Polycondensation Resins by Flavonoid Tannins Reaction with Amines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reactions
- (1)
- Catechin (0.5 g) was mixed with 0.5 g of hexamethylenediamine (HMDA) (70% solution in water). Three samples were prepared with the proportions above. Then, each sample was reacted in an oven at 65, 100, and 185 °C overnight, respectively.
- (2)
- Catechin (0.5 g) was mixed with 0.5 g of HMDA (70% solution in water) and 0.15 g of a 65 wt % aqueous solution of p-toluenesulfonic acid (pTSA). Again, three samples were prepared with the proportions above, and they were reacted in an oven at 65, 100, and 185 °C overnight, respectively.
- (3)
- Catechin (0.5 g) was mixed with 0.5 g of HMDA (70% solution in water) and 0.15 g of a 33 wt % aqueous solution of NaOH. Three samples were prepared with the proportions above. After that, they were reacted in an oven at 65, 100, and 185 °C overnight, respectively.
- (4)
- Mimosa tannin (2 g) was mixed with 2 g of hexamethylenediamine (HMDA) (70% solution in water). Three samples were prepared with the proportions above, and they were reacted in an oven at 65, 100, and 185 °C overnight, respectively.
- (5)
- Mimosa tannin (2 g) was mixed with 2 g of HMDA (70% solution in water) and 0.6 g of a 65 wt % aqueous solution p-toluenesulfonic acid (pTSA). Three samples were prepared with the proportions above. Then, they were reacted in an oven at 65, 100, and 185 °C overnight, respectively.
- (6)
- Mimosa tannin (2 g) was mixed with 2 g of HMDA (70% solution in water) and 0.6 g of a 33 wt % aqueous solution NaOH. Again, three samples were prepared with the proportions above, and they were reacted in an oven at 65, 100, and 185 °C overnight, respectively.
2.2. Matrix-Assisted Laser Desorption Ionisation Time-of-Flight (MALDI-ToF) Mass Spectrometry Analysis
2.3. CP-MAS 13C NMR
3. Results and Discussion
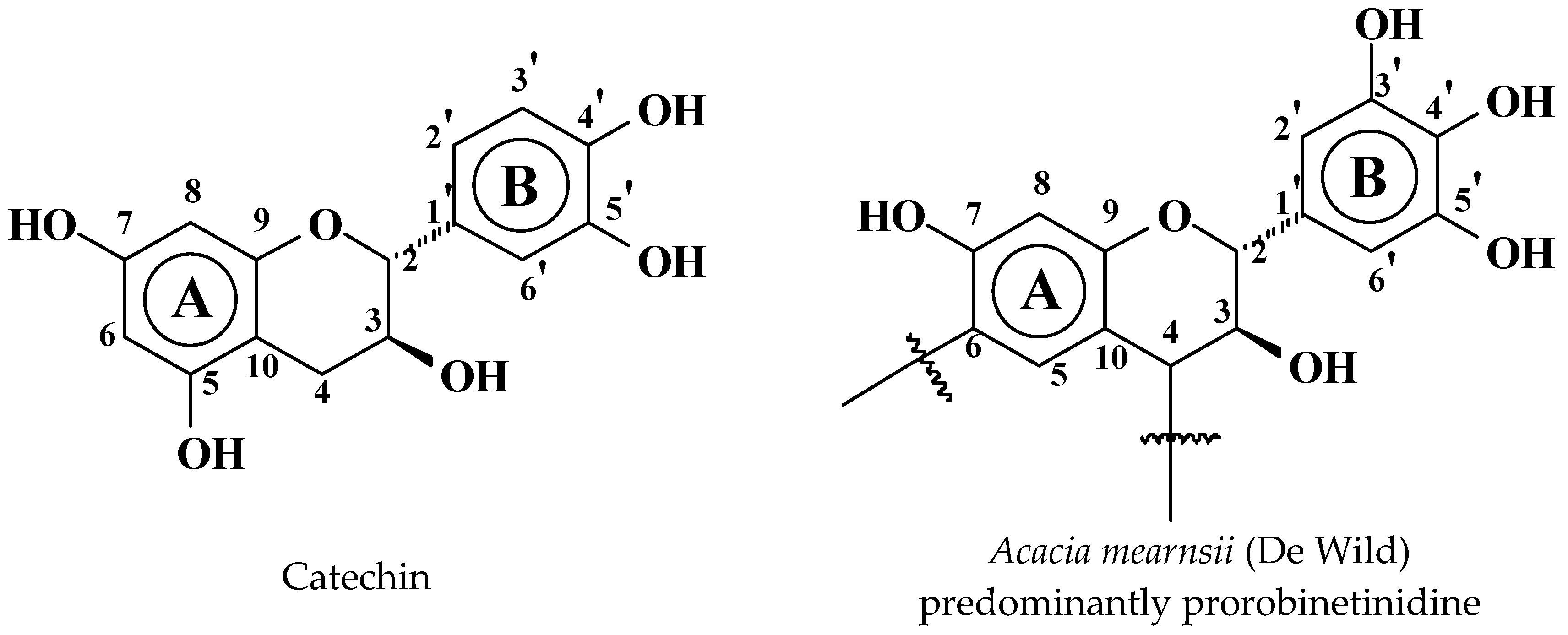
3.1. Reactions of Catechin with Hexamethylene Diamine
3.1.1. MALDI-ToF
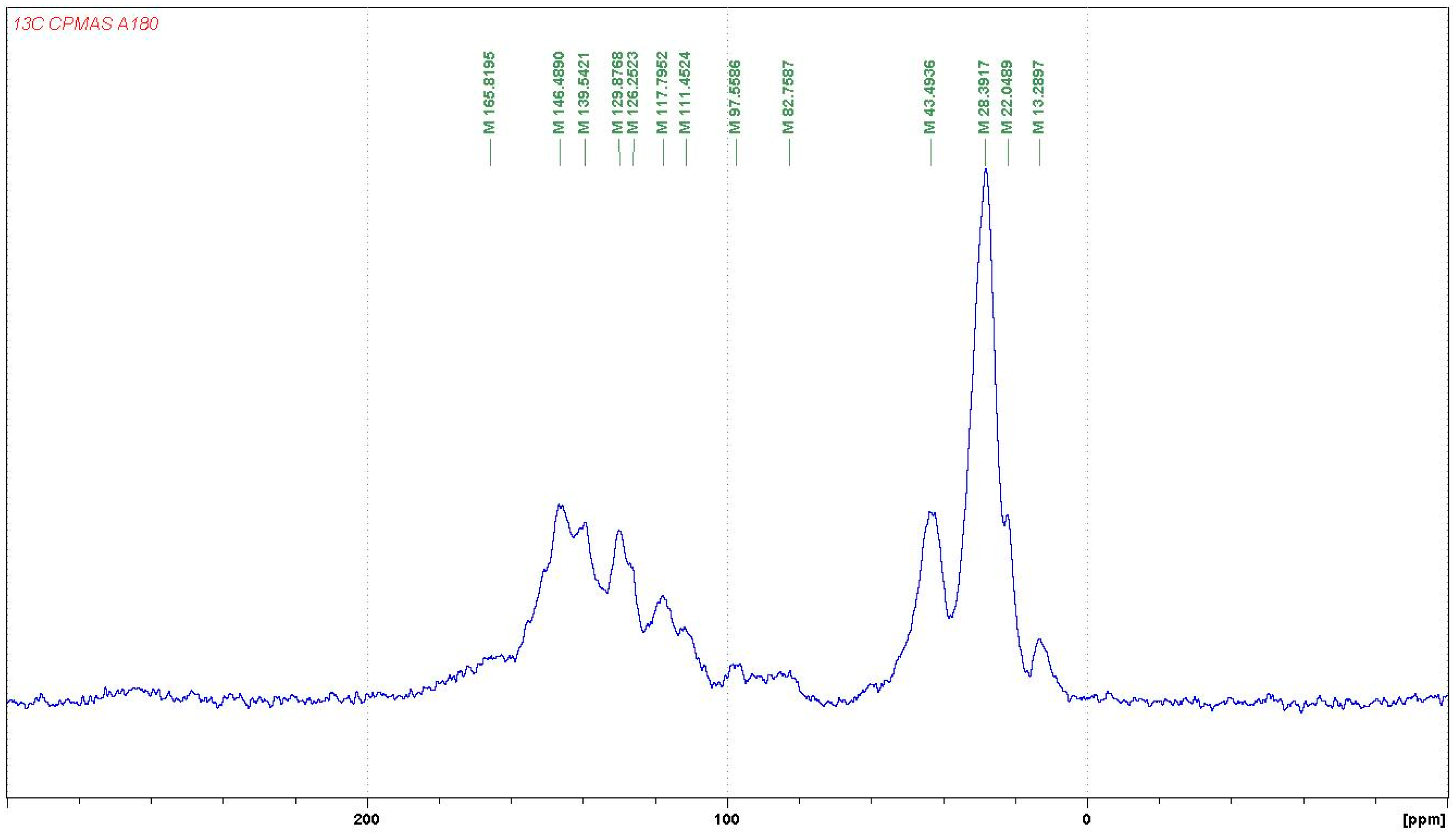
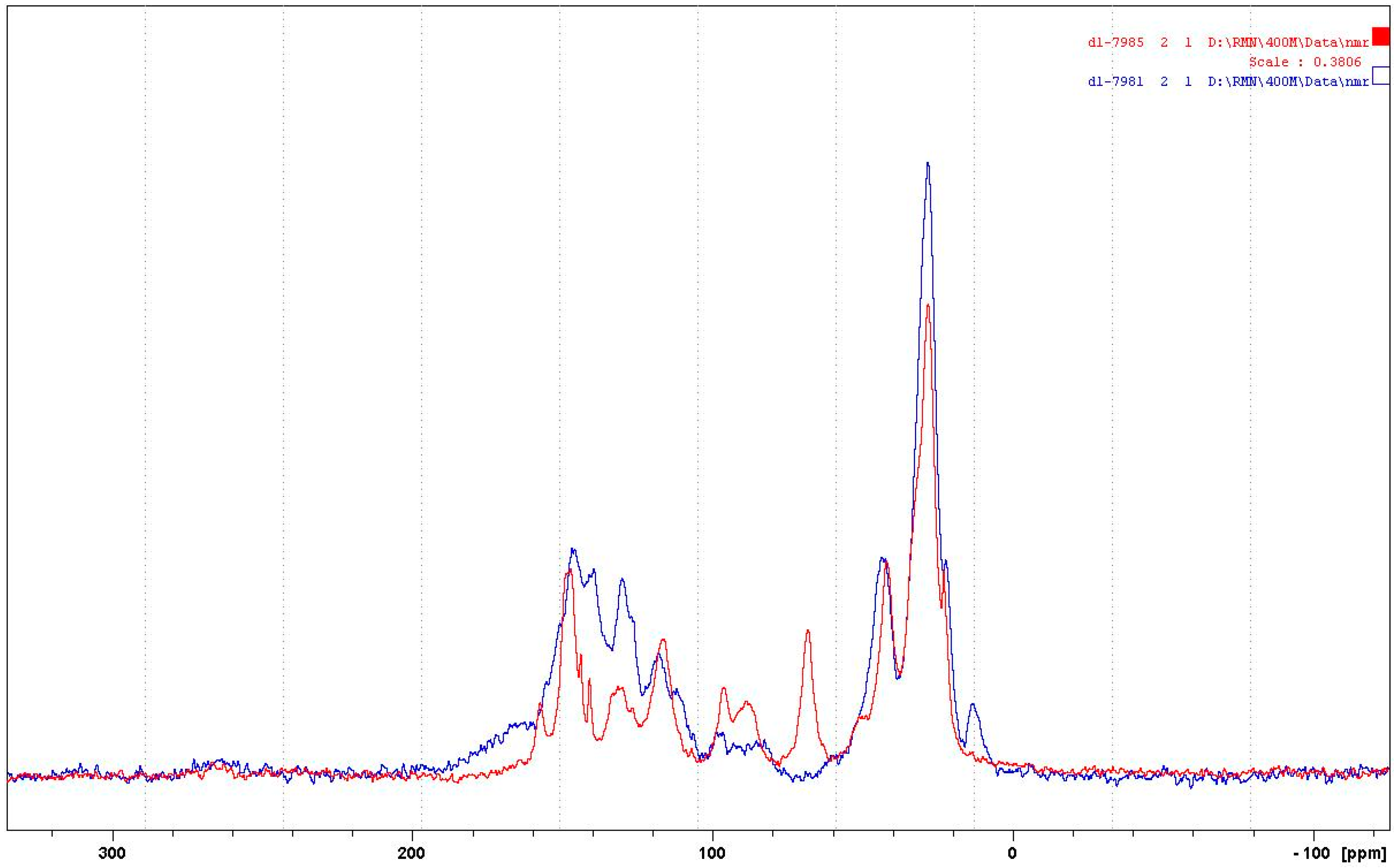
3.1.2. CP-MAS 13C NMR
3.2. Reaction of Mimosa Tannin Extract with Hexamethylene Diamine
4. Conclusions
- -
- At 185 °C, there are covalent bonds between the amine and the A- and B-rings of the catechin and also with the aliphatic C3 site of the catechin. The A-ring appears to react first, before the B-rings.
- -
- At 185 °C, there is a high proportion of ionic bonds between the amine and the A- and B-rings of the catechin, while presence of some unreacted amine cannot be excluded.
- -
- At 100 °C, the proportion of ionic bonds appears to predominate.
- -
- At 100 °C, there are covalent bonds between the amine and the A- and B-rings of the catechin for the pTSA-catalysed reaction (reaction A), with the A-rings having reacted more.
- -
- At 100 °C, there are covalent bonds between the amine and the A-rings of the catechin for the NaOH-catalysed reaction, but less or even no reaction on the B-ring. There are even fewer covalent bonds in the uncatalysed reaction.
- -
- At 180 °C, and to a lesser extent also at 100 °C, mimosa tannin reacting with hexamethylene diamine forms hard, condensed solids, be it uncatalysed or alkali- or acid-catalysed.
- -
- At 180 °C, the condensation solid formed is hardly soluble in acetone water.
- -
- The reaction of mimosa tannin or similar condensed tannins with a diamine is fast.
- -
- In regard to the influence of different catalysts, their influence is minimal other than to accelerate the reaction. To this purpose, reactions at three different temperatures (but without any catalysts) were done with similar results, and the analysis results are shown in the Supplementary Material.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pizzi, A. Tannin-based wood adhesives. In Wood Adhesives Chemistry and Technology; Pizzi, A., Ed.; Marcel Dekker: New York, NY, USA, 1983; pp. 177–246. Available online: https://www.amazon.fr/Wood-Adhesives-Pizzi/dp/0824715799/ref=sr_1_5?s=english-books&ie=UTF8&qid=1482513734&sr=1-5&keywords=Wood+Adhesives+Chemistry+and+Technology (accessed on November 1983).
- Pizzi, A. Advanced Wood Adhesives Technology; Marcel Dekker: New York, NY, USA, 1994; pp. 1–304. Available online: https://books.google.fr/books?hl=fr&lr=&id=xpZVVkiaQDsC&oi=fnd&pg=PA19&dq=2.%09Pizzi,+A.+Advanced+wood+adhesives+technology,+Marcel+Dekker,+New+York,+1994&ots=9ktO_3O1NO&sig=3mcurFkvpjL9SrStZElkRavCI1Y#v=onepage&q=2.%09Pizzi%2C%20A.%20Advanced%20wood%20adhesives%20technology%2C%20Marcel%20Dekker%2C%20New%20York%2C%201994&f=false (accessed on August 1994).
- Garcia, R.; Pizzi, A. Polycondensation and autocondensation networks in polyflavonoid tannins. I. Final networks. J. Appl. Polym. Sci. 1998, 70, 1083–1091. [Google Scholar] [CrossRef]
- Garcia, R.; Pizzi, A. Polycondensation and autocondensation networks in polyflavonoid tannins. II. Polycondensation vs. Autocondensation. J. Appl. Polym. Sci. 1998, 70, 1093–1110. [Google Scholar] [CrossRef]
- Masson, E.; Merlin, A.; Pizzi, A. Comparative kinetics of the induced radical autocondensation of polyflavonoid tannins. I. Modified and non-modified tannins. J. Appl. Polym. Sci. 1996, 60, 263–269. [Google Scholar] [CrossRef]
- Masson, E.; Pizzi, A.; Merlin, A. Comparative kinetics of the induced radical autocondensation of polyflavonoid tannins. III. Micellar reactions vs. cellulose surface catalysis. J. Appl. Polym. Sci. 1996, 60, 1655–1664. [Google Scholar] [CrossRef]
- Masson, E.; Pizzi, A.; Merlin, A. Comparative kinetics of: The induced radical autocondensation of polyflavonoid tannins. II. Flavonoid units effects. J. Appl. Polym. Sci. 1997, 64, 243–265. [Google Scholar] [CrossRef]
- Itoh, M.; Hattori, H.; Tanabe, K. The acidic properties of TiO2-SiO2 and its catalytic activities for the amination of phenol, the hydration of ethylene and the isomerization of butane. J. Catal. 1974, 35, 225–231. [Google Scholar] [CrossRef]
- Iranpoor, N.; Panahi, F. Direct Nickel-catalyzed amination of phenols via C-O Bond activation using 2,4,6-Trichloro-1,3,5-triazine (TCT) as reagent. Adv. Synth. Catal. 2014, 356, 3067–3073. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Zhang, P.; Wu, J. Direct amination of phenols under metal-free conditions. Synlett 2013, 24, 1448–1454. [Google Scholar]
- Kim, H.J.; Kim, J.; Cho, S.H.; Chang, S. Intermolecular oxidative C-N bond formation under metal-free conditions: Control of chemoselectivity between aryl sp2 and benzylic sp3 C-H Bond imidation. J. Am. Chem. Soc. 2011, 133, 16382–16385. [Google Scholar] [CrossRef] [PubMed]
- Hashida, K.; Makino, R.; Ohara, S. Amination of pyrogallol nucleus of condensed tannins and related polyphenols by ammonia water treatment. Holzforschung 2009, 63, 319–326. [Google Scholar] [CrossRef]
- Kida, K.; Suzuki, M.; Takagaki, A.; Nanjo, F. Deodorizing effects of tea catechins on amines and ammonia. Biosci. Biotechnol. Biochem. 2002, 66, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, F.; Fierro, V.; Pizzi, A.; Rode, K.; Radke, W.; Delmotte, L.; Parmentier, J.; Celzard, A. Condensation reactions of flavonoid tannins with ammonia. Ind. Crop. Prod. 2013, 44, 330–335. [Google Scholar] [CrossRef]
- Binev, Y.; Marques, M.M.; Aires-de-Sousa, J. Prediction of 1H NMR coupling constants with associative neural networks trained for chemical shifts. J. Chem. Inf. Model. 2007, 47, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Hemingway, R.W. Condensed tannins: The formation of a diarylpropanol-catechinic acid dimer from base-catalyzed reactions of (+)-catechin. J. Wood Chem. Technol. 1991, 11, 195–208. [Google Scholar] [CrossRef]
- Hashida, K.; Ohara, S.; Makino, R. Base-catalyzed reactions of (−)-epicatechin: Formation of enantiomers of base-catalyzed reaction products from (+)-catechin. J. Wood Chem. Technol. 2003, 23, 227–232. [Google Scholar] [CrossRef]
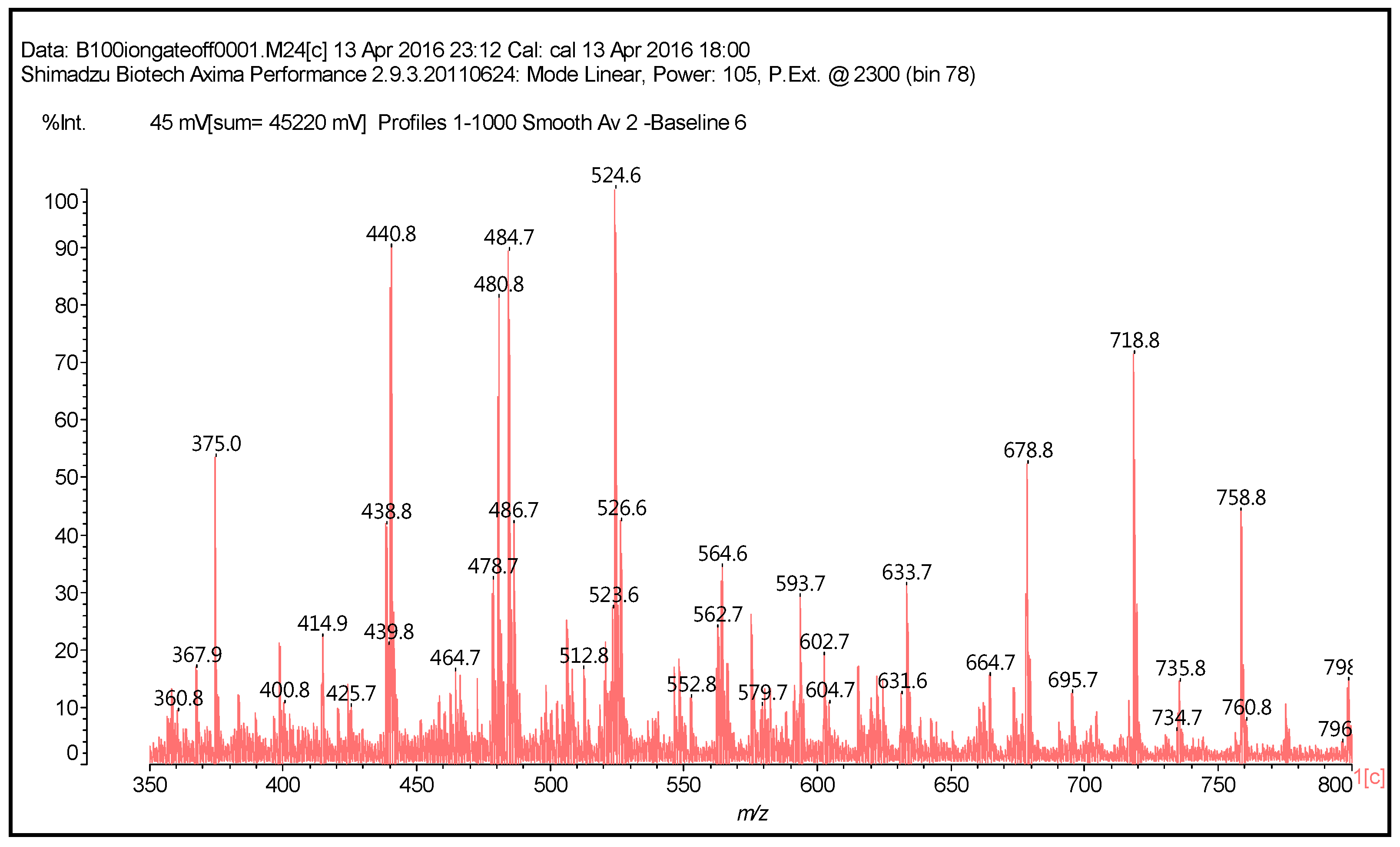


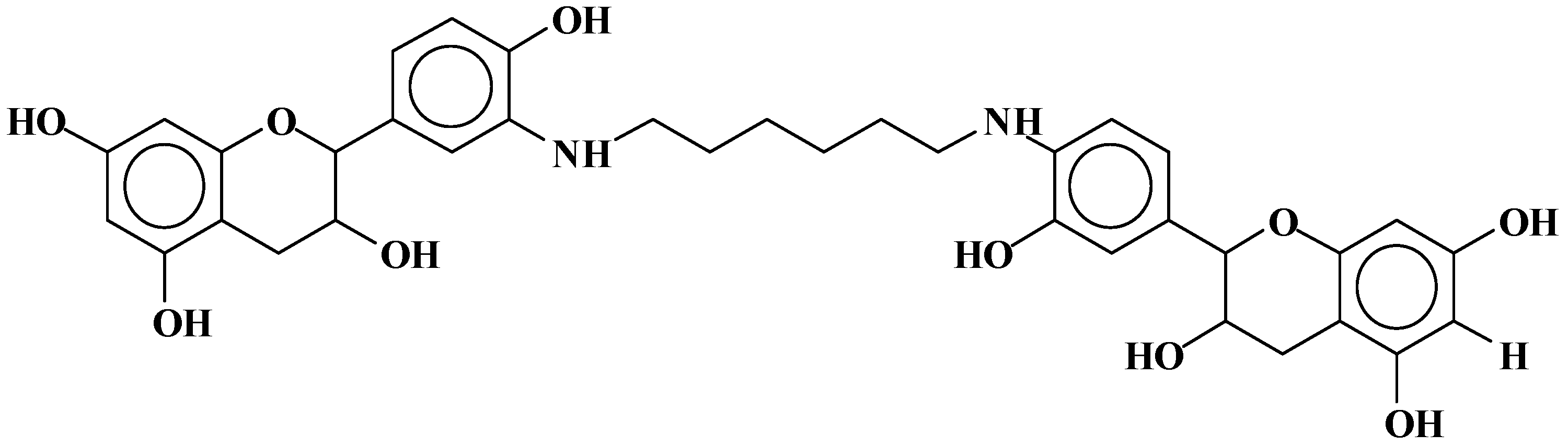
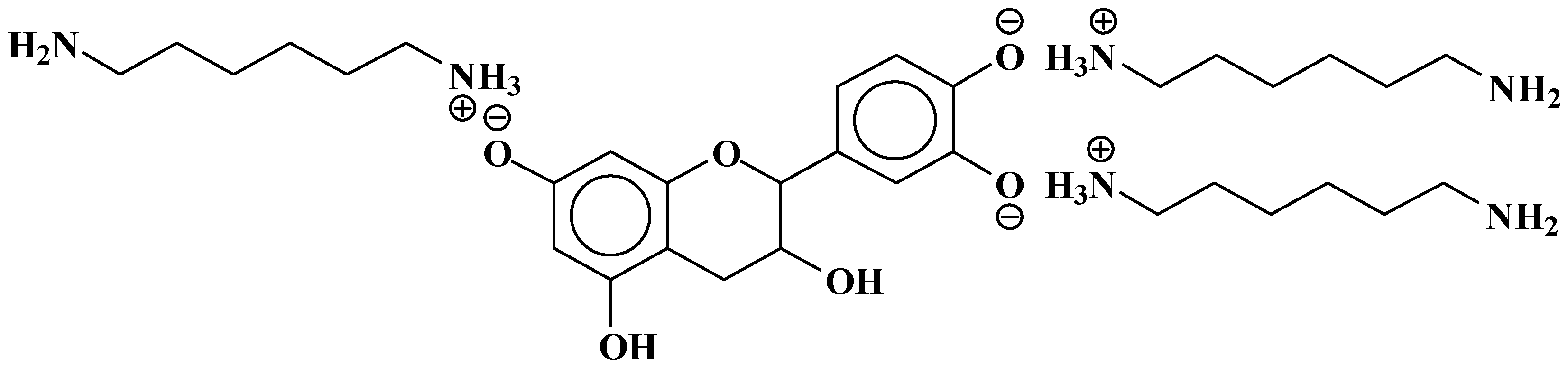
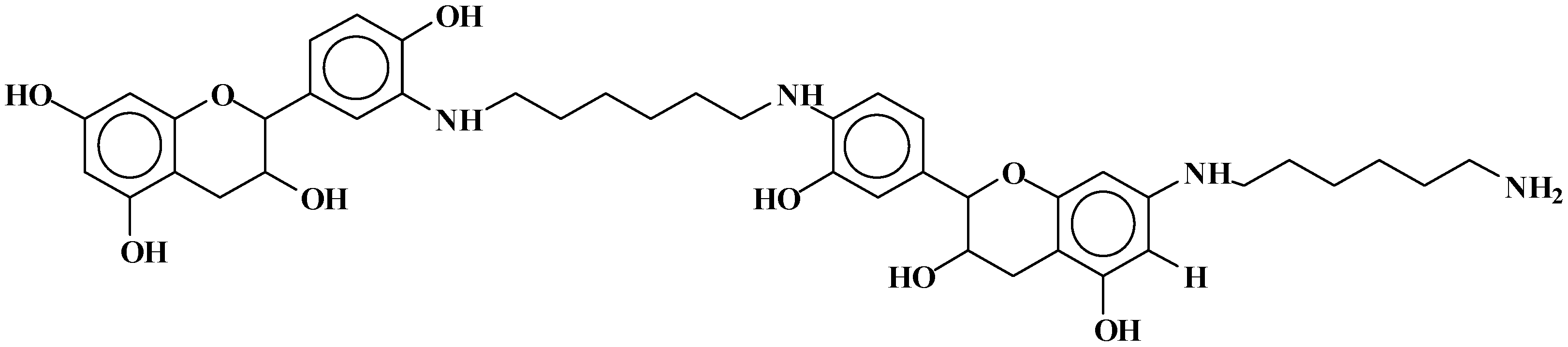
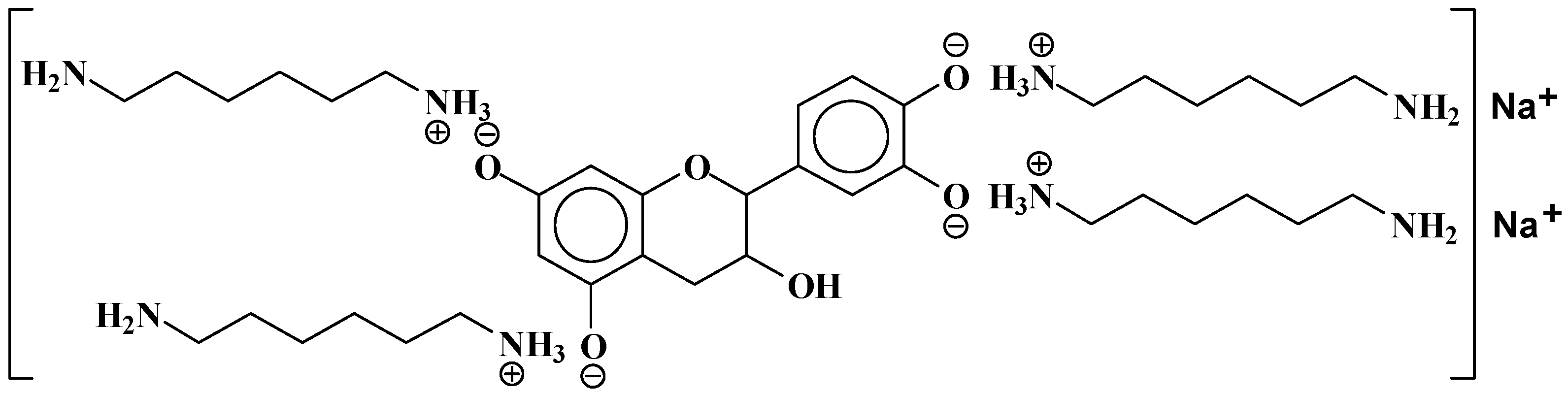
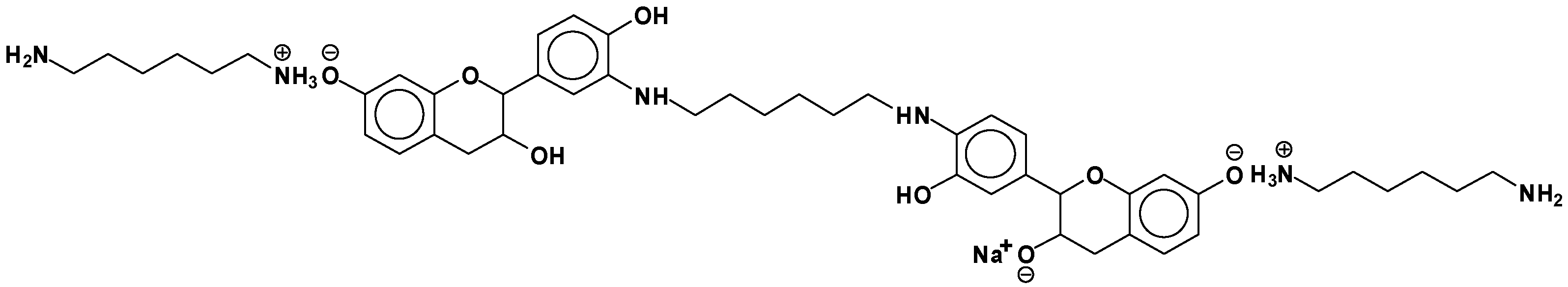

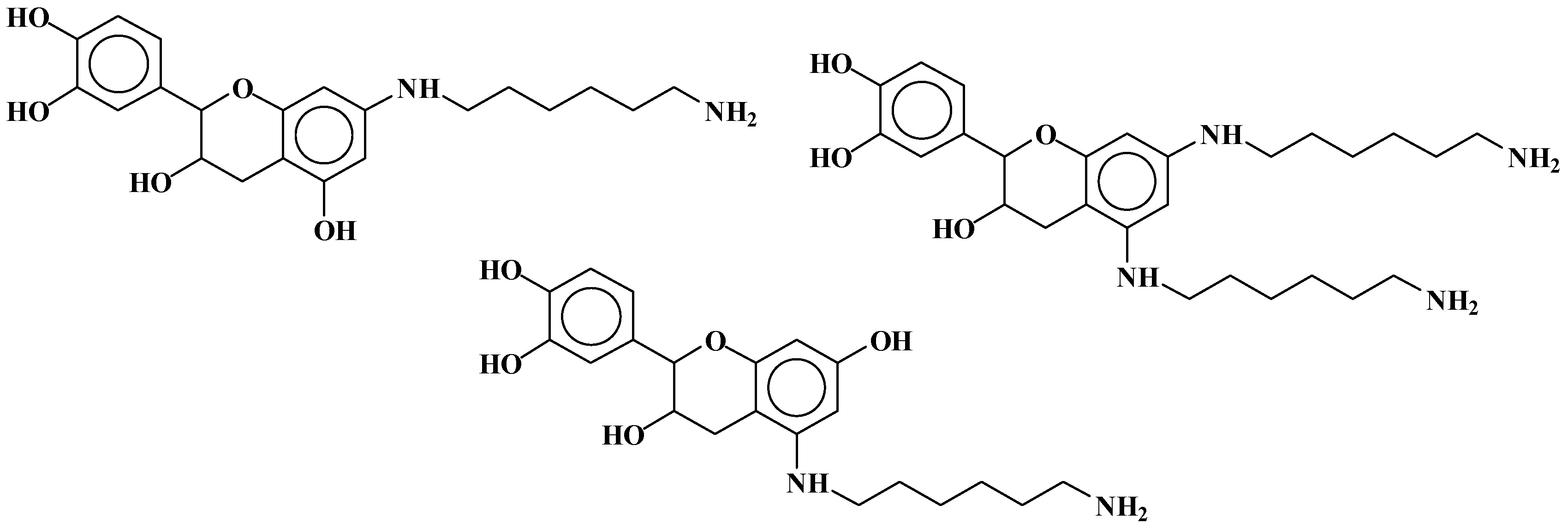
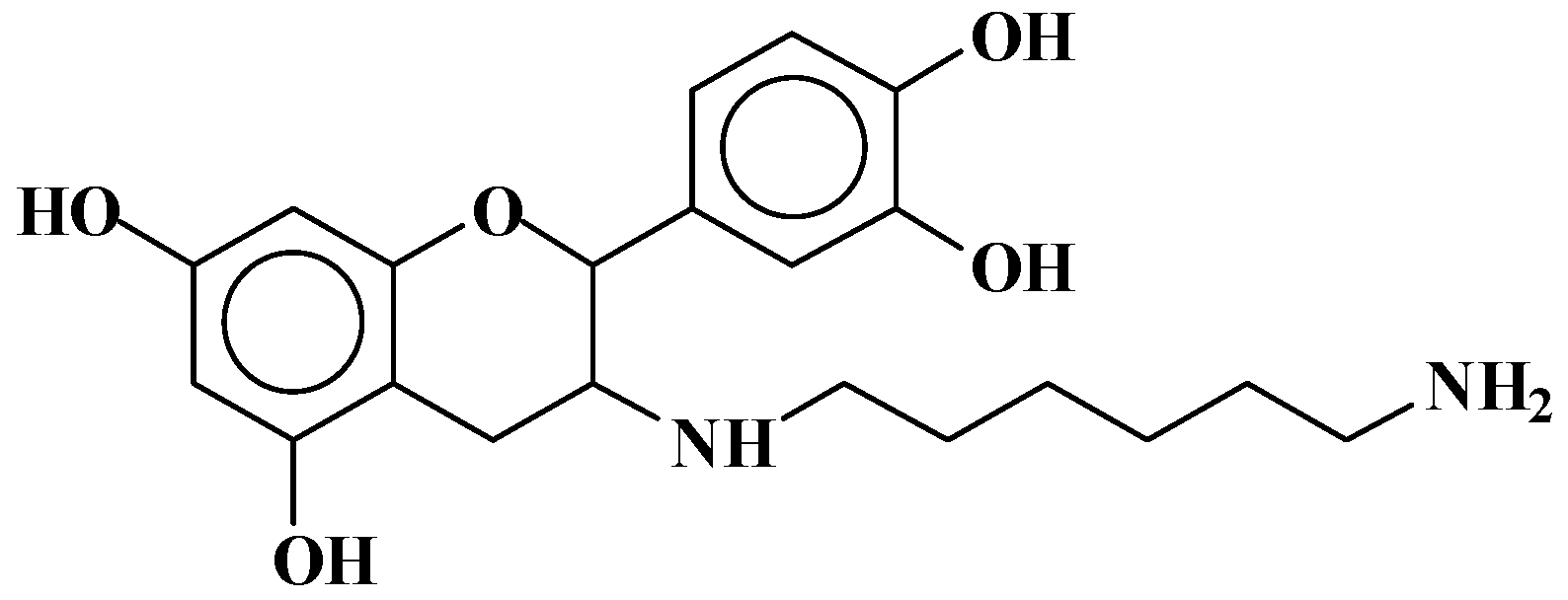
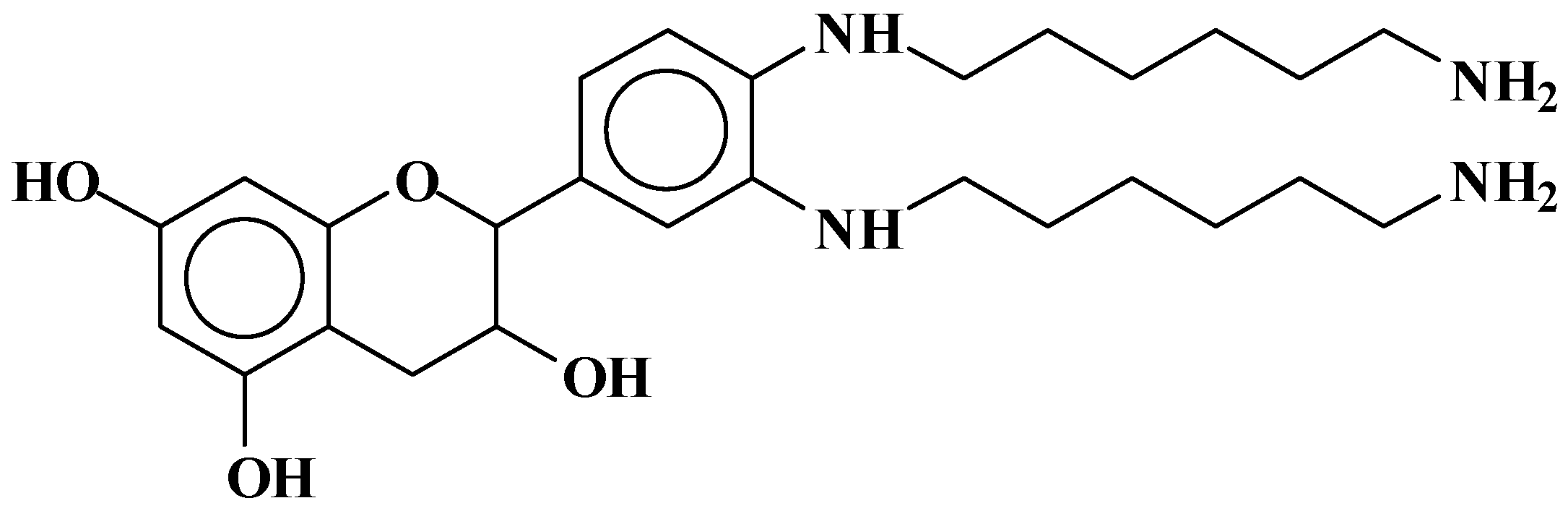
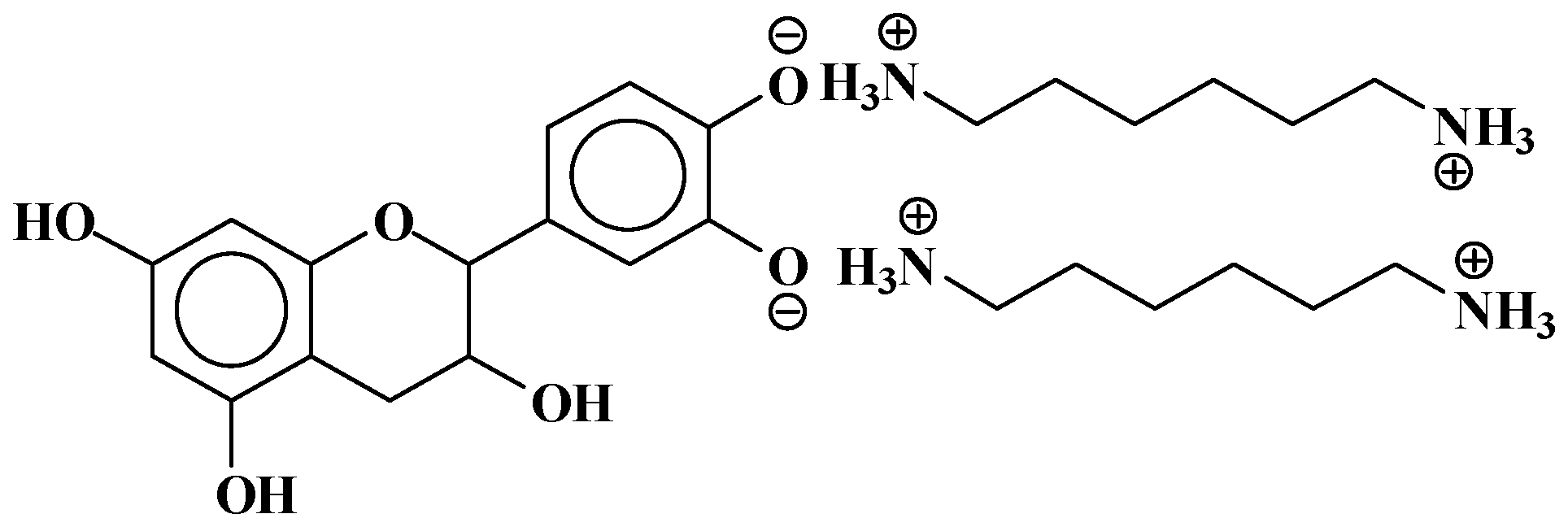
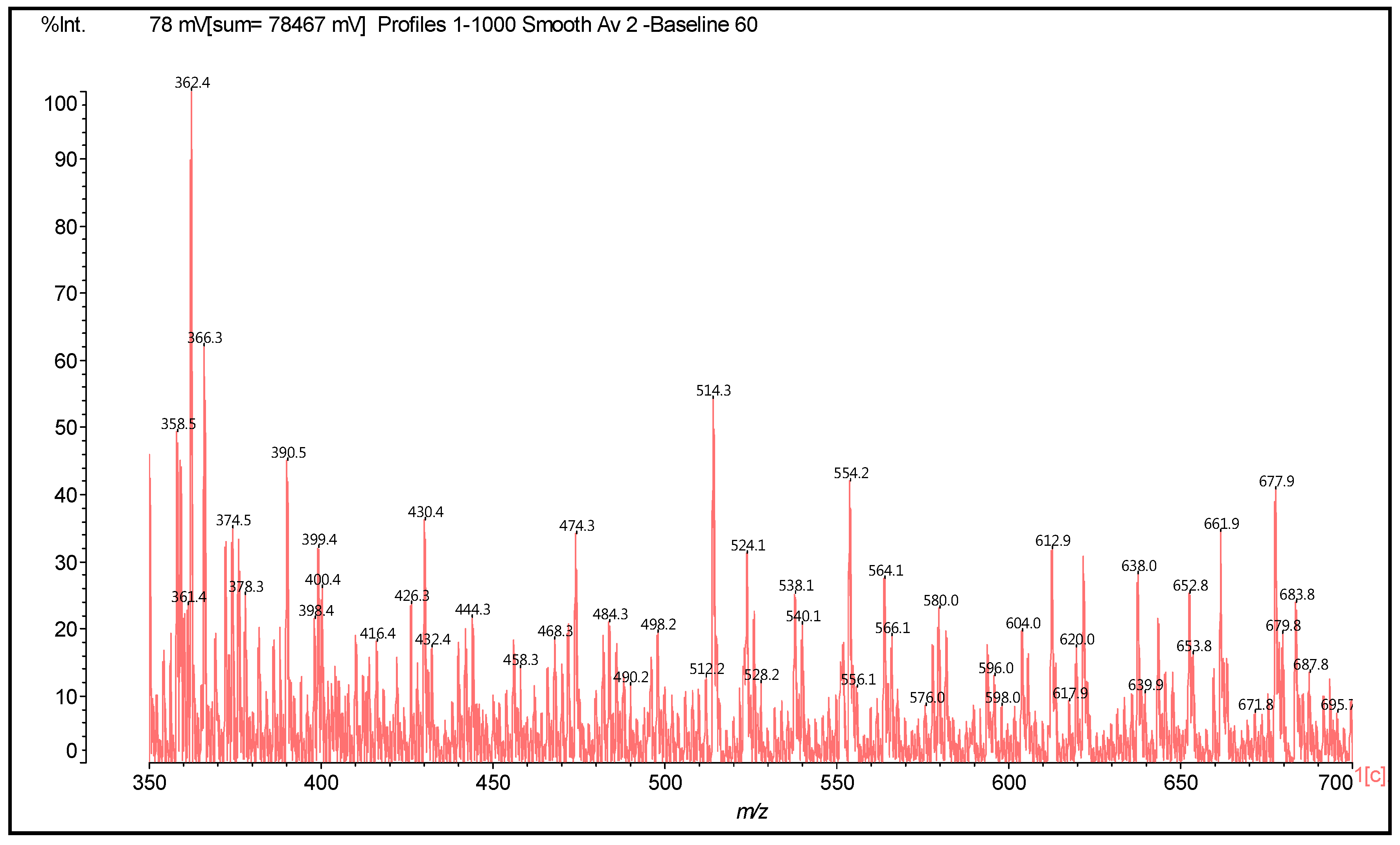
| 289.9 Da = Catechin alone |
| 509–512 Da = catechin-(hexamethylenediamine)2 (509 Da calculated) |
| 524.6 Da = catechin-(hexamethylenediamine)2 ionic salt |
| 526 Da = 524 diprotonated |
| 552.8 Da = catechin-(hexamethylenediamine)2 + 3 × Na+ (calculated 553 Da) |
| 579.7 Da = catechin-(hexamethylenediamine)2 + 4 × Na+ (calculated 582 Da) |
| 602.7 Da = catechin dimer + Na+ (calculated 601 Da) |
| 605 Da = catechin-(hexamethylenediamine)3 + Na+ |
| 631.6–633.7 Da = catechin-(hexamethylenediamine)3 + 2 × Na+ (calculated 630 Da) |
| 664 Da = catechin-hexamethylenediamine-catechin (660 Da calculated) |
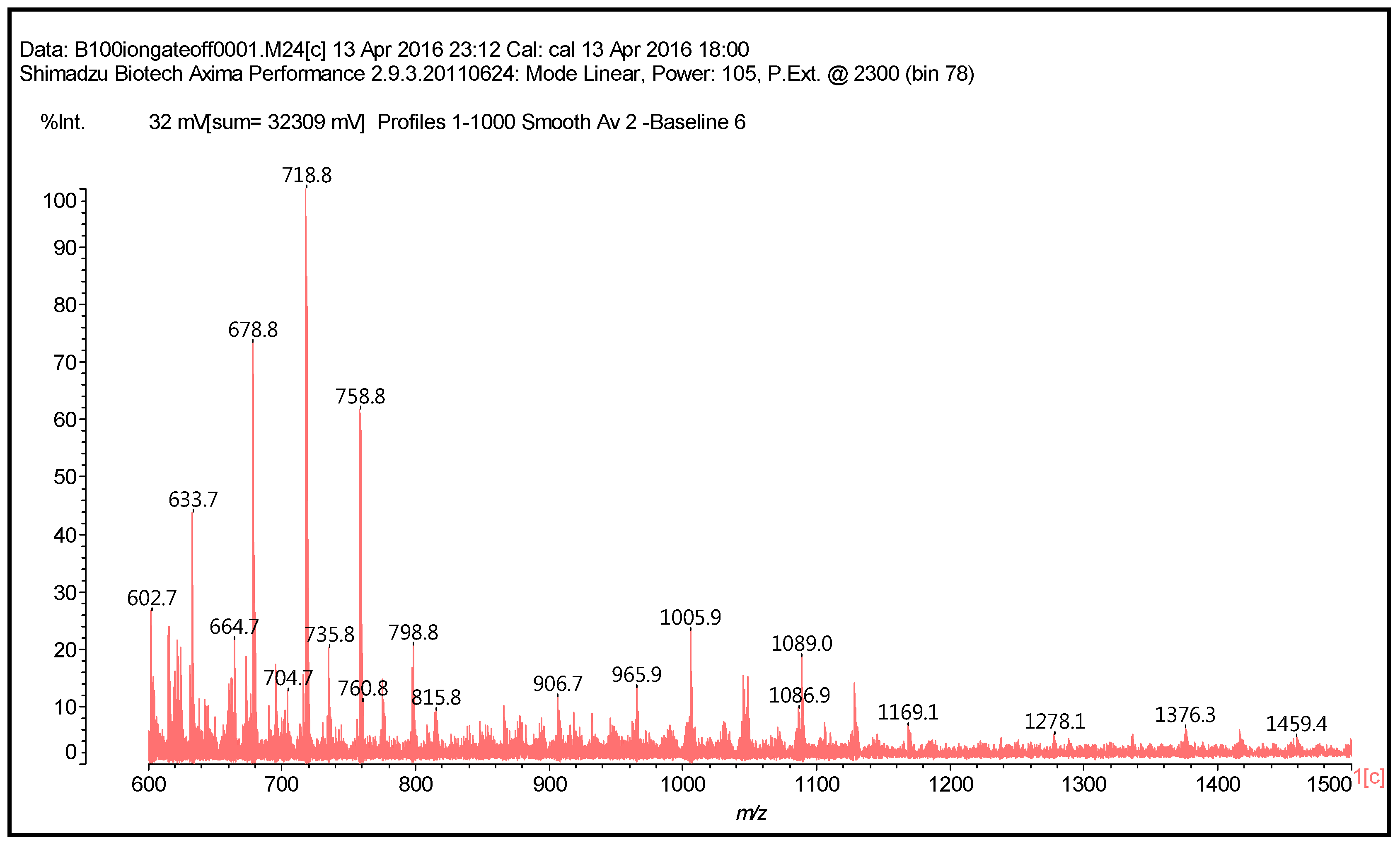
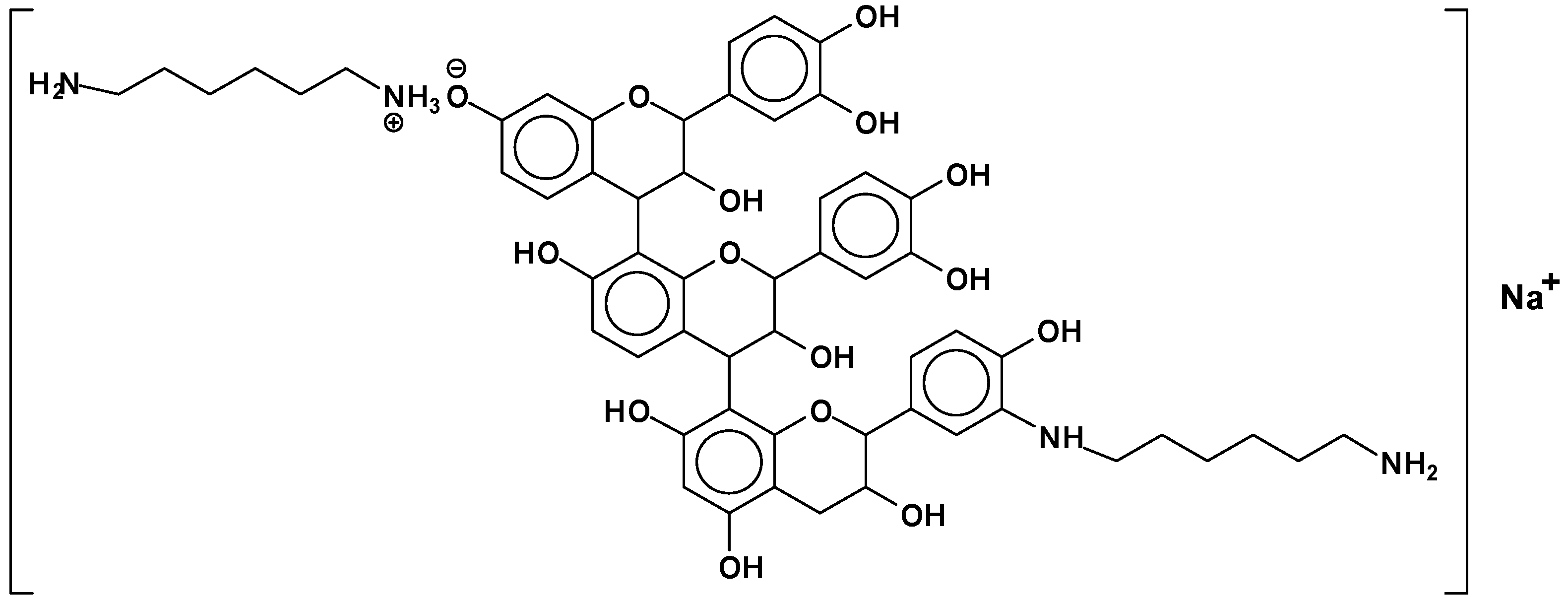
| However, also: |
| 638 + 1 × Na+ = catechin-(hexamethylenediamine)3 ionic salt (calculated 661 Da) |
| 678 Da = catechin-(hexamethylenediamine)3 + 4 × Na+ (calculated 675 Da) |
| 758 Da = catechin-hexamethylenediamine-catechin-hexamethylenediamine |
| 798 Da = catechin-(hexamethylenediamine)4 + 2 × Na+, ionic salt. |
| 1169 Da = hexamethylenediamine-catechindimer-hexamethylenediamine-catechin-hexamethylenediamine + 1 × Na+ |
| 1459 Da = hexamethylenediamine-catechindimer-hexamethylenediamine-catechindimer-hexamethylenediamine, diprotonated |
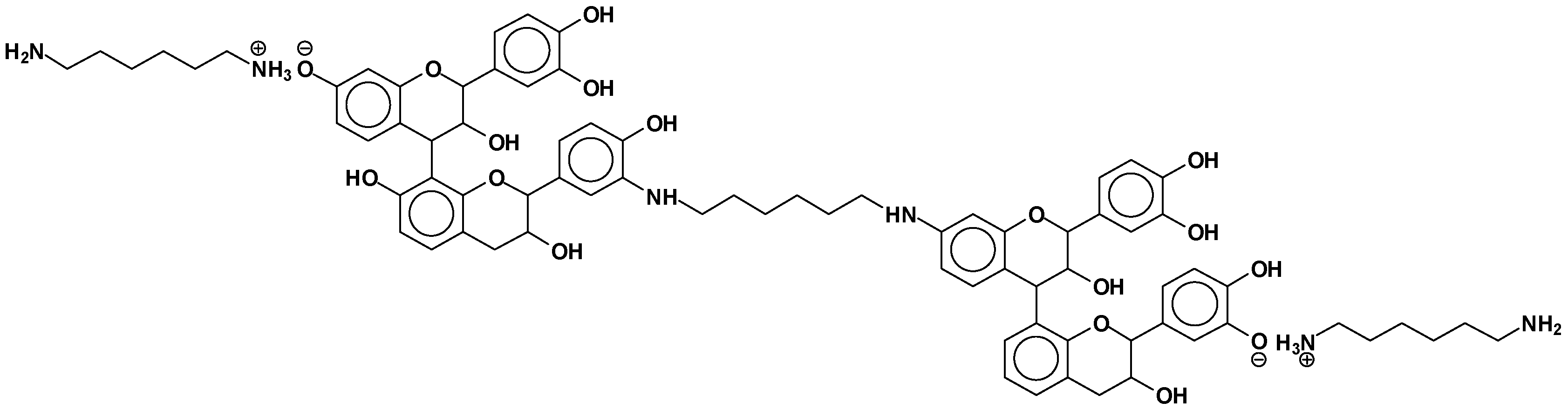
| Experimental | Calculated (Da) | Description of Calculation (Da) | Description | Number Ionic Bonds | Number Covalent Bonds |
|---|---|---|---|---|---|
| 372–374 | 372 | 274 + 116 − 18 | F-HMDA | - | 1 |
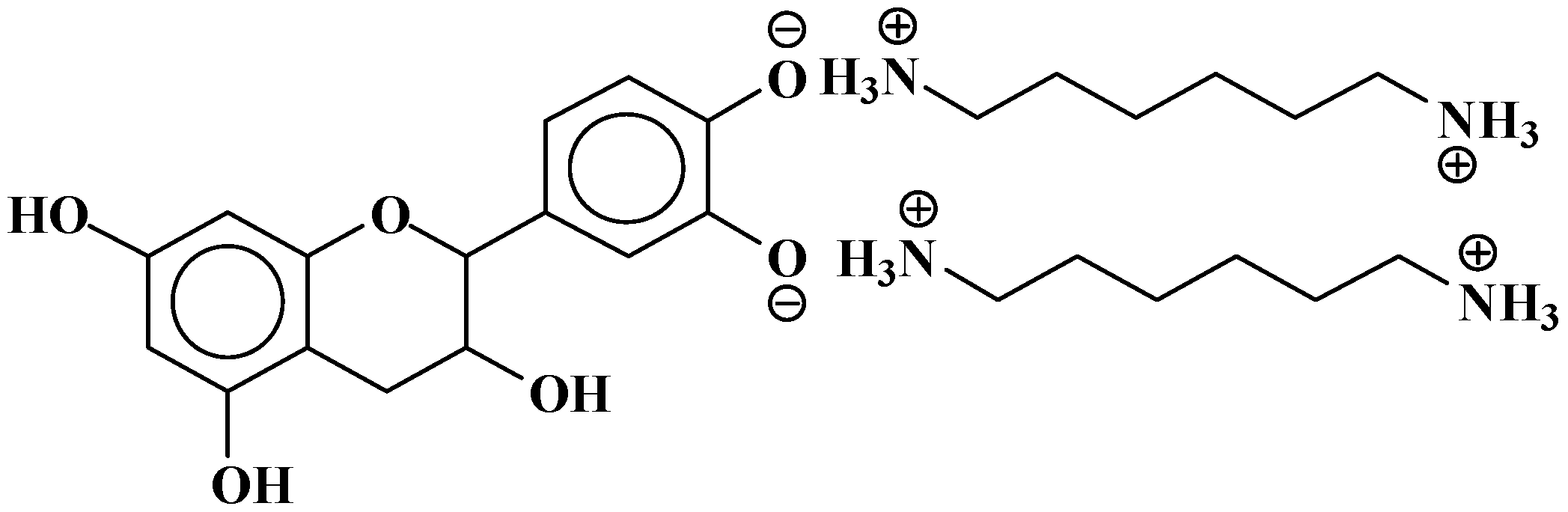
| 390 | 390 | 274 + 116 | F(−)(+)HMDA | 1 | - |
| 410 | 410 | 290 − 17 + 114 + 23 | F-HMDA(−)(+)Na | - | 1 |
| 426 | 426 | 306 + 114 − 17 + 23 | G-HMDA(−)(+)Na | - | 1 |
| 428–430 | 428 | 290 + 115 + 23 | C(−)(+)HMDA(−)(+)Na | 1 | - |
| 444 | 444 | 306 + 115 + 23 | G(−)(+)HMDA(−)(+)Na | 1 | - |
| 488 | 488 | 274 − 17 + 115 + 116 | HMDA(+)(−)F-HMDA | 1 | 1 |
| 468 | 468 | 274 + 114 × 2 − 17 × 2 | (HMDA-F-HMDA) less 2H+ | - | 2 |
| 484 | 484 | 290 + 114 × 2 − 17 × 2 | (HMDA-C-HMDA) less 2H+ | - | 2 |
| 500 | 500 | 304 + 2 + 114 × 2 − 17 − 17 | (HMDA-G-HMDA) less 2H+ | - | 2 |
| 524 | 524 | 306 + 114 + 115 – 17 − 17 + 23 | (G[HMDA]2)(−)(+)Na | - | 2 |
| 525 | 525 | 290 + 114 + 115 − 17 + 23 | HMDA-C(−)(+)HMDA(−)(+)Na | 1 | 1 |
| 528 | 528 | 274 + 116 + 114 + 23 | HMDA(+)(−)F(−)(+)HMDA(−)(+)Na | 2 | - |
| 540 | 542 | 306 + 115 × 2 − 17 + 23 | [HMDA(+)(−)G-HMDA](−)(+)Na | 1 | 1 |
| 562 | 562 | 272 + 288 + 2 | Dimer | - | - |
| 564 | 564 | 306 + 114 + 115 − 17 + 23 × 2 | Na(+)(−)HMDA(+)(−)G-HMDA(−)(+)Na | 1 | 1 |
| 578 | 578 | 288 + 288 + 2 | Dimer | - | - |
| 612 | 610 | 304 + 304 + 2 | Dimer | - | - |
| 617 | 618 | 306 + 115 × 2 − 17 × 2 + 116 | HMDA(+)(−)G(HMDA)2 | 1 | 2 |
| 621 | 622 | 306 + 115 × 2+ 114 − 17 × 3 + 23 | [G(HMDA)3](−)(+)Na | - | 3 |
| 638 | 640 | 306 + 115 × 3 − 17 − 17 + 23 | [HMDA(+)(−)G(HMDA)2](−)(+)Na | 1 | 2 |
| 643 | 644 | 272 + 272 + 2 + 114 − 17 | F-F-HMDA | - | 1 |
| 642 | 642 | 272 + 288 + 2 + 114 − 17 × 2 | F-HMDA-C | - | 2 |
| 653 | 654 | 306 + 116 × 3 | G[(−)(+)HMDA]3 | 3 | - |
| 661 | 660 | 272 + 288 + 2 + 115 − 17 | F-C-HMDA | - | 1 |
| 661 | 662 | 272 + 272 + 2 + 116 | F-F(−)(+)HMDA | 1 | - |
| 677 | 676 | 306 + 116 × 2+ 115 + 23 | [HMDA(+)(−)]2 G (−)(+)HMDA(−)(+)Na | 3 | - |
| 677 | 678 | 272 + 288 + 2 + 116 | F-C(−)(+)HMDA | 1 | - |
| 682 | 682 | 272 + 288 + 2 + 114 − 17 + 23 | [F-C-HMDA] | - | 1 |
| 687 | 688 | 272 + 2 + 115 × 3 + 114 − 17 × 4 + 23 | [F(HMDA)4](−)(+)Na | - | 4 |
| 695 | 694 | 288 + 288 + 2 + 116 | C-C(−)(+)HMDA | 1 | - |
| 701 | 701 | 272 + 288 + 2 + 116 + 23 | [F-C(−)(+)HMDA](−)(+)Na | 1 | - |
| 716 | 717 | 288 + 288 + 2 + 116 + 23 | [C-C(−)(+)HMDA](−)(+)Na | 1 | - |
| 723 | 723 | 272 + 272 + 2 + 114 + 115 − 17 × 3 | F-HMDA-F-HMDA | - | 3 |
| 745 | 744 | 274 + 274 + 114 + 116 − 17 × 2 | F-HMDA-F(−)(+)HMDA | - | - |
| 740–743 | 742 | 274 + 274 + 2 + 115 × 2 − 17 × 2 | HMDA-F-F-HMDA | - | 2 |
| 757 | 758 | 272 + 288 + 2 + 115 × 2 − 17 × 2 | HMDA-F-C-HMDA | - | 2 |
| 761 | 760 | 274 + 290 + 114 − 17 × 2 + 116 | F-HMDA-C(−)(+)HMDA | 1 | 2 |
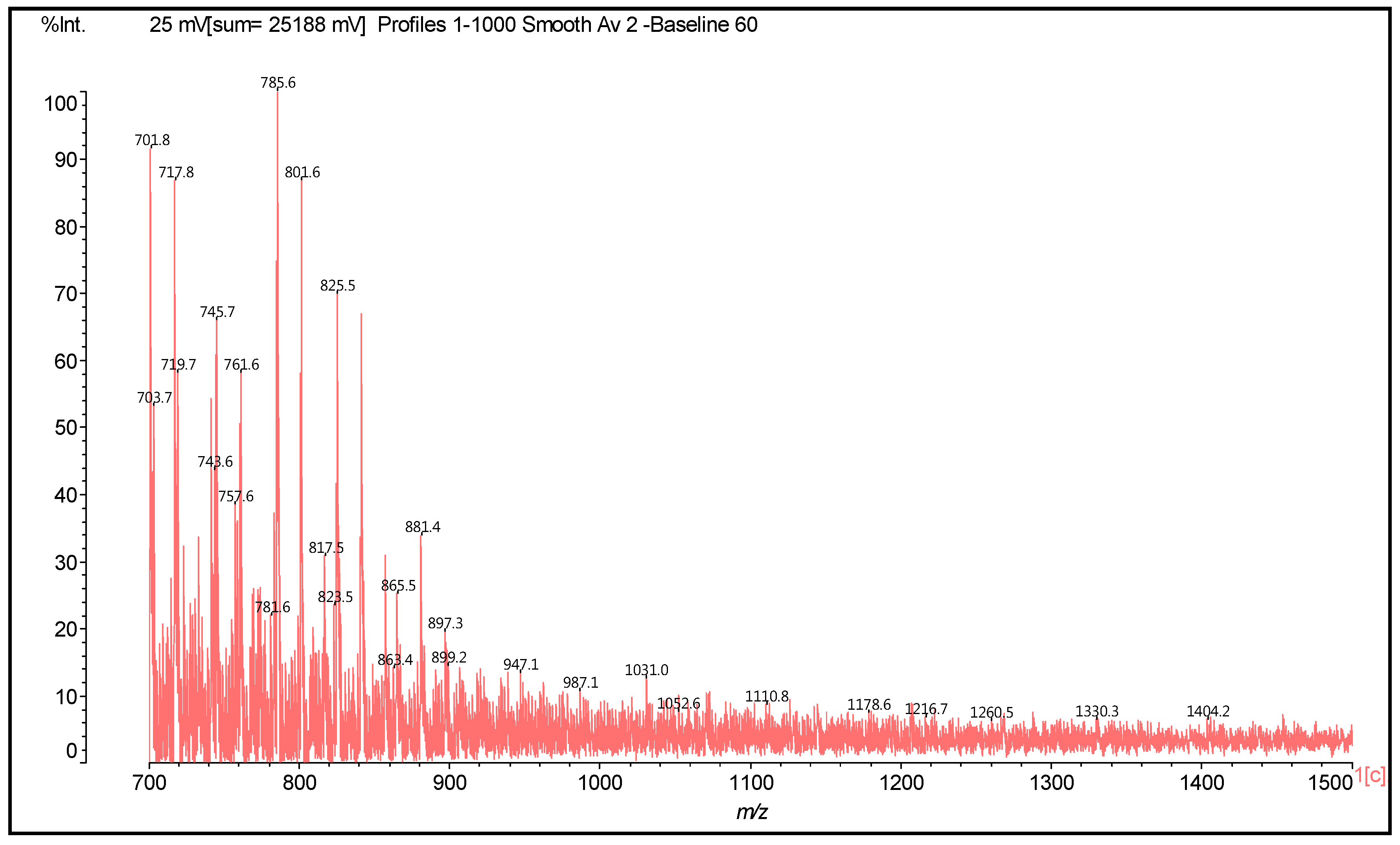
| 857 | 857 | 272 + 272 + 288 + 2 + 23 | Trimer | - | - |
| 865 | 866 | 272 + 288 + 304 + 2 | Trimer | - | - |
| 881 | 880 | 272 × 2 + 2 + 115 × 3 − 17 × 2 + 23 | [(HMDA(+)(−))2 (F-HMDA-F)](−)(+)Na | 2 | 2 |
| 881 | 882 | 288 × 2 + 304 + 2 | Trimer | - | - |
| 898 | 898 | 272 × 2 + 2+ 114 × 3 − 17 + 23 | [(HMDA(+)(−))2 F-F-HMDA](−)(+)Na | 2 | 1 |
| 898 | 898 | 288 + 304 × 2 + 2 | Trimer | - | - |
| 906 | 905 | 288 × 2 + 304 + 2 + 23 | Trimer | - | - |
| 921 | 921 | 288 + 304 × 2 + 2 + 23 | Trimer | - | - |
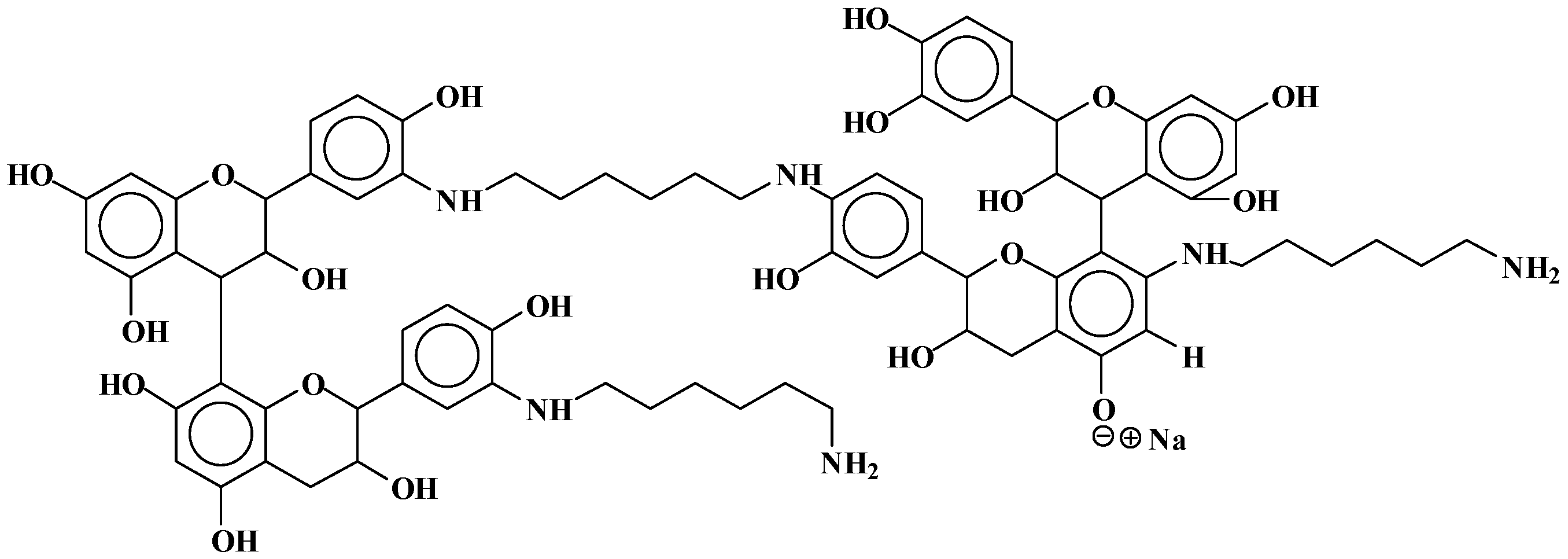
| 1052 | 1052 | 857 + 114 + 115 − 17 × 2 | [(F-C-F)(HMDA)2](−)(+)Na | - | 2 |
| 1069 | 1070 | 857 + 115 × 2 − 17 | [HMDA-(F-C-F)(−)(+)HMDA](−)(+)Na | 1 | 1 |
| 1178 | 1178 | 288 × 4 + 2 + 23 | Tetramer | - | - |
| 1260 | 1258 | 288 × 2 + 2 + 116 − 18 × 2 + 288 × 2 + 2 + 23 − 1 | [C-C-HMDA-C-C](−)(+)Na | - | 2 |
| 1330 | 1334 | 288 × 2 + 2 + 116 × 2 − 18 × 3 + 288 × 2 + 2 | C-C-HMDA-C-C-HMDA | - | 3 |
| 1404 | 1404 | 272 × 2 + 2 + 116 × 3 − 18 × 2 + 272 × 2 + 2 | HMDA(+)(−)F-F-HMDA-F-F(−)(+)HMDA | 2 | 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Medina, F.-J.; Pizzi, A.; Basso, M.C.; Delmotte, L.; Celzard, A. Polycondensation Resins by Flavonoid Tannins Reaction with Amines. Polymers 2017, 9, 37. https://doi.org/10.3390/polym9020037
Santiago-Medina F-J, Pizzi A, Basso MC, Delmotte L, Celzard A. Polycondensation Resins by Flavonoid Tannins Reaction with Amines. Polymers. 2017; 9(2):37. https://doi.org/10.3390/polym9020037
Chicago/Turabian StyleSantiago-Medina, Francisco-Jose, Antonio Pizzi, Maria Cecilia Basso, Luc Delmotte, and Alain Celzard. 2017. "Polycondensation Resins by Flavonoid Tannins Reaction with Amines" Polymers 9, no. 2: 37. https://doi.org/10.3390/polym9020037







