A Facile and Effective Method to Fabricate Superhydrophobic/Superoeophilic Surface for the Separation of Both Water/Oil Mixtures and Water-in-Oil Emulsions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of Superhydrophobic/Superoleophilic Surfaces
2.3. Preparation of Water-in-Oil Emulsions
2.4. Setup of Water-in-Oil Emulsion Separation
2.5. Characterization
3. Results and Discussion
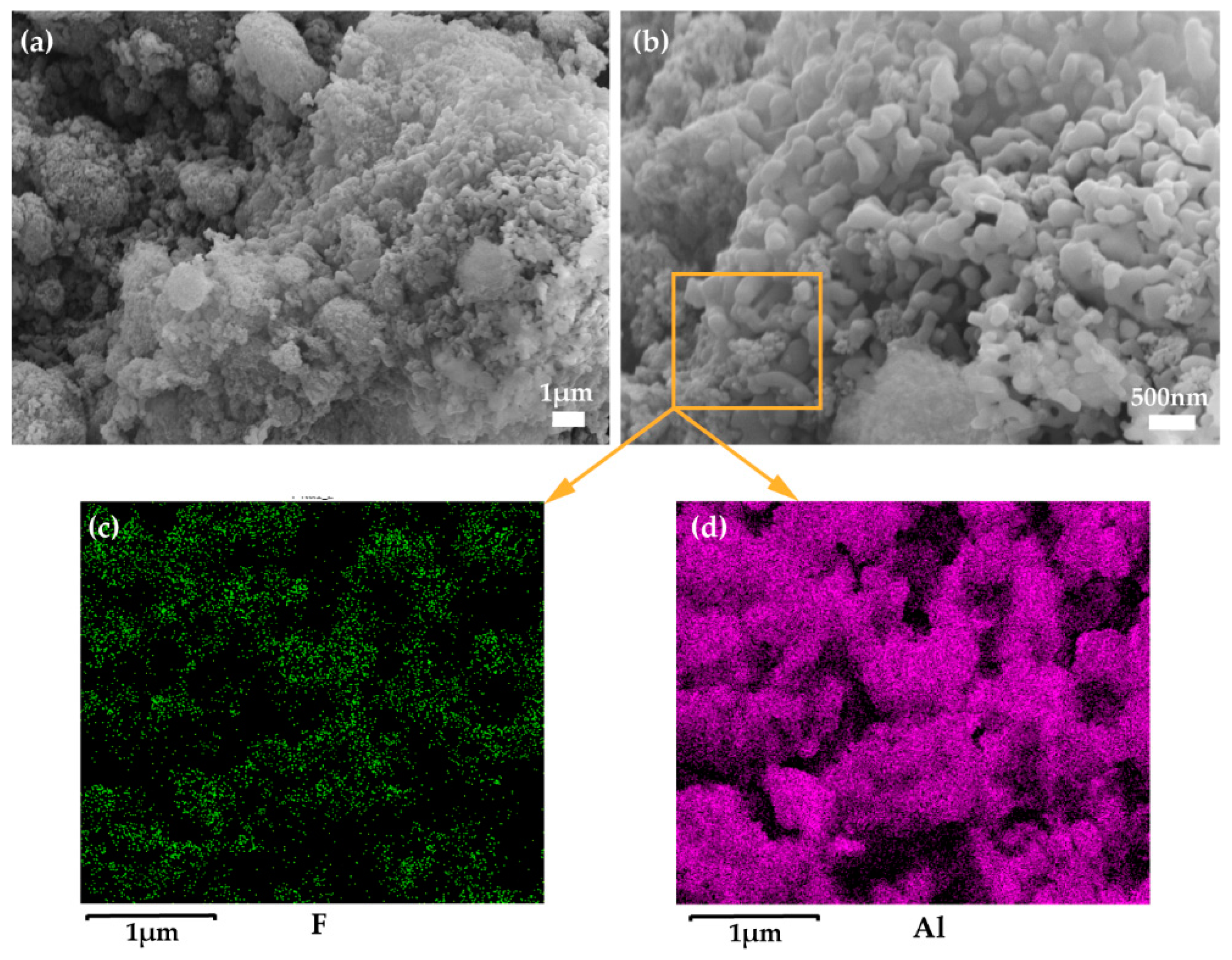
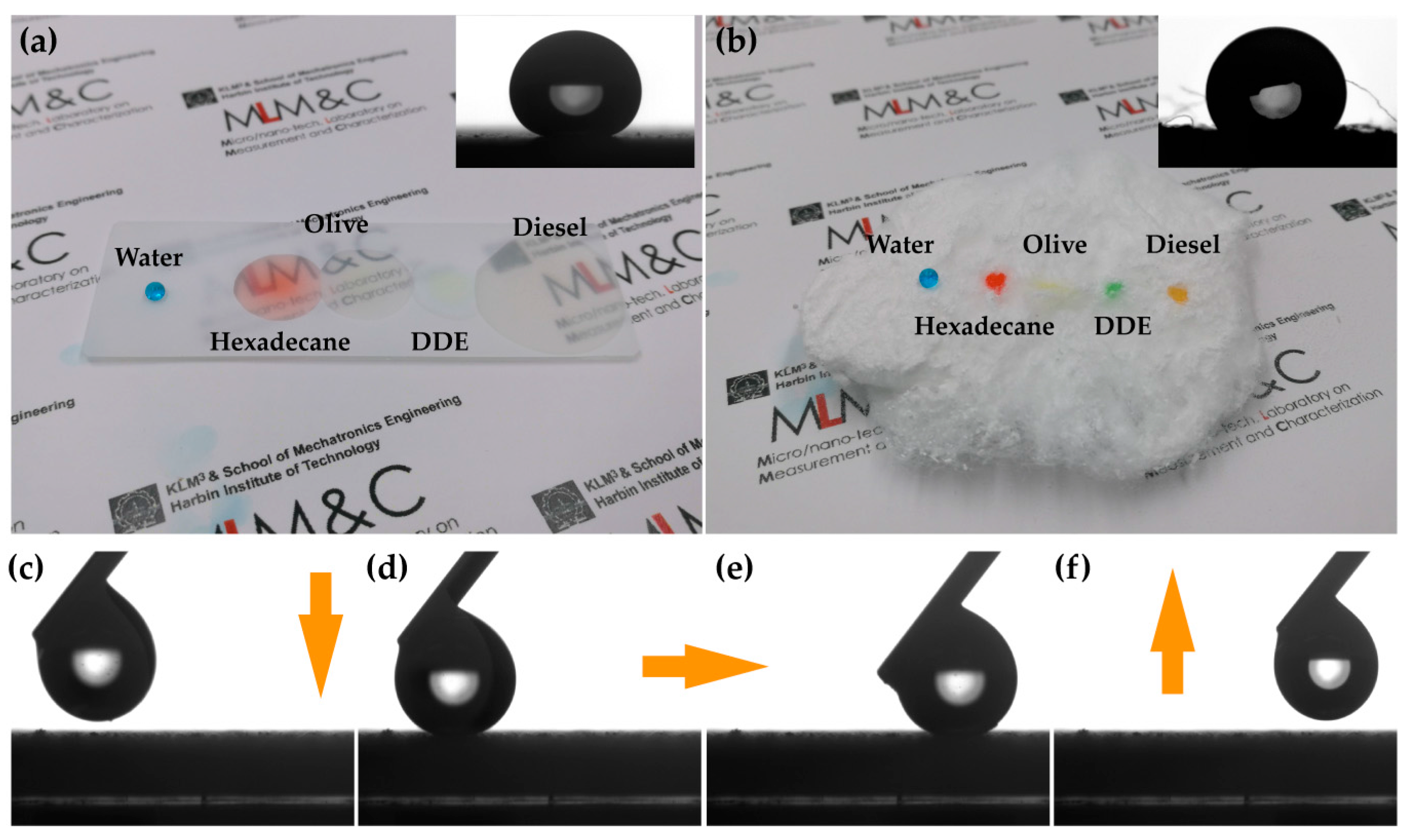
3.1. The Characterization of the Superhydrophobic/Superoleophilic Surface
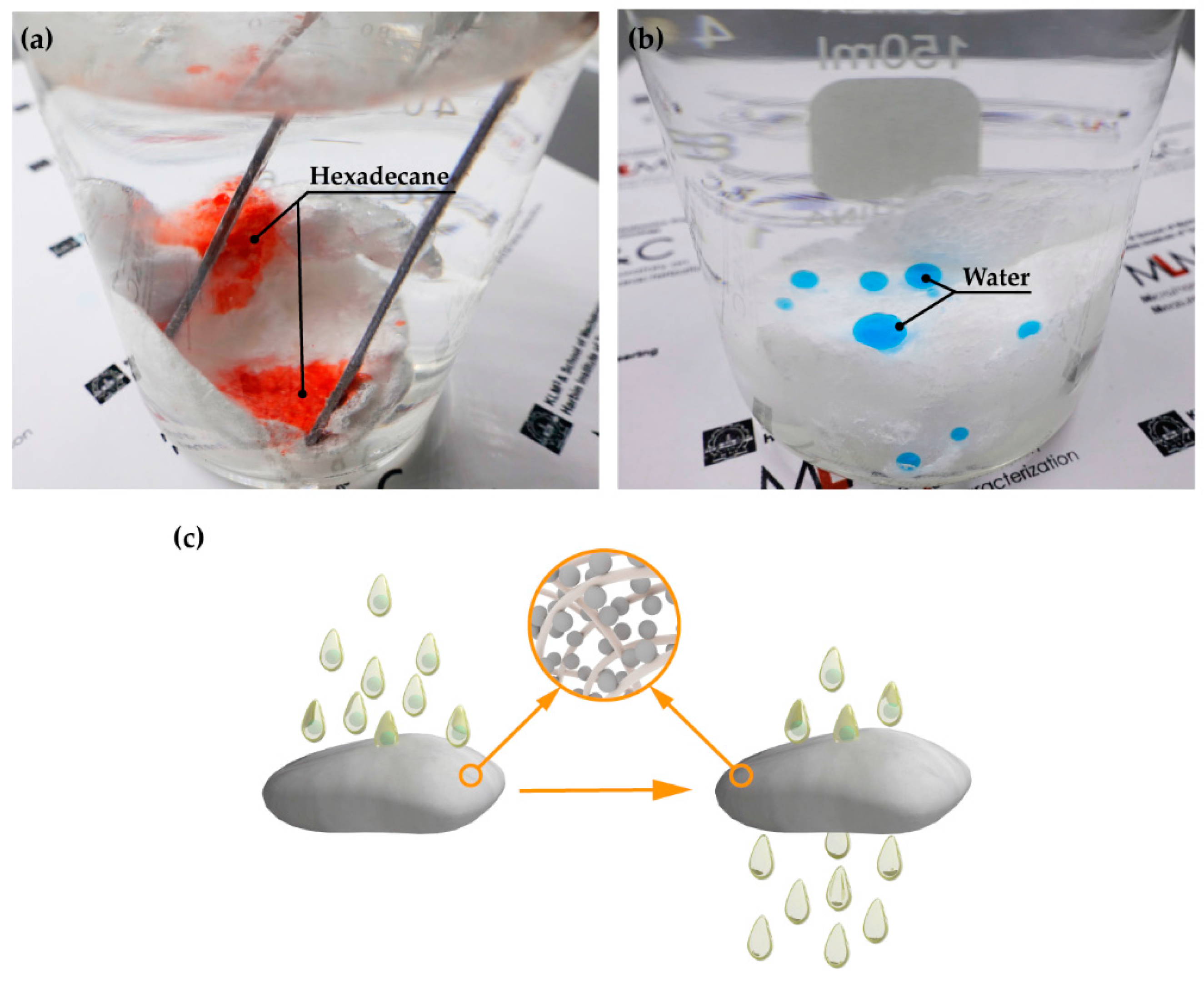
3.2. Separation of Oil/Water Mixtures
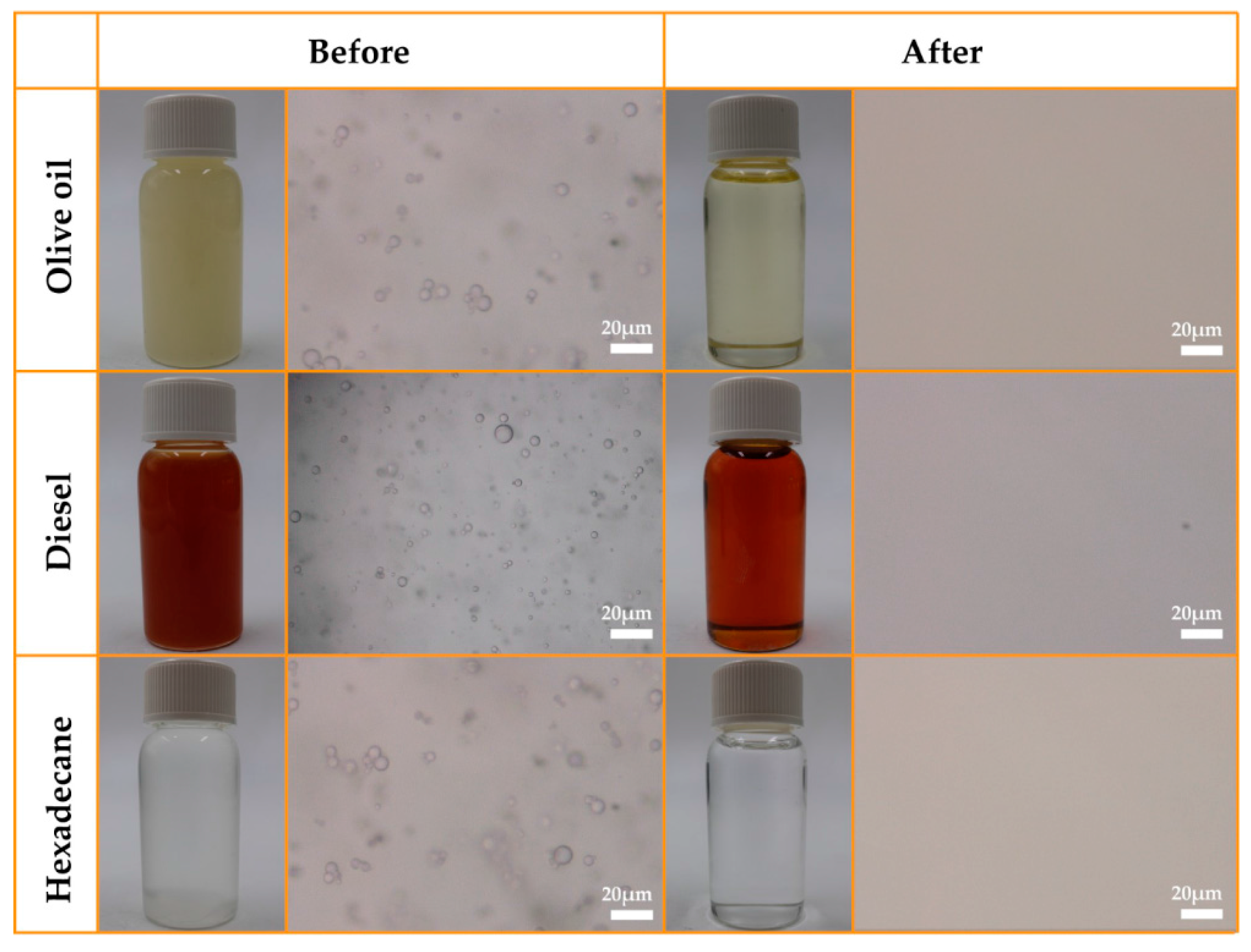
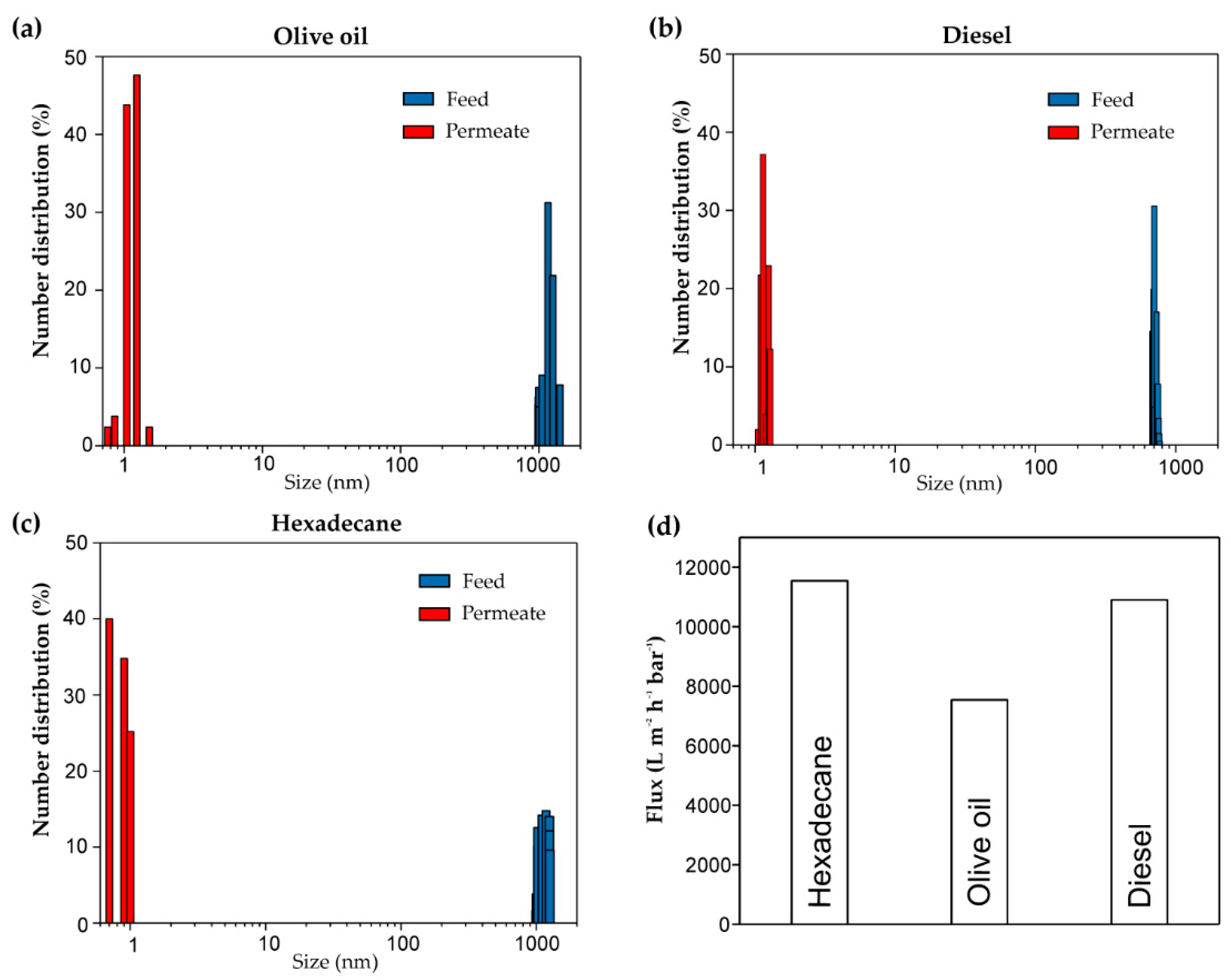
3.3. Demulsification of Surfactant-Stabilized Water-in-Oil Emulsions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, B.; Zhang, Y.; Shi, L.; Li, J.; Guo, Z. Advances in the theory of superhydrophobic surfaces. J. Mater. Chem. 2012, 22, 20112–20127. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Bioinspired multiscale surfaces with special wettability. MRS Bull. 2013, 38, 375–382. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, X.; Bin, J.; Wang, M.; Peng, C.; Xing, S.; Xiao, J.; Zeng, J.; Chen, H. Controllable fabrication of lotus-leaf-like superhydrophobic surface on copper foil by self-assembly. Appl. Phys. A 2014, 116, 1613–1620. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Tuukka, V.; Chris, B.; Piers, A.; Sami, F.; Olli, I.; Ras, R.H.A. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2011, 23, 673. [Google Scholar] [CrossRef]
- Bhushan, B.; Yong, C.J. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Shi, L.; Li, J.; Guo, Z. Recent progress of double-structural and functional materials with special wettability. J. Mater. Chem. 2011, 22, 799–815. [Google Scholar] [CrossRef]
- Lau, K.K.; Bico, J.; Teo, K.B.; Chhowalla, M.; Amaratunga, G.A.; Milne, W.I.; McKinley, G.H.; Gleason, K.K. Superhydrophobic carbon nanotube forests. Nano Lett. 2003, 3, 1701–1705. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Erbil, H.Y.; Demirel, A.L.; Avci, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. 2015, 54, 2328. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Yao, T.; Wang, C.; Lin, Q.; Li, X.; Chen, X.; Wu, J.; Zhang, J.; Yu, K.; Yang, B. Fabrication of flexible superhydrophobic films by lift-up soft-lithography and decoration with ag nanoparticles. Nanotechnology 2009, 20, 065304. [Google Scholar] [CrossRef] [PubMed]
- Ferchichi, A.K.; Panabière, M.; Desplats, O.; Gourgon, C. Fabrication of superhydrophobic surfaces on flexible fluorinated foils by using dual-scale patterning. Mater. Res. Express 2014, 1, 025704. [Google Scholar] [CrossRef]
- Zahner, D.; Abagat, J.; Svec, F.; Fréchet, J.M.; Levkin, P.A. A facile approach to superhydrophilic-superhydrophobic patterns in porous polymer films. Adv. Mater. 2011, 23, 3030–3034. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Tuominen, M.T.; Rothstein, J.P. Hierarchical superhydrophobic surfaces fabricated by dual-scale electron-beam-lithography with well-ordered secondary nanostructures. Adv. Funct. Mater. 2011, 21, 3715–3722. [Google Scholar] [CrossRef]
- Li, X.M.; Reinhoudt, D.; Cregocalama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 38, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Thieme, M.; Streller, F.; Simon, F.; Frenzel, R.; White, A.J. Superhydrophobic aluminium-based surfaces: Wetting and wear properties of different CVD-generated coating types. Appl. Surf. Sci. 2013, 283, 1041–1050. [Google Scholar] [CrossRef]
- Vilaró, I.; Yagüe, J.L.; Borros, S. Superhydrophobic copper surfaces with anti-corrosion properties fabricated by solventless CVD methods. ACS Appl. Mater. Interfaces 2016, 9. [Google Scholar] [CrossRef]
- Rahmawan, Y.; Xu, L.; Yang, S. Self-assembly of nanostructures towards transparent, superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv. Funct. Mater. 2015, 21, 4699–4704. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, K.; Park, J.; Hwang, B.G.; Ko, Y.; Kim, H.; Han, J.; Seo, E.; Park, Y.; Lee, S.J. Nearly perfect durable superhydrophobic surfaces fabricated by a simple one-step plasma treatment. Sci. Rep. 2017, 7, 1981. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blázquez, J.P.; Fell, D.; Bonaccurso, E.; Del, C.A. Superhydrophilic and superhydrophobic nanostructured surfaces via plasma treatment. J. Colloid Interface Sci. 2011, 357, 234. [Google Scholar] [CrossRef] [PubMed]
- Barshilia, H.C.; Ananth, A.; Gupta, N.; Anandan, C. Superhydrophobic nanostructured kapton® surfaces fabricated through Ar + O2 plasma treatment: Effects of different environments on wetting behaviour. Appl. Surf. Sci. 2013, 268, 464–471. [Google Scholar] [CrossRef]
- Ellinas, K.; Tsougeni, K.; Petrou, P.S.; Boulousis, G.; Tsoukleris, D.; Pavlatou, E.; Tserepi, A.; Kakabakos, S.E.; Gogolides, E. Three-dimensional plasma micro–nanotextured cyclo-olefin-polymer surfaces for biomolecule immobilization and environmentally stable superhydrophobic and superoleophobic behavior. Chem. Eng. J. 2016, 300, 394–403. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Travlos, A.; Psycharis, V.P.; Gogolides, E. Superhydrophobic paper by facile and fast atmospheric pressure plasma etching. Plasma Process. Polym. 2016, 14. [Google Scholar] [CrossRef]
- Ellinas, K.; Chatzipetrou, M.; Zergioti, I.; Tserepi, A.; Gogolides, E. Superamphiphobic polymeric surfaces sustaining ultrahigh impact pressures of aqueous high- and low-surface-tension mixtures, tested with laser-induced forward transfer of drops. Adv. Mater. 2015, 27, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Song, J.; Liu, Z.; Liu, J.; Zheng, H.; Huang, S.; Sun, J.; Xu, W.; Liu, X. Atmospheric pressure plasma functionalized polymer mesh: An environment-friendly and efficient tool for oil/water separation. ACS Sustain. Chem. Eng. 2016, 4. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Satulu, V.; Ionita, M.D.; Vizireanu, S.; Mitu, B.; Dinescu, G. Plasma processing with fluorine chemistry for modification of surfaces wettability. Molecules 2016, 21, 1711. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Wang, C.F.; Yang, S.Y.; Kuo, S.W. Eco-friendly superwetting material for highly effective separations of oil/water mixtures and oil-in-water emulsions. Sci. Rep. 2017, 7, 43053. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Khan, F.A.; Baik, S. Janus graphene oxide sponges for high-purity fast separation of both water-in-oil and oil-in-water emulsions. ACS Appl. Mater. Interfaces 2017, 9, 16694. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Ge, B.; Men, X.; Xue, Q. A versatile and efficient approach to separate both surfactant-stabilized water-in-oil and oil-in-water emulsions. Sep. Purif. Technol. 2017, 176, 1–7. [Google Scholar] [CrossRef]
- Duan, C.; Zhu, T.; Guo, J.; Wang, Z.; Liu, X.; Wang, H.; Xu, X.; Jin, Y.; Zhao, N.; Xu, J. Smart enrichment and facile separation of oil from emulsions and mixtures by superhydrophobic/superoleophilic particles. ACS Appl. Mater. Interfaces 2015, 7, 10475. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro-responsive membranes for effective oil–water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Kota, A.K.; Li, Y.; Sohani, A.; Mabry, J.M.; Tuteja, A. On-demand separation of oil-water mixtures. Adv. Mater. 2012, 24, 3666. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Wetting behavior of water and oil droplets in three-phase interfaces for hydrophobicity/philicity and oleophobicity/philicity. J. Surf. Colloids 2009, 25, 14165. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- De Gennes, P.G.; Brochard-Wyart, F.; Quéré, D. Special Interfaces. In Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves; Springer: New York, NY, USA, 2013. [Google Scholar]
- Dufour, R.; Perry, G.; Harnois, M.; Coffinier, Y.; Thomy, V.; Senez, V.; Boukherroub, R. From micro to nano reentrant structures: Hysteresis on superomniphobic surfaces. Colloid Polym. Sci. 2013, 291, 409–415. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Mabry, J.M.; Mckinley, G.H.; Cohen, R.E. Robust omniphobic surfaces. Proc. Natl. Acad. Sci. USA 2008, 105, 18200. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S.; Fragouli, D.; Martorana, P.J.; Martiradonna, L.; Cingolani, R.; Athanassiou, A. Solvent resistant superhydrophobic films from self-emulsifying carnauba wax-alcohol emulsions. Soft Matter 2011, 7, 7939–7943. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-based aerogel membrane for robust oil-in-water emulsion separation. ACS Appl. Mater. Interfaces 2015, 7, 24957–24962. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jia, S.; Wang, S.; Yan, Q.; Yan, N.; Wang, Q.; Meng, T. A facile and novel emulsion for efficient and convenient fabrication of durable superhydrophobic materials. Chem. Eng. J. 2017. [Google Scholar] [CrossRef]
- Bayer, I.S.; Steele, A.; Martorana, P.J.; Loth, E.; Miller, L. Superhydrophobic cellulose-based bionanocomposite films from pickering emulsions. Appl. Phys. Lett. 2009, 94, 1100. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An intelligent superwetting pvdf membrane showing switchable transport performance for oil/water separation. Adv. Mater. 2014, 26, 2943. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, W.; Zhang, F.; Liu, X.; Wang, D.; Jin, J.; Jiang, L. Ultrafast Separation of Emulsified Oil/Water Mixtures by Ultrathin Free-Standing Single-Walled Carbon Nanotube Network Films. Adv. Mater. 2013, 25, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Liu, F.; Pan, Q. Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J. Mater. Chem. A 2013, 1, 5386–5393. [Google Scholar] [CrossRef]

| Water-in-Oil Emulsion | Density of Feed (g/cm3) | Density of Filtrate (g/cm3) | Density of Pure Oil (g/cm3) |
|---|---|---|---|
| Water-in-n-octane | 0.982 | 0.700 | 0.703 |
| Water-in-n-dodecane | 0.984 | 0.753 | 0.750 |
| Water-in-hexadecane | 0.985 | 0.772 | 0.770 |
| Water-in-diesel | 0.990 | 0.840 | 0.840 |
| Water-in-olive oil | 0.996 | 0.925 | 0.920 |
| Water-in-dichloroethane | 1.012 | 1.263 | 1.260 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wang, Z.; Pan, Y.; Zhao, X. A Facile and Effective Method to Fabricate Superhydrophobic/Superoeophilic Surface for the Separation of Both Water/Oil Mixtures and Water-in-Oil Emulsions. Polymers 2017, 9, 563. https://doi.org/10.3390/polym9110563
Li F, Wang Z, Pan Y, Zhao X. A Facile and Effective Method to Fabricate Superhydrophobic/Superoeophilic Surface for the Separation of Both Water/Oil Mixtures and Water-in-Oil Emulsions. Polymers. 2017; 9(11):563. https://doi.org/10.3390/polym9110563
Chicago/Turabian StyleLi, Feiran, Ziran Wang, Yunlu Pan, and Xuezeng Zhao. 2017. "A Facile and Effective Method to Fabricate Superhydrophobic/Superoeophilic Surface for the Separation of Both Water/Oil Mixtures and Water-in-Oil Emulsions" Polymers 9, no. 11: 563. https://doi.org/10.3390/polym9110563





