Engineering Cell Surfaces with Polyelectrolyte Materials for Translational Applications
Abstract
:1. Introduction
1.1. Introduction to Cell Surface Engineering
1.2. Key Challenges in Cell Surface Engineering
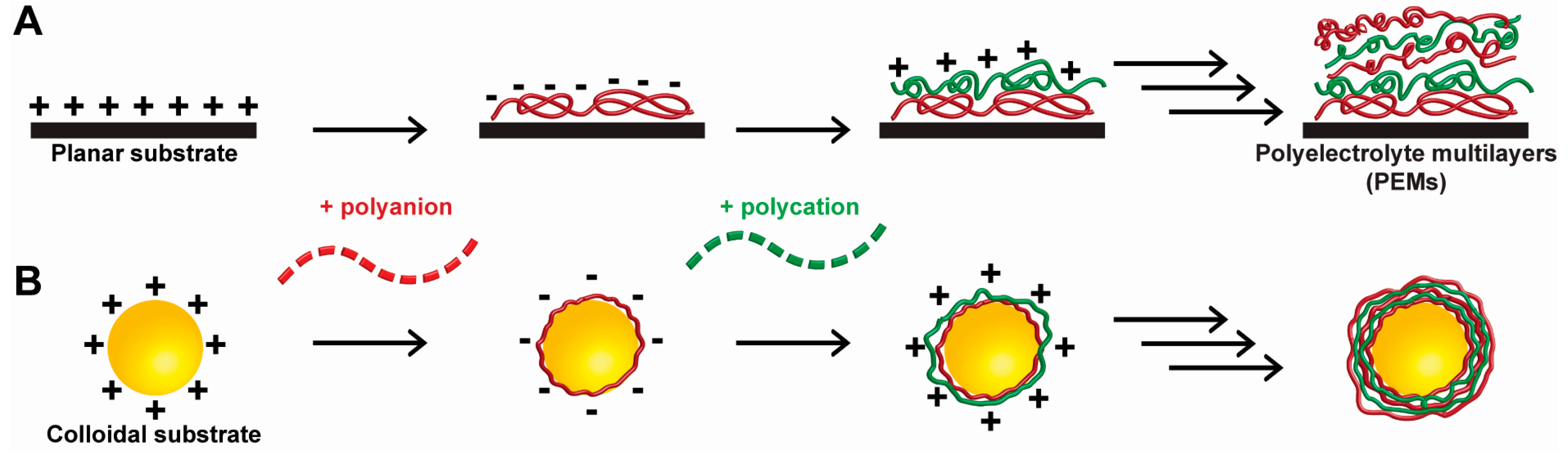
1.3. Introduction of Polyelectrolyte Materials
1.4. Cell Surface Engineering with Polyelectrolyte Materials
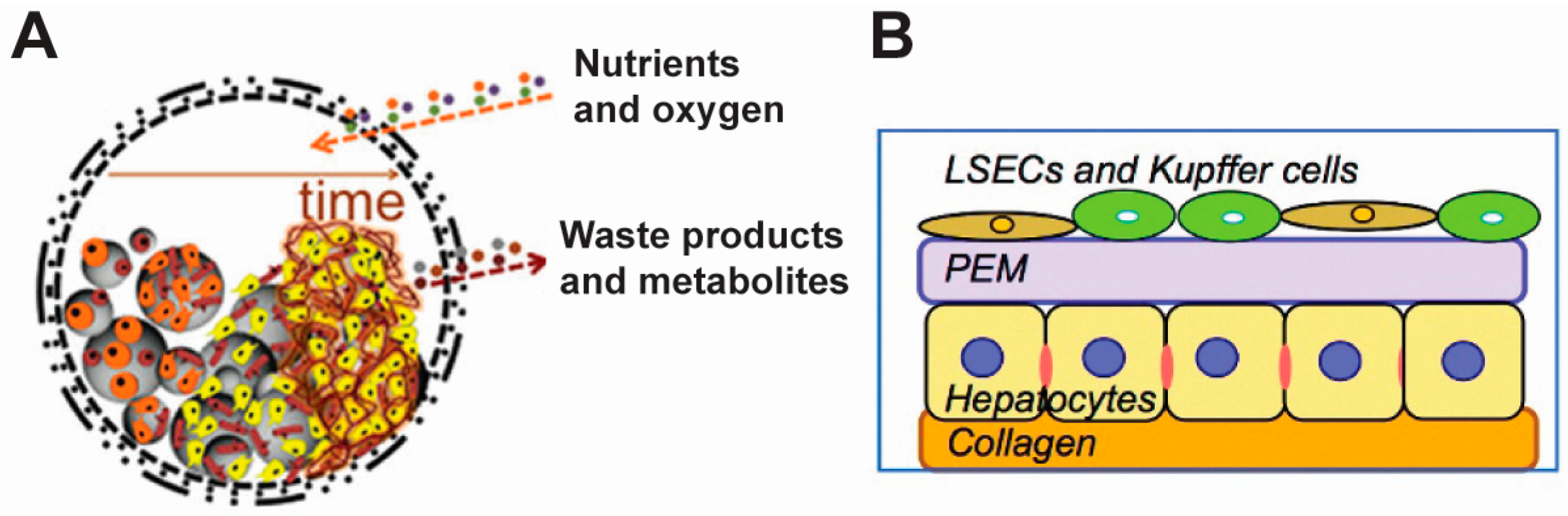
2. Translational Applications of Polyelectrolyte-Based Cell Surface Engineering
2.1 Conferring New Functions without Limiting Viability
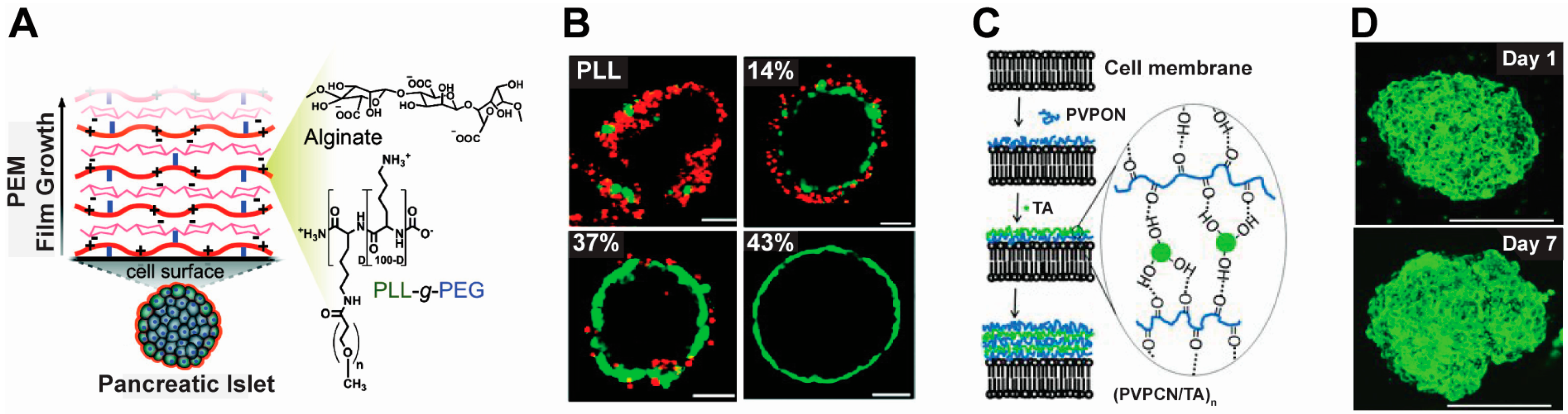
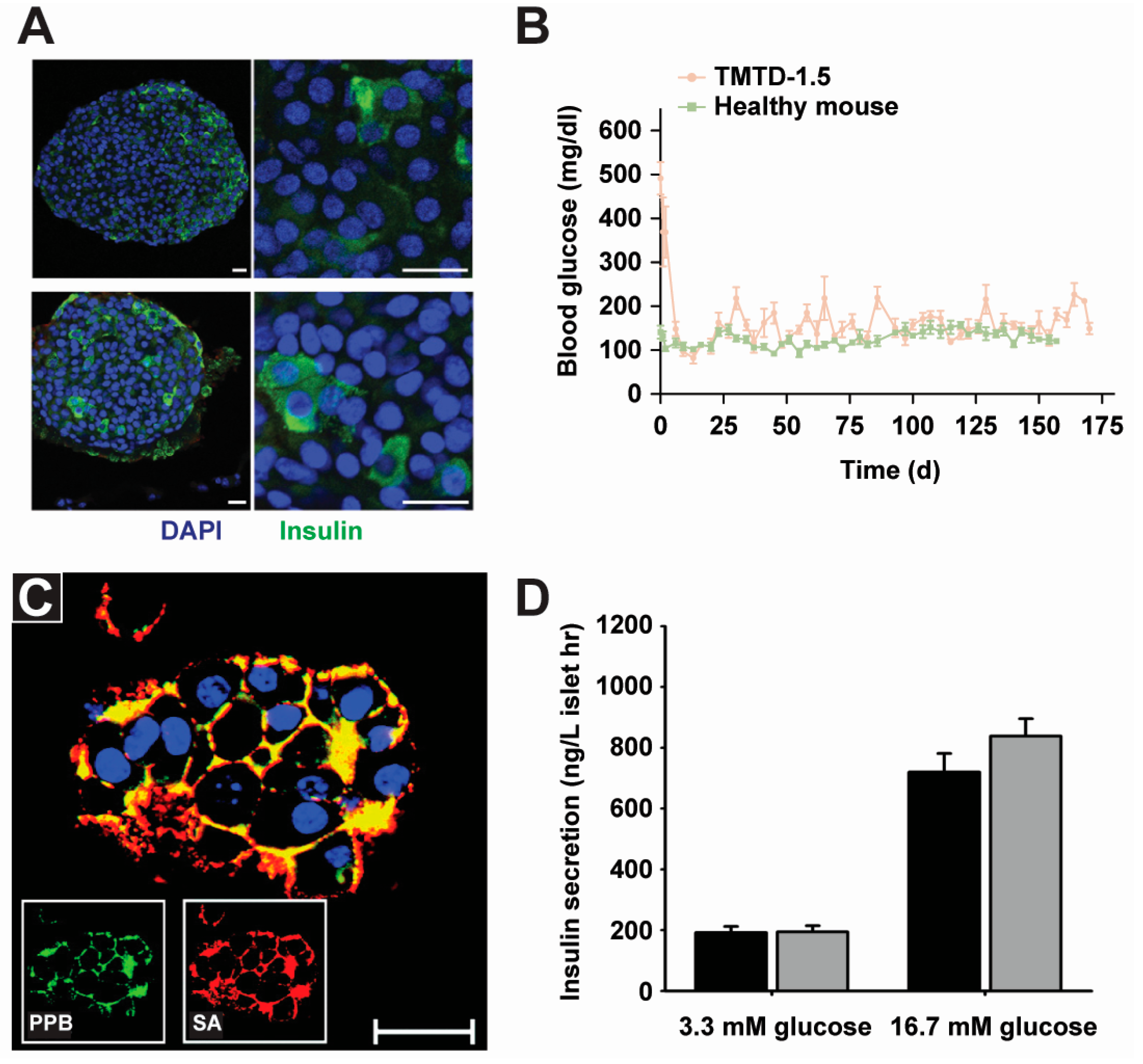
2.2. Cell Therapy
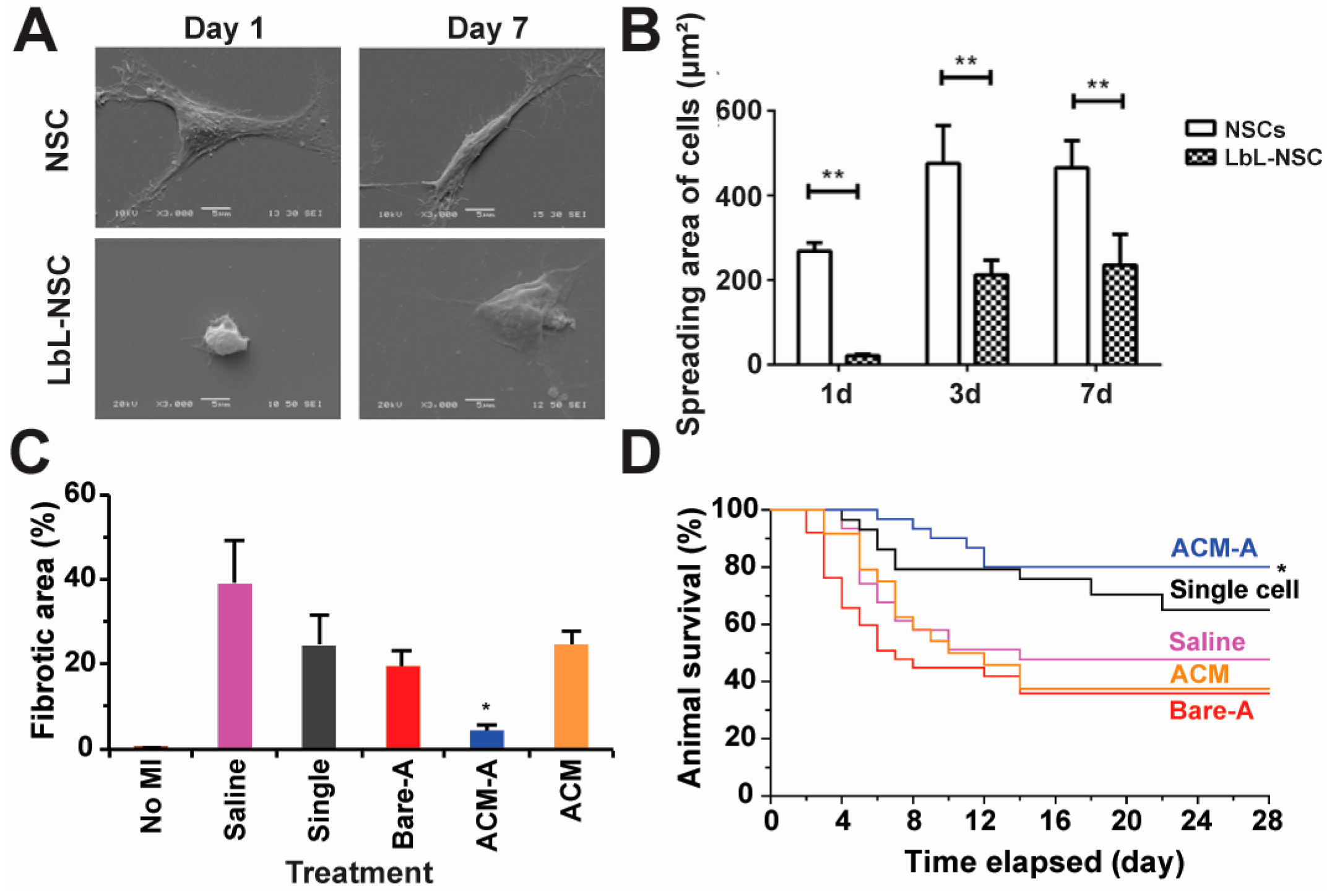
2.3. Tissue Engineering
2.4. Cell Tracking and Sensing
2.5. Cell-Based Drug Delivery
2.6. Immune Modulation
3. Comparison of Polyelectrolytes to Other Cell Surface Engineering Approaches
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, W.; Schafer, S.; Choi, J.; Yamanaka, Y.J.; Lombardi, M.L.; Bose, S.; Carlson, A.L.; Phillips, J.A.; Teo, W.; Droujinine, I.A.; et al. Cell-surface sensors for real-time probing of cellular environments. Nat. Nanotechnol. 2011, 6, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, S.; Regmi, S.; Gupta, B.; Poudel, B.K.; Pham, T.T.; Kim, J.R.; Park, P.H.; Yong, C.S.; Kim, J.O.; Bae, Y.K.; et al. Hybrid Congregation of Islet Single Cells and Curcumin-Loaded Polymeric Microspheres as an Interventional Strategy to Overcome Apoptosis Associated with Pancreatic Islets Transplantation. ACS Appl. Mater. Interfaces 2016, 8, 25702–25713. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.L.; Kizhakkedathu, J.N. The mechanism and modulation of complement activation on polymer grafted cells. Acta Biomater. 2016, 31, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Sackstein, R.; Merzaban, J.S.; Cain, D.W.; Dagia, N.M.; Spencer, J.A.; Lin, C.P.; Wohlgemuth, R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Stephan, M.T.; Moon, J.J.; Um, S.H.; Bershteyn, A.; Irvine, D.J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010, 16, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, J.B.; Garmendia, J.V.; Radzioch, A.; Radzioch, D. New frontiers in the therapeutic management of transplant rejection. Curr. Pharm. Des. 2006, 12, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Gibly, R.F.; Graham, J.G.; Luo, X.; Lowe, W.L.; Hering, B.J.; Shea, L.D. Advancing islet transplantation: From engraftment to the immune response. Diabetologia 2011, 54, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.R.; Pirraco, R.P.; Cerqueira, M.T.; Marques, A.P.; Reis, R.L.; Mano, J.F. Semipermeable Capsules Wrapping a Multifunctional and Self-regulated Co-culture Microenvironment for Osteogenic Differentiation. Sci. Rep. 2016, 6, 21883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, C.R.; Gil, S.; Reis, R.L.; Mano, J.F. A Closed Chondromimetic Environment within Magnetic-Responsive Liquified Capsules Encapsulating Stem Cells and Collagen II/TGF-beta3 Microparticles. Adv. Healthc. Mater. 2016, 5, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.R.; Reis, R.L.; Mano, J.F. Multilayered hierarchical capsules providing cell adhesion sites. Biomacromolecules 2013, 14, 743–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.M.; Georgi, N.; Costa, R.; Sher, P.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Nanostructured 3D constructs based on chitosan and chondroitin sulphate multilayers for cartilage tissue engineering. PLoS ONE 2013, 8, e55451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselmo, A.C.; Gilbert, J.B.; Kumar, S.; Gupta, V.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: A generalized strategy to deliver drugs to treat inflammation. J. Control. Release 2015, 199, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Zhao, W.A.; Gupta, A.; Loh, W.L.; Karnik, R.; Karp, J.M. Cell Surface Engineering of Mesenchymal Stem Cells. Methods Mol. Biol. 2011, 698, 505–523. [Google Scholar] [PubMed]
- Elliott, R.B.; Escobar, L.; Tan, P.L.J.; Muzina, M.; Zwain, S.; Buchanan, C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 year after xenotransplantation. Xenotransplantation 2007, 14, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.A.; Sauter, C.S.; Armand, P. Fast Cars and No Brakes: Autologous Stem Cell Transplantation as a Platform for Novel Immunotherapies. Biol. Blood Marrow Transplant. 2016, 22, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, D.; Kaiktsis, L.; Chaniotis, A.; Pantos, J.; Efstathopoulos, E.P.; Marmarelis, V. Wall shear stress: Theoretical considerations and methods of measurement. Prog. Cardiovasc. Dis. 2007, 49, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Shintaku, H.; Okitsu, T.; Kawano, S.; Matsumoto, S.; Suzuki, T.; Kanno, I.; Kotera, H. Effects of fluid dynamic stress on fracturing of cell-aggregated tissue during purification for islets of Langerhans transplantation. J. Phys. D Appl. Phys. 2008, 41, 11. [Google Scholar] [CrossRef]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.M.; Weitzman, M.D. Gene therapy: Twenty-first century medicine. Annu. Rev. Biochem. 2005, 74, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.B.; Sadelain, M.; Glorioso, J.C. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat. Rev. Cancer 2003, 3, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Custodio, C.A.; Mano, J.F. Cell Surface Engineering to Control Cellular Interactions. ChemNanoMat 2016, 2, 376–384. [Google Scholar] [CrossRef]
- Boudou, T.; Crouzier, T.; Ren, K.; Blin, G.; Picart, C. Multiple functionalities of polyelectrolyte multilayer films: New biomedical applications. Adv. Mater. 2010, 22, 441–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lv, F. Functionalized Conjugated Polyelectrolytes: Design and Biomedical Applications. In Functionalized Conjugated Polyelectrolytes: Design and Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–87. [Google Scholar]
- Scranton, A.B.; Rangarajan, B.; Klier, J. Biomedical applications of polyelectrolytes. In Biopolymers II; Springer: Berlin/Heidelberg, Germany, 1995; Volume 122, pp. 1–54. [Google Scholar]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Xiao, F.X.; Pagliaro, M.; Xu, Y.J.; Liu, B. Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: A new perspective for rational construction of multilayer assemblies. Chem. Soc. Rev. 2016, 45, 3088–3121. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, 6233. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.M.; Lynn, D.M. Multilayered polyelectrolyte assemblies as platforms for the delivery of DNA and other nucleic acid-based therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 979–999. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Carmagnola, I.; Nardo, T.; Chiono, V. Layer-by-layer assembly for biomedical applications in the last decade. Nanotechnology 2015, 26, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chiu, Y.C.; Tostanoski, L.H.; Jewell, C.M. Polyelectrolyte Multilayers Assembled Entirely from Immune Signals on Gold Nanoparticle Templates Promote Antigen-Specific T Cell Response. ACS Nano 2015, 9, 6465–6477. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; Mano, J.F. Molecular Interactions Driving the Layer-by-Layer Assembly of Multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, K.R.; Andorko, J.I.; Whipple, R.A.; Zhang, P.; Sooklal, E.L.; Martin, S.S.; Jewell, C.M. Lipid tethering of breast tumor cells enables real-time imaging of free-floating cell dynamics and drug response. Oncotarget 2016, 7, 10486–10497. [Google Scholar] [PubMed]
- Chiu, Y.C.; Gammon, J.M.; Andorko, J.I.; Tostanoski, L.H.; Jewell, C.M. Assembly and Immunological Processing of Polyelectrolyte Multilayers Composed of Antigens and Adjuvants. ACS Appl. Mater. Interfaces 2016, 8, 18722–18731. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.M.; Fuchs, S.M.; Flessner, R.M.; Raines, R.T.; Lynn, D.M. Multilayered films fabricated from an oligoarginine-conjugated protein promote efficient surface-mediated protein transduction. Biomacromolecules 2007, 8, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Zhao, Y.; Sato, K.; Bin Ihsan, A.; Li, X.F.; Guo, H.L.; Gong, J.P. Oppositely Charged Polyelectrolytes Form Tough, Self-Healing, and Rebuildable Hydrogels. Adv. Mater. 2015, 27, 2722–2727. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Zavgorodnya, O.; Chen, Y.; Ellis, K.; Tse, H.M.; Cui, W.X.; Thompson, J.A.; Kharlampieva, E. Ultrathin Polymeric Coatings Based on Hydrogen-Bonded Polyphenol for Protection of Pancreatic Islet Cells. Adv. Funct. Mater. 2012, 22, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Cui, W.; Chaikof, E.L. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008, 8, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- D'Souza, S.; Murata, H.; Jose, M.V.; Askarova, S.; Yantsen, Y.; Andersen, J.D.; Edington, C.D.; Clafshenkel, W.P.; Koepsel, R.R.; Russell, A.J. Engineering of cell membranes with a bisphosphonate-containing polymer using ATRP synthesis for bone targeting. Biomaterials 2014, 35, 9447–9458. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Iwata, H. Cell surface modification with polymers for biomedical studies. Soft Matter 2010, 6, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.T.; Cui, W.; Kozlovskaya, V.; Kharlampieva, E.; Pan, D.; Qu, Z.; Krishnamurthy, V.R.; Mets, J.; Kumar, V.; Wen, J.; et al. Cell surface engineering with polyelectrolyte multilayer thin films. J. Am. Chem. Soc. 2011, 133, 7054–7064. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, S.J.; Lee, S.; Nam, J.H.; Byun, Y. Highly poly(ethylene) glycolylated islets improve long-term islet allograft survival without immunosuppressive medication. Tissue Eng. 2007, 13, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Lee, K.B.; Kong, B.; Kim, J.H.; Kim, H.S.; Choi, I.S. Biomimetic encapsulation of individual cells with silica. Angew. Chem. Int. Ed. Engl. 2009, 48, 9160–9163. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, L.; Liu, P.; Yan, Y.; Xu, X.; Tang, R. Extracellular silica nanocoat confers thermotolerance on individual cells: A case study of material-based functionalization of living cells. ChemBioChem 2010, 11, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Zamaleeva, A.I.; Sharipova, I.R.; Porfireva, A.V.; Evtugyn, G.A.; Fakhrullin, R.F. Polyelectrolyte-mediated assembly of multiwalled carbon nanotubes on living yeast cells. Langmuir 2010, 26, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Kempaiah, R.; Chung, A.; Maheshwari, V. Graphene as cellular interface: Electromechanical coupling with cells. ACS Nano 2011, 5, 6025–6031. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullin, R.F.; Shlykova, L.V.; Zamaleeva, A.I.; Nurgaliev, D.K.; Osin, Y.N.; Garcia-Alonso, J.; Paunov, V.N. Interfacing living unicellular algae cells with biocompatible polyelectrolyte-stabilised magnetic nanoparticles. Macromol. Biosci. 2010, 10, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Frasca, G.; Gazeau, F.; Wilhelm, C. Formation of a Three-Dimensional Multicellular Assembly Using Magnetic Patterning. Langmuir 2009, 25, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Vegas, A.J.; Veiseh, O.; Gurtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guan, T.; Zhang, X.; Wang, Z.; Wang, M.; Zhong, W.; Feng, H.; Xing, M.; Kong, J. The Effect of Layer-by-Layer Assembly Coating on the Proliferation and Differentiation of Neural Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.; Shen, C.J.; Berthiaume, F.; Tilles, A.W.; Toner, M.; Yarmush, M.L. Polyelectrolyte nano-scaffolds for the design of layered cellular architectures. Tissue Eng. 2006, 12, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Rajagopalan, P. 3D hepatic cultures simultaneously maintain primary hepatocyte and liver sinusoidal endothelial cell phenotypes. PLoS ONE 2010, 5, e15456. [Google Scholar] [CrossRef] [PubMed]
- Larkin, A.L.; Rodrigues, R.R.; Murali, T.M.; Rajagopalan, P. Designing a multicellular organotypic 3D liver model with a detachable, nanoscale polymeric Space of Disse. Tissue Eng. Part C Methods 2013, 19, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Brandy, M.L.; Cayre, O.J.; Fakhrullin, R.F.; Velev, O.D.; Paunov, V.N. Directed assembly of yeast cells into living yeastosomes by microbubble templating. Soft Matter 2010, 6, 3494–3498. [Google Scholar] [CrossRef]
- Pasparakis, G.; Cockayne, A.; Alexander, C. Control of bacterial aggregation by thermoresponsive glycopolymers. J. Am. Chem. Soc. 2007, 129, 11014–11015. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.J.R.; Pasparakis, G. Rapid Formation of Cell Aggregates and Spheroids Induced by a “Smart” Boronic Acid Copolymer. ACS Appl. Mater. Interfaces 2016, 8, 22930–22941. [Google Scholar] [CrossRef] [PubMed]
- Swiston, A.J.; Cheng, C.; Um, S.H.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Surface functionalization of living cells with multilayer patches. Nano Lett. 2008, 8, 4446–4453. [Google Scholar] [CrossRef] [PubMed]
- Swiston, A.J.; Gilbert, J.B.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Freely suspended cellular “backpacks” lead to cell aggregate self-assembly. Biomacromolecules 2010, 11, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Guan, J.J. Fabrication of Multilayered Microparticles by Integrating Layer-by-Layer Assembly and MicroContact Printing. Small 2011, 7, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-based drug delivery devices using phagocytosis-resistant backpacks. Adv. Mater. 2011, 23, H105–H109. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; Lim, R.M.; Beppu, M.M.; Pitombo, R.N.; Cohen, R.E.; Rubner, M.F. Liposome-Loaded Cell Backpacks. Adv. Healthc. Mater. 2015, 4, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Xia, J.; Wang, Z.; Kirkland, B.; Guan, J. Top-down fabrication of polyelectrolyte-thermoplastic hybrid microparticles for unidirectional drug delivery to single cells. Adv. Healthc. Mater. 2013, 2, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.J.; Wang, J.; Miao, Y.; Liu, Y.Q.; Jiang, W.; Fan, Z.X.; Darabi, M.A.; Hu, Z.Q.; Xing, M. Cytokine loaded layer-by-layer ultrathin matrices to deliver single dermal papilla cells for spot-by-spot hair follicle regeneration. J. Mater. Chem. B 2016, 4, 489–504. [Google Scholar] [CrossRef]
- Sakai, S.; Hashimoto, I.; Tanaka, S.; Salmons, B.; Kawakami, K. Small Agarose Microcapsules With Cell-Enclosing Hollow Core for Cell Therapy: Transplantation of Ifosfamide-Activating Cells to the Mice With Preestablished Subcutaneous Tumor. Cell Transplant. 2009, 18, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Xia, J.F.; Wang, Z.B.; Guan, J.J. Gold nanoparticle-packed microdisks for multiplex Raman labelling of cells. Nanoscale 2014, 6, 8762–8768. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullin, R.F.; Zamaleeva, A.I.; Morozov, M.V.; Tazetdinova, D.I.; Alimova, F.K.; Hilmutdinov, A.K.; Zhdanov, R.I.; Kahraman, M.; Culha, M. Living Fungi Cells Encapsulated in Polyelectrolyte Shells Doped with Metal Nanoparticles. Langmuir 2009, 25, 4628–4634. [Google Scholar] [CrossRef] [PubMed]
- Krol, S.; del Guerra, S.; Grupillo, M.; Diaspro, A.; Gliozzi, A.; Marchetti, P. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006, 9, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Andorko, J.I.; Hess, K.L.; Pineault, K.G.; Jewell, C.M. Intrinsic immunogenicity of rapidly-degradable polymers evolves during degradation. Acta Biomater. 2016, 32, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Gammon, J.M.; Dold, N.M.; Jewell, C.M. Improving the clinical impact of biomaterials in cancer immunotherapy. Oncotarget 2016, 7, 15421–15443. [Google Scholar] [CrossRef] [PubMed]
- Tostanoski, L.H.; Gosselin, E.A.; Jewell, C.M. Design of Polyelectrolyte Multilayers to Promote Immunological. ACS Nano 2016, 10, 9334–9345. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; Allen, R.S.; Guan, X.; Jin, P.; Uchida, N.; Dovey, M.; Harris, J.M.; Metzger, M.E.; Bonifacino, A.C.; Stroncek, D.; et al. Prostaglandin E2 Enhances Human Cord Blood Stem Cell Xenotransplants and Shows Long-Term Safety in Preclinical Nonhuman Primate Transplant Models. Cell Stem Cell 2011, 8, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.; Heimfeld, S.; Brashem-Stein, C.; Voorhies, H.; Manger, R.L.; Bernstein, I.D. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat. Med. 2010, 16, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Quintarelli, C.; Savoldo, B.; Dotti, G. Gene therapy to improve function of T cells for adoptive immunotherapy. Methods Mol. Biol. 2010, 651, 119–130. [Google Scholar] [PubMed]
- Goldberg, G.L.; Zakrzewski, J.L.; Perales, M.A.; van den Brink, M.R. Clinical strategies to enhance T cell reconstitution. Semin. Immunol. 2007, 19, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.Y.; Kronenfeld, J.P.; Stabler, C.L. Engineering biomimetic materials for islet transplantation. Curr. Diabetes Rev. 2015, 11, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Asif, S.; Ekdahl, K.N.; Nilsson, B. Cell Surface Engineering for Regulation of Immune Reactions in Cell Therapy. Adv. Exp. Med. Biol. 2015, 865, 189–209. [Google Scholar] [PubMed]
- Eich, T.; Eriksson, O.; Sundin, A.; Estrada, S.; Brandhorst, D.; Brandhorst, H.; Langstrom, B.; Nilsson, B.; Korsgren, O.; Lundgren, T. Positron emission tomography: A real-time tool to quantify early islet engraftment in a preclinical large animal model. Transplantation 2007, 84, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Alexander, M.; Robles, L.; Foster, C.E.; Lakey, J.R., III. Islet and stem cell encapsulation for clinical transplantation. Rev. Diabet. Stud. 2014, 11, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Roy, S. Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: Cells, biomaterials, and devices. Biotechnol. Bioeng. 2016, 113, 1381–1402. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Iwata, H. Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv. Drug Deliv. Rev. 2010, 62, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Sumi, S.; Yoshimatsu, G.; Goto, M.; Egawa, S.; Unno, M. Encapsulated islets transplantation: Past, present and future. World J. Gastrointest. Pathophysiol. 2012, 3, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Santos, E.; Poncelet, D.; Hernandez, R.M.; Pedraz, J.L.; Wahlberg, L.U.; De Vos, P.; Emerich, D. Cell encapsulation: Technical and clinical advances. Trends Pharmacol. Sci. 2015, 36, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.T.; Xu, Z.B.; Wang, H.; Reese, B.E.; Gushchina, L.V.; Jiang, M.; Agarwal, P.; Xu, J.S.; Zhang, M.J.; Shen, R.L.; et al. Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction. Nat. Commun. 2016, 7, 13306. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.L.; Andorko, J.I.; Tostanoski, L.H.; Jewell, C.M. Polyplexes assembled from self-peptides and regulatory nucleic acids blunt toll-like receptor signaling to combat autoimmunity. Biomaterials 2017, 118, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Veerabadran, N.G.; Goli, P.L.; Stewart-Clark, S.S.; Lvov, Y.M.; Mills, D.K. Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol. Biosci. 2007, 7, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mendicino, M.; Bailey, A.M.; Wonnacott, K.; Puri, R.K.; Bauer, S.R. MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell 2014, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Li, X.; Chung, U.I.; Sakai, T. Design of Hydrogels for Biomedical Applications. Adv. Healthc. Mater. 2015, 4, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Kravtzoff, R.; Colombat, P.H.; Desbois, I.; Linassier, C.; Muh, J.P.; Philip, T.; Blay, J.Y.; Gardenbas, M.; Poumier-Gaschard, P.; Lamagnere, J.P.; et al. Tolerance evaluation of l-asparaginase loaded in red blood cells. Eur. J. Clin. Pharmacol. 1996, 51, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.E.; Bain, M.D.; Fairbanks, L.D.; Simmonds, H.A.; Webster, A.D.; Chalmers, R.A. Carrier erythrocyte entrapped adenosine deaminase therapy in adenosine deaminase deficiency. Adv. Exp. Med. Biol. 2000, 486, 47–50. [Google Scholar] [PubMed]
- Annese, V.; Latiano, A.; Rossi, L.; Lombardi, G.; Dallapiccola, B.; Serafini, S.; Damonte, G.; Andriulli, A.; Magnani, M. Erythrocytes-mediated delivery of dexamethasone in steroid-dependent IBD patients—A pilot uncontrolled study. Am. J. Gastroenterol. 2005, 100, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Zarrin, A.; Foroozesh, M.; Mohammadi-Samani, S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J. Control. Release 2007, 118, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, Z.; Huang, D.; Yan, Y.; Li, Y.; Guan, J. Asymmetric biodegradable microdevices for cell-borne drug delivery. ACS Appl. Mater. Interfaces 2015, 11, 6293–6299. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, Z.; Yan, Y.; Cheng, Z.; Sun, L.; Li, Y.; Ren, Y.; Guan, J. Catalase-laden Microdevices for Cell-mediated Enzyme Delivery. Langmuir 2016, 32, 13386–13393. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.X.; Xie, Z.W.; Kim, G.B.; Dong, C.; Yang, J. Design Strategies and Applications of Circulating Cell-Mediated Drug Delivery Systems. ACS Biomater. Sci. Eng. 2015, 1, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Andorko, J.I.; Hess, K.L.; Jewell, C.M. Harnessing biomaterials to engineer the lymph node microenvironment for immunity or tolerance. AAPS J. 2015, 17, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Sharp, F.A.; Ruane, D.; Claass, B.; Creagh, E.; Harris, J.; Malyala, P.; Singh, M.; O’Hagan, D.T.; Petrilli, V.; Tschopp, J.; et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Tostanoski, L.H.; Chiu, Y.C.; Gammon, J.M.; Simon, T.; Andorko, J.I.; Bromberg, J.S.; Jewell, C.M. Reprogramming the Local Lymph Node Microenvironment Promotes Tolerance that Is Systemic and Antigen Specific. Cell Rep. 2016, 16, 2940–2952. [Google Scholar] [CrossRef] [PubMed]
- Lybaert, L.; De Vlieghere, E.; De Rycke, R.; Vanparijs, N.; De Wever, O.; De Koker, S.; De Geest, B.G. Bio-Hybrid Tumor Cell-Templated Capsules: A Generic Formulation Strategy for Tumor Associated Antigens in View of Immune Therapy. Adv. Funct. Mater. 2014, 24, 7139–7150. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, L.; Han, J.; Chu, J.; Wang, H.; Chen, X.; Wang, Y.; Tun, N.; Lu, L.; Bai, X.F.; et al. Conformal Nanoencapsulation of Allogeneic T Cells Mitigates Graft-versus-Host Disease and Retains Graft-versus-Leukemia Activity. ACS Nano 2016, 10, 6189–6200. [Google Scholar] [CrossRef] [PubMed]
- Penn, M.S.; Mangi, A.A. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ. Res. 2008, 102, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Drachuk, I.; Gupta, M.K.; Tsukruk, V.V. Biomimetic Coatings to Control Cellular Function through Cell Surface Engineering. Adv. Funct. Mater. 2013, 23, 4437–4453. [Google Scholar] [CrossRef]
- Stephan, M.T.; Irvine, D.J. Enhancing cell therapies from the outside in: Cell surface engineering using synthetic nanomaterials. Nano Today 2011, 6, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, S.J.; Nam, J.H.; Byun, Y. A new strategy toward improving immunoprotection in cell therapy for diabetes mellitus: Long-functioning PEGylated islets in vivo. Tissue Eng. 2006, 12, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Spencer, J.A.; Phillips, J.A.; Zhao, W.A.; Schafer, S.; Spelke, D.P.; Mortensen, L.J.; Ruiz, J.P.; Vemula, P.K.; Sridharan, R.; et al. Engineered cell homing. Blood 2011, 118, E184–E191. [Google Scholar] [CrossRef] [PubMed]
- Howarth, M.; Takao, K.; Hayashi, Y.; Ting, A.Y. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. USA 2005, 102, 7583–7588. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.L.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.B.; Jiang, W.Y.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 6121, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
| Opportunities | Cell Modification Strategies | References |
|---|---|---|
| Viable cells with novel functionalities | Engineering cell surfaces without toxicity | [44,47,48] |
| Generating cells with novel functionality | [49,50,51,52,53,54] | |
| Cell therapy | Encapsulating islet cells with bulk materials | [55] |
| Encapsulating islet cells with PEM shells | [42] | |
| Encapsulating stem cells with PEM shells | [56] | |
| Tissue engineering | Generating cell-laden semi-permeable capsules | [8,9,10,11] |
| Fabricating liquefied multilayer hierarchical capsules | [57,58,59] | |
| Assembing cells in an electric filed | [60] | |
| Forming celluar spheroids with polymers | [61,62] | |
| Assembling cells with multilayer patches | [63,64,65] | |
| Cell-based drug delivery | Using cells as drug delivery vehiclesEncapsulating cells with drug-loaded PEMsEncapsulating drug secreting cells with capsules | [12,63,66,67] [68,69] [70] |
| Sensing and tracking | SERS-based cell sensing and tracking | [71,72] |
| Immune modulation | Protecting cells from immune responsesModulating complement activation with polymer grafting | [3,42] [66] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Bookstaver, M.L.; Jewell, C.M. Engineering Cell Surfaces with Polyelectrolyte Materials for Translational Applications. Polymers 2017, 9, 40. https://doi.org/10.3390/polym9020040
Zhang P, Bookstaver ML, Jewell CM. Engineering Cell Surfaces with Polyelectrolyte Materials for Translational Applications. Polymers. 2017; 9(2):40. https://doi.org/10.3390/polym9020040
Chicago/Turabian StyleZhang, Peipei, Michelle L. Bookstaver, and Christopher M. Jewell. 2017. "Engineering Cell Surfaces with Polyelectrolyte Materials for Translational Applications" Polymers 9, no. 2: 40. https://doi.org/10.3390/polym9020040






