approach involving coupling reaction of living anionic polymer and functionalization. Macromolecules 2004, 37, 6283–6290. [Google Scholar] [CrossRef]
- Advincula, R.; Zhou, Q.; Park, M.; Wang, S.; Mays, J.; Sakellariou, G.; Pispas, S.; Hadjichristidis, N. Polymer brushes by living anionic surface initiated polymerization on flat silicon (sio x) and gold surfaces: Homopolymers and block copolymers. Langmuir 2002, 18, 8672–8684. [Google Scholar] [CrossRef]
- Hirao, A.; Hayashi, M.; Haraguchi, N. Synthesis of well-defined functionalized polymers and star branched polymers by means of living anionic polymerization using specially designed 1,1-diphenylethylene derivatives. Macromol. Rapid Commun. 2000, 21, 1171–1184. [Google Scholar] [CrossRef]
- Zhou, Q.; Fan, X.; Xia, C.; Mays, J.; Advincula, R. Living anionic surface initiated polymerization (sip) of styrene from clay surfaces. Chem. Mater. 2001, 13, 2465–2467. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Feng, H.; Fu, Y.; Cheng, S.; Carroll, B.; Kumar, R.; Novikov, V.N.; Kisliuk, A.M.; Saito, T.; Kang, N.-G.; et al. Effect of chain rigidity on the decoupling of ion motion from segmental relaxation in polymerized ionic liquids: Ambient and elevated pressure studies. Macromolecules 2017, 50, 6710–6721. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Feng, H.; Diaz, M.; Ortiz, A.; Ortiz, I.; Knapik-Kowalczuk, J.; Vilas, M.; Verdía, P.; Tojo, E.; Saito, T. Revealing the charge transport mechanism in polymerized ionic liquids: Insight from high pressure conductivity studies. Chem. Mater. 2017. [Google Scholar] [CrossRef]
- Matsuo, Y.; Konno, R.; Ishizone, T.; Goseki, R.; Hirao, A. Precise synthesis of block polymers composed of three or more blocks by specially designed linking methodologies in conjunction with living anionic polymerization system. Polymers 2013, 5, 1012–1040. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef] [PubMed]
- Ekizoglou, N.; Hadjichristidis, N. Synthesis of model linear tetrablock quaterpolymers and pentablock quintopolymers of ethylene oxide. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2166–2170. [Google Scholar] [CrossRef]
- Bellas, V.; Iatrou, H.; Pitsinos, E.N.; Hadjichristidis, N. Heterofunctional linking agents for the synthesis of well-defined block copolymers of dimethylsiloxane and tert-butyl methacrylate or 2-vinylpyridine. Macromolecules 2001, 34, 5376–5378. [Google Scholar] [CrossRef]
- Iatrou, H.; Mays, J.W.; Hadjichristidis, N. Regular comb polystyrenes and graft polyisoprene/polystyrene copolymers with double branches (“centipedes”). Quality of (1,3-phenylene) bis (3-methyl-1-phenylpentylidene) dilithium initiator in the presence of polar additives. Macromolecules 1998, 31, 6697–6701. [Google Scholar] [CrossRef]
- Quirk, R.P.; Yoo, T.; Lee, Y.; Kim, J.; Lee, B. Applications of 1,1-diphenylethylene chemistry in anionic synthesis of polymers with controlled structures. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Berlin, Germany, 2000; pp. 67–162. [Google Scholar]
- Handlin, D.L.; Hansen, D.R.; Wright, K.J.; Trenor, S.R. Industrial applications. In Controlled and Living Polymerizations: From Mechanisms to Applications; John Wiley & Sons: Weinheim, Germany, 2010; pp. 555–603. [Google Scholar]
- Wang, H.; Lu, W.; Wang, W.; Shah, P.N.; Misichronis, K.; Kang, N.-G.; Mays, J.W. Design and synthesis of multigraft copolymer thermoplastic elastomers: Superelastomers. Macromol. Chem. Phys. 2017. [Google Scholar] [CrossRef]
- Aydogan, C.; Kutahya, C.; Allushi, A.; Yilmaz, G.; Yagci, Y. Block copolymer synthesis in one shot: Concurrent metal-free atrp and rop processes under sunlight. Polym. Chem. 2017, 8, 2899–2903. [Google Scholar] [CrossRef]
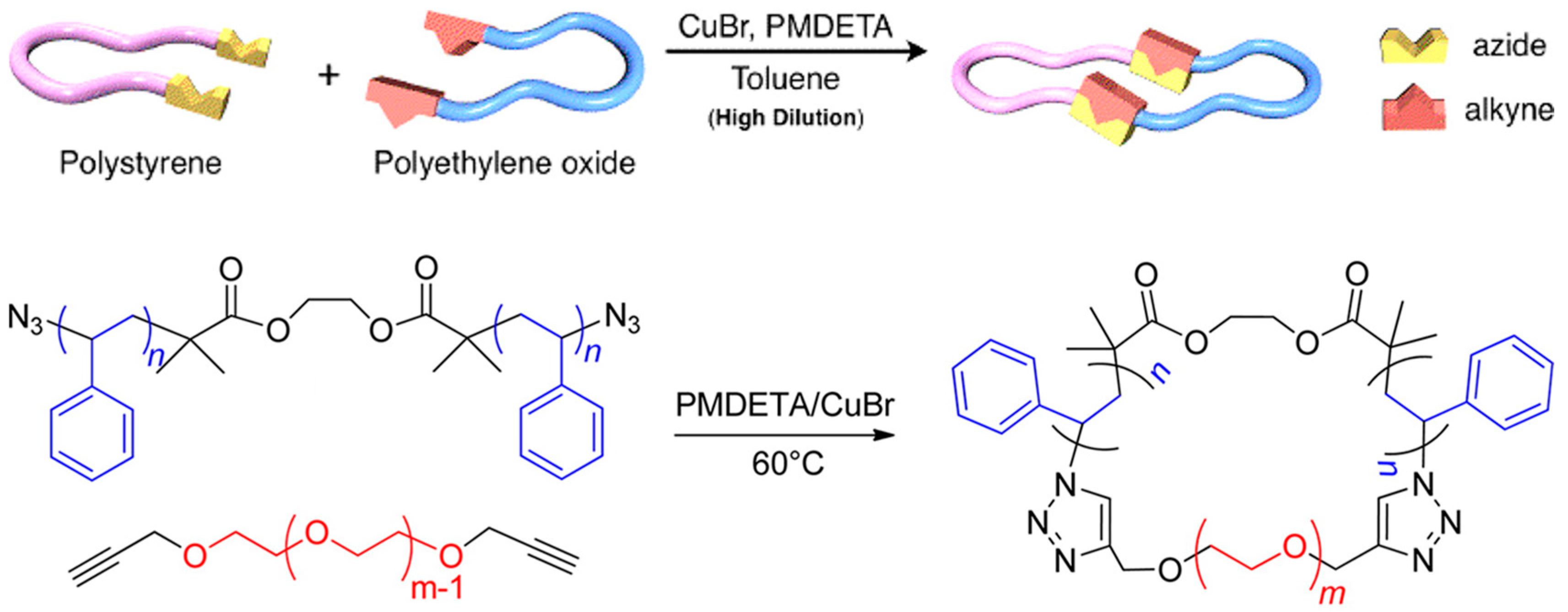
- Opsteen, J.A.; van Hest, J.C. Modular synthesis of block copolymers via cycloaddition of terminal azide and alkyne functionalized polymers. Chem. Commun. (Camb.) 2005, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tezuka, Y. Topological polymer chemistry: A cyclic approach toward novel polymer properties and functions. Polym. Chem. 2011, 2, 1930–1941. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (raft) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Inglis, A.J.; Sinnwell, S.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Reversible addition fragmentation chain transfer (raft) and hetero-diels–alder chemistry as a convenient conjugation tool for access to complex macromolecular designs. Macromolecules 2008, 41, 4120–4126. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.; Schubert, U.S. Supramolecular engineering with macromolecules: An alternative concept for block copolymers. Angew. Chem. Int. Ed. 2002, 41, 3825–3829. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, G.; Qiu, F.; Zhang, H.; Yang, Y.; Shi, A.-C. Discovering ordered phases of block copolymers: New results from a generic fourier-space approach. Phys. Rev. Lett. 2008, 101, 028301. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Plantenberg, T. From self-organizing polymers to nanohybrid and biomaterials. Angew. Chem. Int. Ed. 2002, 41, 688–714. [Google Scholar] [CrossRef]
- Kim, J.K.; Yang, S.Y.; Lee, Y.; Kim, Y. Functional nanomaterials based on block copolymer self-assembly. Prog. Polym. Sci. 2010, 35, 1325–1349. [Google Scholar] [CrossRef]
- Bates, F.S. Polymer-polymer phase behavior. Science 1991, 251, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Matsen, M.W.; Schick, M. Stable and unstable phases of a diblock copolymer melt. Phys. Rev. Lett. 1994, 72, 2660–2663. [Google Scholar] [CrossRef] [PubMed]
- Honeker, C.C.; Thomas, E.L. Impact of morphological orientation in determining mechanical properties in triblock copolymer systems. Chem. Mater. 1996, 8, 1702–1714. [Google Scholar] [CrossRef]
- DeRouchey, J.; Thurn-Albrecht, T.; Russell, T.; Kolb, R. Block copolymer domain reorientation in an electric field: An in-situ small-angle x-ray scattering study. Macromolecules 2004, 37, 2538–2543. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Baugh, D. Poly (styrene-b-isobutylene-b-styrene) block copolymers and ionomers therefrom: Morphology as determined by small-angle x-ray scattering and transmission electron microscopy. Polymer 2000, 41, 3205–3211. [Google Scholar] [CrossRef]
- Elabd, Y.A.; Napadensky, E.; Walker, C.W.; Winey, K.I. Transport properties of sulfonated poly (styrene-b-isobutylene-b-styrene) triblock copolymers at high ion-exchange capacities. Macromolecules 2006, 39, 399–407. [Google Scholar] [CrossRef]
- Goswami, M.; Sumpter, B.G.; Huang, T.; Messman, J.M.; Gido, S.P.; Isaacs-Sodeye, A.; Mays, J.W. Tunable morphologies from charged block copolymers. Soft Matter 2010, 6, 6146–6154. [Google Scholar] [CrossRef]
- Sanoja, G.E.; Popere, B.C.; Beckingham, B.S.; Evans, C.M.; Lynd, N.A.; Segalman, R.A. Structure–conductivity relationships of block copolymer membranes based on hydrated protic polymerized ionic liquids: Effect of domain spacing. Macromolecules 2016, 49, 2216–2223. [Google Scholar] [CrossRef]
- Patel, S.N.; Javier, A.E.; Beers, K.M.; Pople, J.A.; Ho, V.; Segalman, R.A.; Balsara, N.P. Morphology and thermodynamic properties of a copolymer with an electronically conducting block: Poly(3-ethylhexylthiophene)-block-poly (ethylene oxide). Nano Lett. 2012, 12, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.W.; Beaucage, P.A.; Sai, H.; Tan, K.W.; Werner, J.G.; Sethna, J.P.; DiSalvo, F.J.; Gruner, S.M.; Van Dover, R.B.; Wiesner, U. Block copolymer self-assembly-directed synthesis of mesoporous gyroidal superconductors. Sci. Adv. 2016, 2, e1501119. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Schulze, M.W.; Arora, A.; Lewis, R.M.; Hillmyer, M.A.; Dorfman, K.D.; Bates, F.S. Thermal processing of diblock copolymer melts mimics metallurgy. Science 2017, 356, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Changez, M.; Hong, K.; Mays, J.W.; Kang, N.-G. 2-isopropenyl-2-oxazoline: Well-defined homopolymers and block copolymers via living anionic polymerization. Macromolecules 2017, 50, 54–62. [Google Scholar] [CrossRef]
- Hamley, I.W. Nanostructure fabrication using block copolymers. Nanotechnology 2003, 14, R39–R54. [Google Scholar] [CrossRef]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-assembled block copolymer aggregates: From micelles to vesicles and their biological applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Eisenberg, A. Multiple morphologies and characteristics of “crew-cut” micelle-like aggregates of polystyrene-b-poly (acrylic acid) diblock copolymers in aqueous solutions. J. Am. Chem. Soc. 1996, 118, 3168–3181. [Google Scholar] [CrossRef]
- Wang, X.; Guerin, G.; Wang, H.; Wang, Y.; Manners, I.; Winnik, M.A. Cylindrical block copolymer micelles and co-micelles of controlled length and architecture. Science 2007, 317, 644–647. [Google Scholar] [CrossRef] [PubMed]
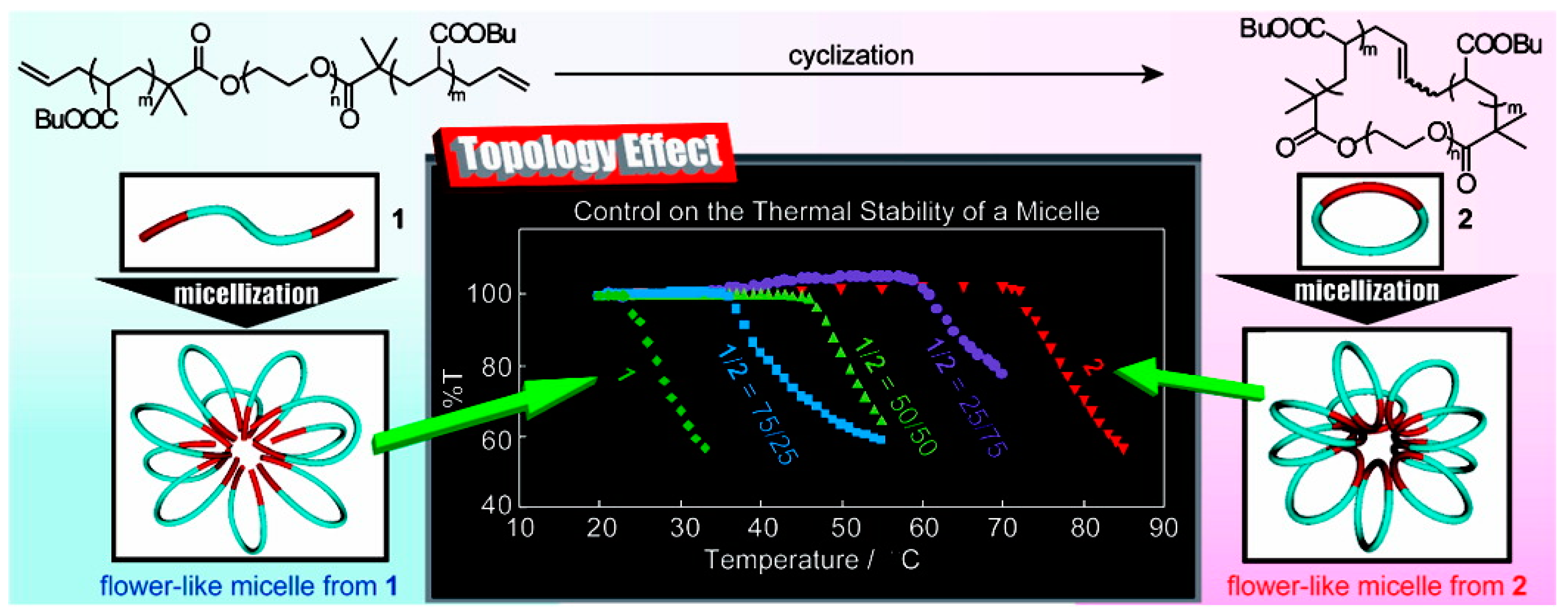
- Honda, S.; Yamamoto, T.; Tezuka, Y. Topology-directed control on thermal stability: Micelles formed from linear and cyclized amphiphilic block copolymers. J. Am. Chem. Soc. 2010, 132, 10251–10253. [Google Scholar] [CrossRef] [PubMed]
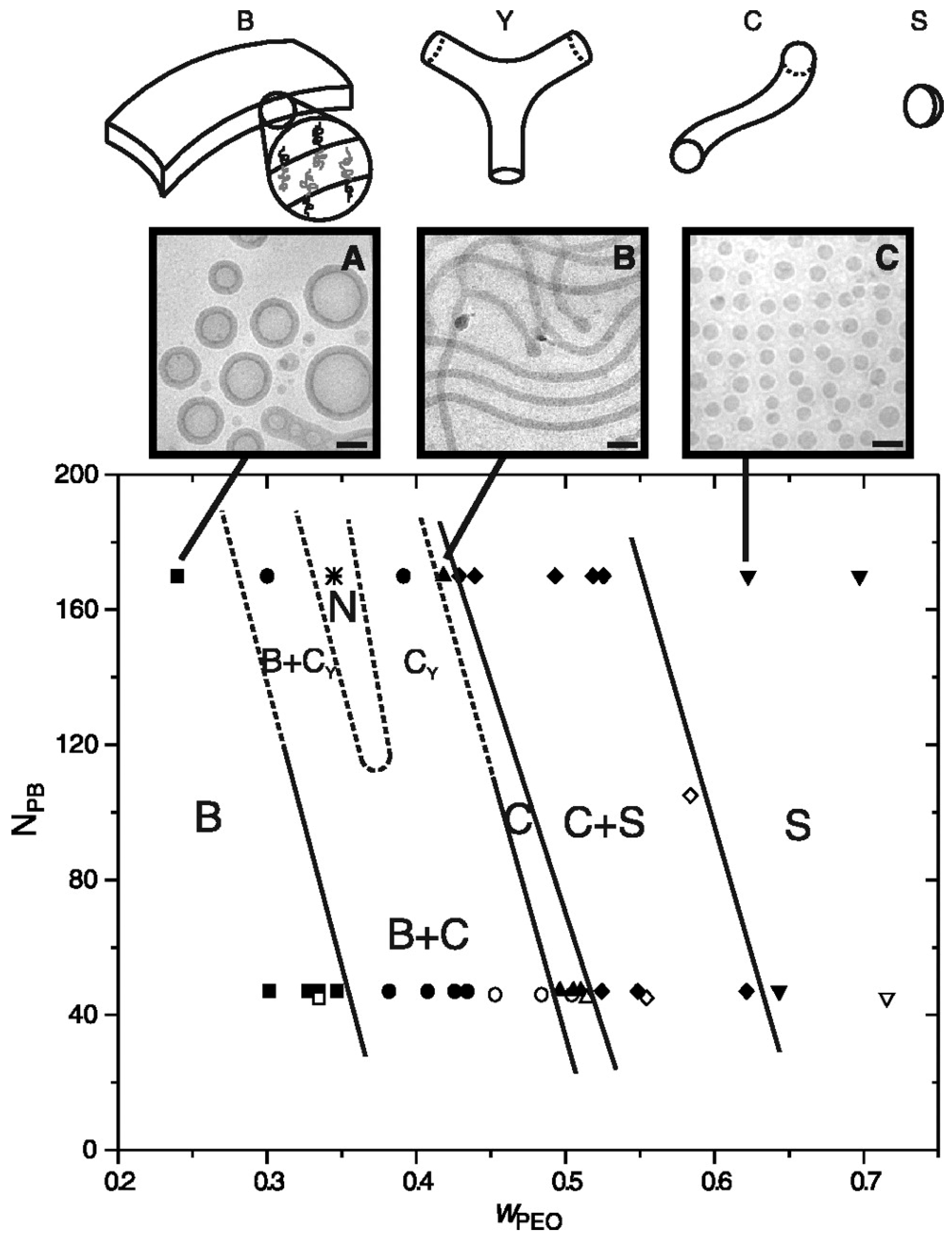
- Jain, S.; Bates, F.S. On the origins of morphological complexity in block copolymer surfactants. Science 2003, 300, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Novel Thermoplastic Elastomers Based on Benzofulvene: Synthesis and Mechanical Properties. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2015. [Google Scholar]
- Bhowmick, A.K.; Stephens, H. Handbook of Elastomers; CRC Press: New York, NY, USA, 2000. [Google Scholar]
- Hadjichristidis, N.; Pispas, S.; Floudas, G. Block Copolymers: Synthetic Strategies, Physical Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Xenidou, M.; Hadjichristidis, N. Synthesis of model multigraft copolymers of butadiene with randomly placed single and double polystyrene branches. Macromolecules 1998, 31, 5690–5694. [Google Scholar] [CrossRef]
- Fetters, L.J.; Morton, M. Synthesis and properties of block polymers. I. Poly-α-methylstyrene-polyisoprene-poly-α-methylstyrene. Macromolecules 1969, 2, 453–458. [Google Scholar] [CrossRef]
- Bolton, J.M.; Hillmyer, M.A.; Hoye, T.R. Sustainable thermoplastic elastomers from terpene-derived monomers. ACS Macro Lett. 2014, 3, 717–720. [Google Scholar] [CrossRef]
- Fetters, L.; Firer, E.; Dafauti, M. Synthesis and properties of block copolymers. 4. Poly(p-tert-butylstyrene-diene-p-tert-butylstyrene) and poly (p-tert-butylstyrene-isoprene-styrene). Macromolecules 1977, 10, 1200–1207. [Google Scholar] [CrossRef]
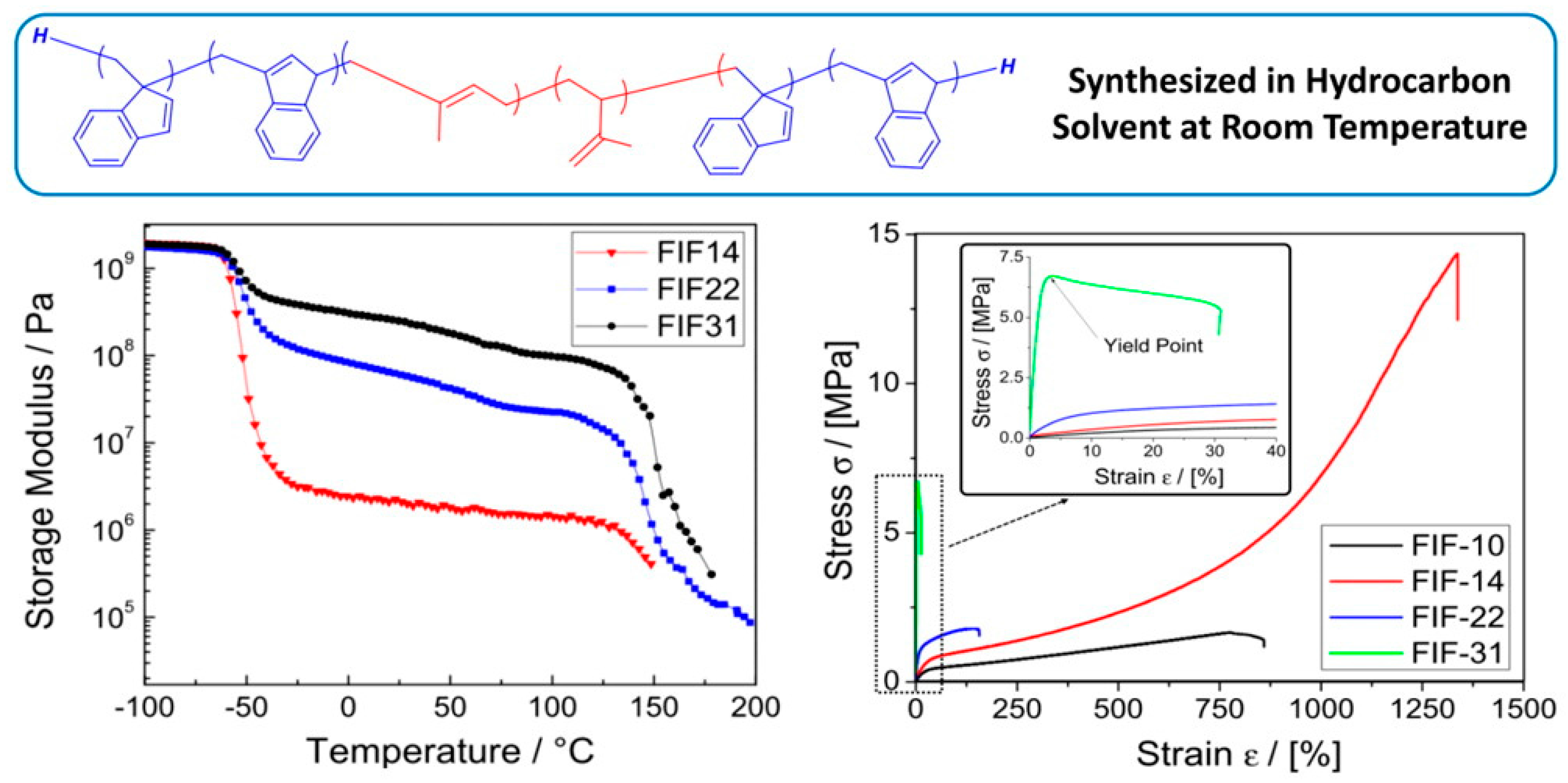
- Wang, W.Y.; Schlegel, R.; White, B.T.; Williams, K.; Voyloy, D.; Steren, C.A.; Goodwin, A.; Coughlin, E.B.; Gido, S.; Beiner, M.; et al. High temperature thermoplastic elastomers synthesized by living anionic polymerization in hydrocarbon solvent at room temperatuie. Macromolecules 2016, 49, 2646–2655. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Y.; Wang, W.; Cheng, S.; Zhu, J.; Xu, Y.; Hong, K.; Kang, N.-G.; Mays, J. All acrylic-based thermoplastic elastomers with high upper service temperature and superior mechanical properties. Polym. Chem. 2017, 8, 5741–5748. [Google Scholar] [CrossRef]
- Martín-Gomis, L.; Fernández-García, M.; de la Fuente, J.L.; Madruga, E.L.; Cerrada, M.L. Physical properties of PBMA-b-PBA-b-PBMA triblock copolymers synthesized by atom transfer radical polymerization. Macromol. Chem. Phys. 2003, 204, 2007–2016. [Google Scholar] [CrossRef]
- Tong, J.; Leclère, P.; Doneux, C.; Brédas, J.-L.; Lazzaroni, R.; Jérôme, R. Morphology and mechanical properties of poly (methylmethacrylate)-b-poly (alkylacrylate)-b-poly (methylmethacrylate). Polymer 2001, 42, 3503–3514. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Burgaz, E.; Gido, S.P.; Staudinger, U.; Weidisch, R.; Uhrig, D.; Mays, J.W. Morphology and tensile properties of multigraft copolymers with regularly spaced tri-, tetra-, and hexafunctional junction points. Macromolecules 2006, 39, 4428–4436. [Google Scholar] [CrossRef]
- Staudinger, U.; Weidisch, R.; Zhu, Y.; Gido, S.; Uhrig, D.; Mays, J.; Iatrou, H.; Hadjichristidis, N. Mechanical properties and hysteresis behaviour of multigraft copolymers. Macromol. Symp. 2006, 233, 42–50. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Lu, X.; Bobade, S.; Chen, J.; Kang, N.-G.; Zhang, Q.; Mays, J. Synthesis and characterization of comb and centipede multigraft copolymers PnBA-g-PS with high molecular weight using miniemulsion polymerization. Macromolecules 2014, 47, 7284–7295. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Li, H.; Lu, X.; Chen, J.; Kang, N.-G.; Zhang, Q.; Mays, J. Synthesis and characterization of graft copolymers poly (isoprene-g-styrene) of high molecular weight by a combination of anionic polymerization and emulsion polymerization. Ind. Eng. Chem. Res. 2015, 54, 1292–1300. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Cross-linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006, 35, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Thermo-sensitive polymeric micelles based on poly (n-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Lee, H.-N.; Bai, Z.; Newell, N.; Lodge, T.P. Micelle/inverse micelle self-assembly of a peo-pnipam block copolymer in ionic liquids with double thermoresponsivity. Macromolecules 2010, 43, 9522–9528. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Son, S.; Lee, D.S.; Park, J.H. Poly(ethylene glycol)-b-poly (lysine) copolymer bearing nitroaromatics for hypoxia-sensitive drug delivery. Acta Biomater. 2016, 29, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of peg–pla and peg–pcl: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Zupancich, J.A.; Bates, F.S.; Hillmyer, M.A. Aqueous dispersions of poly (ethylene oxide)-b-poly (γ-methyl-ε-caprolactone) block copolymers. Macromolecules 2006, 39, 4286–4288. [Google Scholar] [CrossRef]
- Meng, F.; Engbers, G.H.; Feijen, J. Biodegradable polymersomes as a basis for artificial cells: Encapsulation, release and targeting. J. Control. Release 2005, 101, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ghoroghchian, P.P.; Li, G.; Levine, D.H.; Davis, K.P.; Bates, F.S.; Hammer, D.A.; Therien, M.J. Bioresorbable vesicles formed through spontaneous self-assembly of amphiphilic poly(ethylene oxide)-block-polycaprolactone. Macromolecules 2006, 39, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Discher, B.M.; Bermudez, H.; Hammer, D.A.; Discher, D.E.; Won, Y.-Y.; Bates, F.S. Cross-linked polymersome membranes: Vesicles with broadly adjustable properties. J. Phys. Chem. B 2002, 106, 2848–2854. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, W.; Gao, H.; Hu, K.; Chen, J.; Zhang, C.; Gao, X.; Jiang, X.; Zhu, C. Preparation and brain delivery property of biodegradable polymersomes conjugated with ox26. J. Control. Release 2008, 128, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.M.; Maher, M.J.; Janes, D.W.; Ellison, C.J.; Willson, C.G. Block copolymer lithography. Macromolecules 2013, 47, 2–12. [Google Scholar] [CrossRef]
- Li, W.; Muller, M. Defects in the self-assembly of block copolymers and their relevance for directed self-assembly. Annu. Rev. Chem Biomol. 2015, 6, 187–216. [Google Scholar] [CrossRef] [PubMed]
- Seshimo, T.; Maeda, R.; Odashima, R.; Takenaka, Y.; Kawana, D.; Ohmori, K.; Hayakawa, T. Perpendicularly oriented sub-10-nm block copolymer lamellae by atmospheric thermal annealing for one minute. Sci. Rep. 2016, 6, 19481. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.J.; Rettner, C.T.; Bates, C.M.; Blachut, G.; Carlson, M.C.; Durand, W.J.; Ellison, C.J.; Sanders, D.P.; Cheng, J.Y.; Willson, C.G. Directed self-assembly of silicon-containing block copolymer thin films. ACS Appl. Mater. Interfaces 2015, 7, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.W.; McIntosh, L.D.; Hillmyer, M.A.; Lodge, T.P. High-modulus, high-conductivity nanostructured polymer electrolyte membranes via polymerization-induced phase separation. Nano Lett. 2013, 14, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Kennemur, J.G.; Hillmyer, M.A.; Bates, F.S. Synthesis, thermodynamics, and dynamics of poly (4-tert-butylstyrene-b-methyl methacrylate). Macromolecules 2012, 45, 7228–7236. [Google Scholar] [CrossRef]
- Keen, I.; Yu, A.; Cheng, H.-H.; Jack, K.S.; Nicholson, T.M.; Whittaker, A.K.; Blakey, I. Control of the orientation of symmetric poly (styrene)-block-poly (d,l-lactide) block copolymers using statistical copolymers of dissimilar composition. Langmuir 2012, 28, 15876–15888. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Shin, C.; Kim, E.; Ryu, D.Y.; Jeong, U.; Russell, T.P.; Hawker, C.J. Microdomain orientation of ps-b-pmma by controlled interfacial interactions. Macromolecules 2008, 41, 6431–6437. [Google Scholar] [CrossRef]
- Mansky, P.; Liu, Y.; Huang, E.; Russell, T.; Hawker, C. Controlling polymer-surface interactions with random copolymer brushes. Science 1997, 275, 1458–1460. [Google Scholar] [CrossRef]
- Mansky, P.; Russell, T.; Hawker, C.; Mays, J.; Cook, D.; Satija, S. Interfacial segregation in disordered block copolymers: Effect of tunable surface potentials. Phys. Rev. Lett. 1997, 79, 237. [Google Scholar] [CrossRef]
- Zhao, Y.; Sivaniah, E.; Hashimoto, T. Saxs analysis of the order-disorder transition and the interaction parameter of polystyrene-block-poly (methyl methacrylate). Macromolecules 2008, 41, 9948–9951. [Google Scholar] [CrossRef]
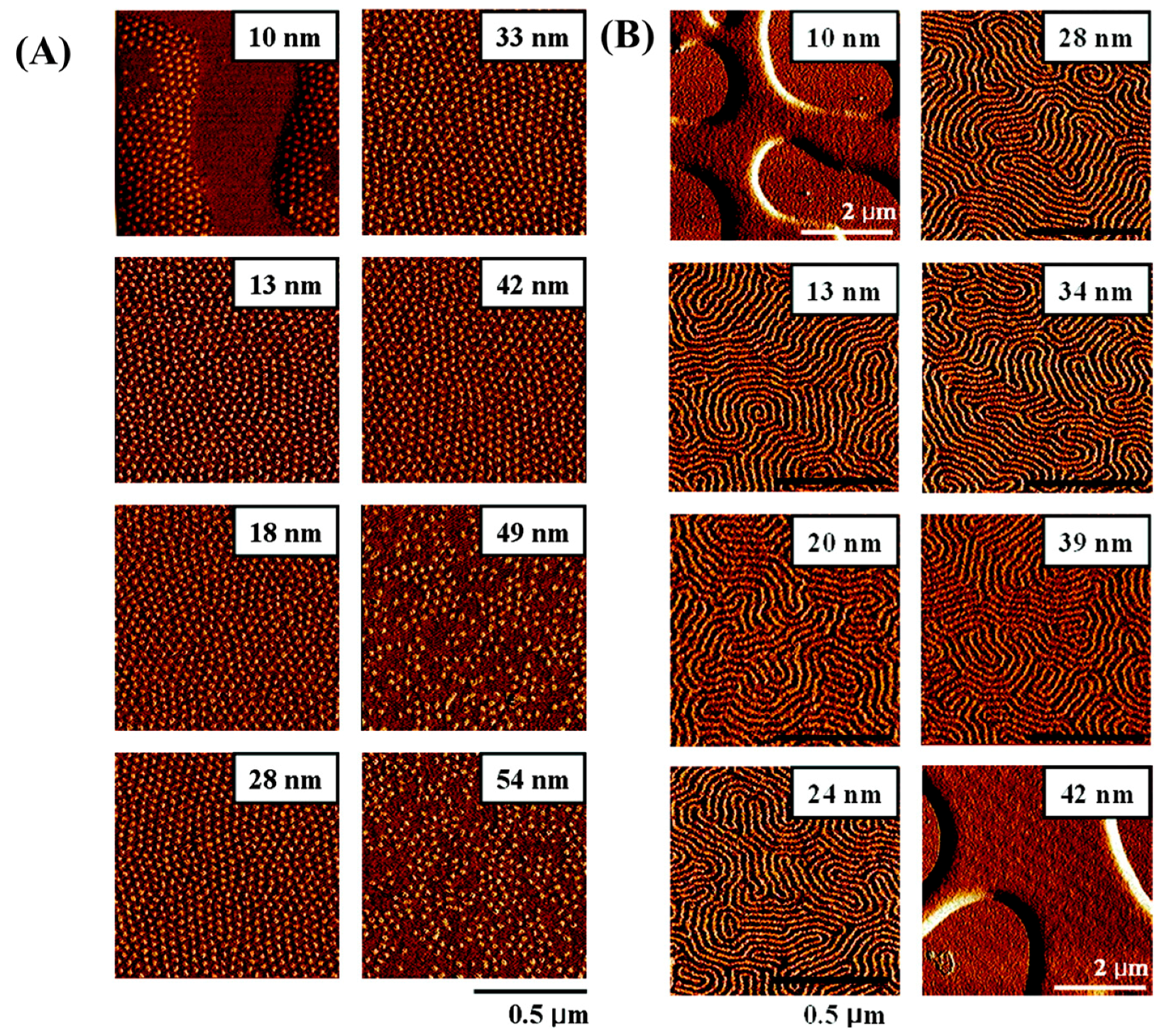
- Park, S.; Lee, D.H.; Xu, J.; Kim, B.; Hong, S.W.; Jeong, U.; Xu, T.; Russell, T.P. Macroscopic 10-terabit–per–square-inch arrays from block copolymers with lateral order. Science 2009, 323, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Rodwogin, M.D.; Spanjers, C.S.; Leighton, C.; Hillmyer, M.A. Polylactide-poly(dimethylsiloxane)-polylactide triblock copolymers as multifunctional materials for nanolithographic applications. ACS Nano 2010, 4, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Almdal, K.; Hillmyer, M.A.; Bates, F.S. Influence of conformational asymmetry on polymer-polymer interactions: An entropic or enthalpic effect? Macromolecules 2002, 35, 7685–7691. [Google Scholar] [CrossRef]
- Yoshida, H.; Suh, H.S.; Ramirez-Herunandez, A.; Lee, J.I.; Aida, K.; Wan, L.; Ishida, Y.; Tada, Y.; Ruiz, R.; de Pablo, J. Topcoat approaches for directed self-assembly of strongly segregating block copolymer thin films. J. Photopolym. Sci. Technol. 2013, 26, 55–58. [Google Scholar] [CrossRef]
- Cushen, J.D.; Otsuka, I.; Bates, C.M.; Halila, S.; Fort, S.; Rochas, C.; Easley, J.A.; Rausch, E.L.; Thio, A.; Borsali, R.; et al. Oligosaccharide/silicon-containing block copolymers with 5 nm features for lithographic applications. ACS Nano 2012, 6, 3424–3433. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.M.; Seshimo, T.; Maher, M.J.; Durand, W.J.; Cushen, J.D.; Dean, L.M.; Blachut, G.; Ellison, C.J.; Willson, C.G. Polarity-switching top coats enable orientation of sub-10-nm block copolymer domains. Science 2012, 338, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.-Y.; Liu, L.; Yuan, Z.-Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Kyotani, T. Templated nanocarbons for energy storage. Adv. Mater. 2012, 24, 4473–4498. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.; Qian, Y.; Stein, A. Porous electrode materials for lithium-ion batteries–how to prepare them and what makes them special. Adv. Energy Mater. 2012, 2, 1056–1085. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Torad, N.L.; Kimura, T.; Yamauchi, Y. Tailored design of functional nanoporous carbon materials toward fuel cell applications. Nano Today 2014, 9, 305–323. [Google Scholar] [CrossRef]
- Rolison, D.R. Catalytic nanoarchitectures—The importance of nothing and the unimportance of periodicity. Science 2003, 299, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Patiño, J.; Gutiérrez, M.; Carriazo, D.; Ania, C.; Fierro, J.; Ferrer, M.; Del Monte, F. Des assisted synthesis of hierarchical nitrogen-doped carbon molecular sieves for selective CO2 versus N2 adsorption. J. Mater. Chem. A 2014, 2, 8719–8729. [Google Scholar] [CrossRef]
- Feng, H.B.; Hong, T.; Mahurin, S.M.; Vogiatzis, K.D.; Gmernicki, K.R.; Long, B.K.; Mays, J.W.; Sokolov, A.P.; Kang, N.G.; Saito, T. Gas separation mechanism of co2 selective amidoxime-poly(1-trimethylsilyl-1-propyne) membranes. Polym. Chem. 2017, 8, 3341–3350. [Google Scholar] [CrossRef]
- Kamegawa, T.; Ishiguro, Y.; Seto, H.; Yamashita, H. Enhanced photocatalytic properties of tio 2-loaded porous silica with hierarchical macroporous and mesoporous architectures in water purification. J. Mater. Chem. A 2015, 3, 2323–2330. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, J.; Peng, X.; Cao, D.; Galaska, A.; Qiu, S.; Liu, J.; Khan, M.A.; Young, D.P.; Ryu, J.E.; et al. Poly(vinylidene fluoride) derived fluorine-doped magnetic carbon nanoadsorbents for enhanced chromium removal. Carbon 2017, 115, 503–514. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, Y.; Wei, J.; Sun, Z.; Gu, D.; Bongard, H.; Liu, C.; Wu, H.; Tu, B.; Schüth, F. Design of amphiphilic abc triblock copolymer for templating synthesis of large-pore ordered mesoporous carbons with tunable pore wall thickness. Chem. Mater. 2009, 21, 3996–4005. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, C.; Gu, D.; Yu, T.; Tu, B.; Zhao, D. Thick wall mesoporous carbons with a large pore structure templated from a weakly hydrophobic PEO-PMMA diblock copolymer. J. Mater. Chem. 2008, 18, 91–97. [Google Scholar] [CrossRef]
- Liang, C.; Hong, K.; Guiochon, G.A.; Mays, J.W.; Dai, S. Synthesis of a large-scale highly ordered porous carbon film by self-assembly of block copolymers. Angew. Chem. Int. Ed. 2004, 43, 5785–5789. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sun, Z.; Luo, W.; Li, Y.; Elzatahry, A.A.; Al-Enizi, A.M.; Deng, Y.; Zhao, D. New insight into the synthesis of large-pore ordered mesoporous materials. J. Am. Chem. Soc. 2017, 139, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Z.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 1998, 396, 152. [Google Scholar]
- Templin, M.; Franck, A.; Du Chesne, A.; Leist, H.; Zhang, Y.; Ulrich, R.; Schädler, V.; Wiesner, U. Organically modified aluminosilicate mesostructures from block copolymer phases. Science 1997, 278, 1795–1798. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhou, D.; Sun, Z.; Deng, Y.; Xia, Y.; Zhao, D. A controllable synthesis of rich nitrogen-doped ordered mesoporous carbon for CO2 capture and supercapacitors. Adv. Funct. Mater. 2013, 23, 2322–2328. [Google Scholar] [CrossRef]
- Wei, J.; Wang, H.; Deng, Y.; Sun, Z.; Shi, L.; Tu, B.; Luqman, M.; Zhao, D. Solvent evaporation induced aggregating assembly approach to three-dimensional ordered mesoporous silica with ultralarge accessible mesopores. J. Am. Chem. Soc. 2011, 133, 20369–20377. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wei, J.; Sun, Z.; Zhao, D. Large-pore ordered mesoporous materials templated from non-pluronic amphiphilic block copolymers. Chem. Soc. Rev. 2013, 42, 4054–4070. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, J.; Li, C.; Li, Y.; Tade, M.O.; Dai, S.; Yamauchi, Y. Synthesis of nitrogen-doped mesoporous carbon spheres with extra-large pores through assembly of diblock copolymer micelles. Angew. Chem. Int. Ed. 2015, 54, 588–593. [Google Scholar] [CrossRef]
- Du, H.; Gan, L.; Li, B.; Wu, P.; Qiu, Y.; Kang, F.; Fu, R.; Zeng, Y. Influences of mesopore size on oxygen reduction reaction catalysis of pt/carbon aerogels. J. Phys. Chem. C 2007, 111, 2040–2043. [Google Scholar] [CrossRef]
- Ma, G.; Yan, X.; Li, Y.; Xiao, L.; Huang, Z.; Lu, Y.; Fan, J. Ordered nanoporous silica with periodic 30–60 nm pores as an effective support for gold nanoparticle catalysts with enhanced lifetime. J. Am. Chem. Soc. 2010, 132, 9596–9597. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Cai, Y.; Sun, Z.; Gu, D.; Wei, J.; Li, W.; Guo, X.; Yang, J.; Zhao, D. Controlled synthesis and functionalization of ordered large-pore mesoporous carbons. Adv. Funct. Mater. 2010, 20, 3658–3665. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, T.; Wan, Y.; Shi, Y.; Meng, Y.; Gu, D.; Zhang, L.; Huang, Y.; Liu, C.; Wu, X. Ordered mesoporous silicas and carbons with large accessible pores templated from amphiphilic diblock copolymer poly (ethylene oxide)-b-polystyrene. J. Am. Chem. Soc. 2007, 129, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T. Colloidal templating fabrication of aluminum-organophosphonate films using high molecular weight PS-b-PEO. Chem. Asian J. 2011, 6, 3236–3242. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of sba-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, J.; Liu, C.; Gu, D.; Sun, Z.; Wei, J.; Zhang, J.; Zhang, L.; Tu, B.; Zhao, D. Ultra-large-pore mesoporous carbons templated from poly (ethylene oxide)-b-polystyrene diblock copolymer by adding polystyrene homopolymer as a pore expander. Chem. Mater. 2008, 20, 7281–7286. [Google Scholar] [CrossRef]
- Yu, K.; Hurd, A.J.; Eisenberg, A.; Brinker, C.J. Syntheses of silica/polystyrene-block-poly (ethylene oxide) films with regular and reverse mesostructures of large characteristic length scales by solvent evaporation-induced self-assembly. Langmuir 2001, 17, 7961–7965. [Google Scholar] [CrossRef]
- Garcia, B.C.; Kamperman, M.; Ulrich, R.; Jain, A.; Gruner, S.M.; Wiesner, U. Morphology diagram of a diblock copolymer–aluminosilicate nanoparticle system. Chem. Mater. 2009, 21, 5397–5405. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Lu, X.; Wang, W.; Kang, N.-G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. https://doi.org/10.3390/polym9100494
Feng H, Lu X, Wang W, Kang N-G, Mays JW. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers. 2017; 9(10):494. https://doi.org/10.3390/polym9100494
Chicago/Turabian StyleFeng, Hongbo, Xinyi Lu, Weiyu Wang, Nam-Goo Kang, and Jimmy W. Mays. 2017. "Block Copolymers: Synthesis, Self-Assembly, and Applications" Polymers 9, no. 10: 494. https://doi.org/10.3390/polym9100494



