Design of Dithiobenzoate RAFT Agent Bearing Hydroxyl Groups and Its Application in RAFT Polymerization for Telechelic Diol Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
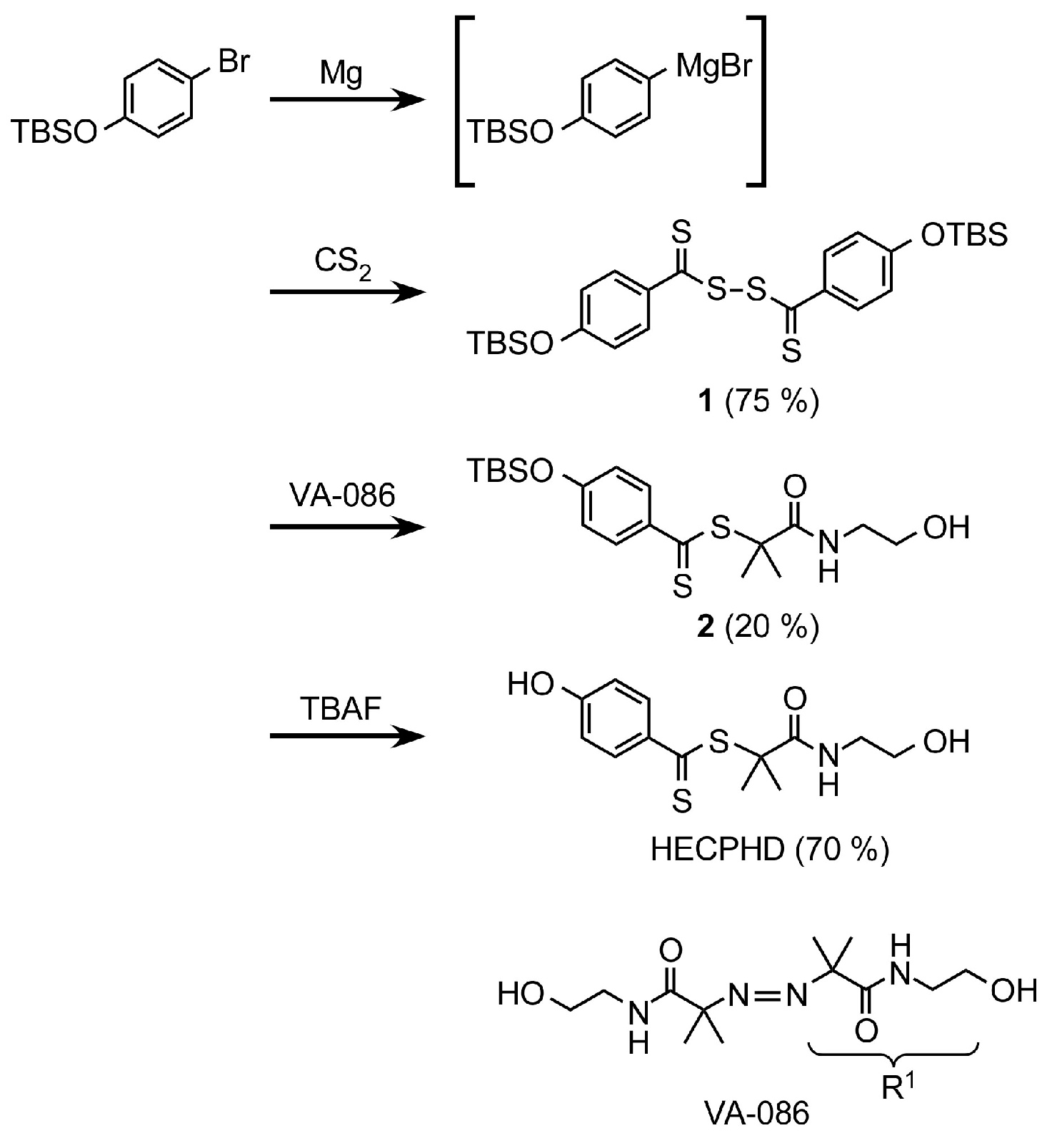
2.2. Synthesis of Bis(4-tert-butyldimethylsiloxythiobenzoyl) Disulfide (1)
2.3. Synthesis of 2-[N-(2-Hydroxyethyl)carbamoyl]prop-2-yl 4-tert-butyldimethylsiloxy-dithiobenzoate 2
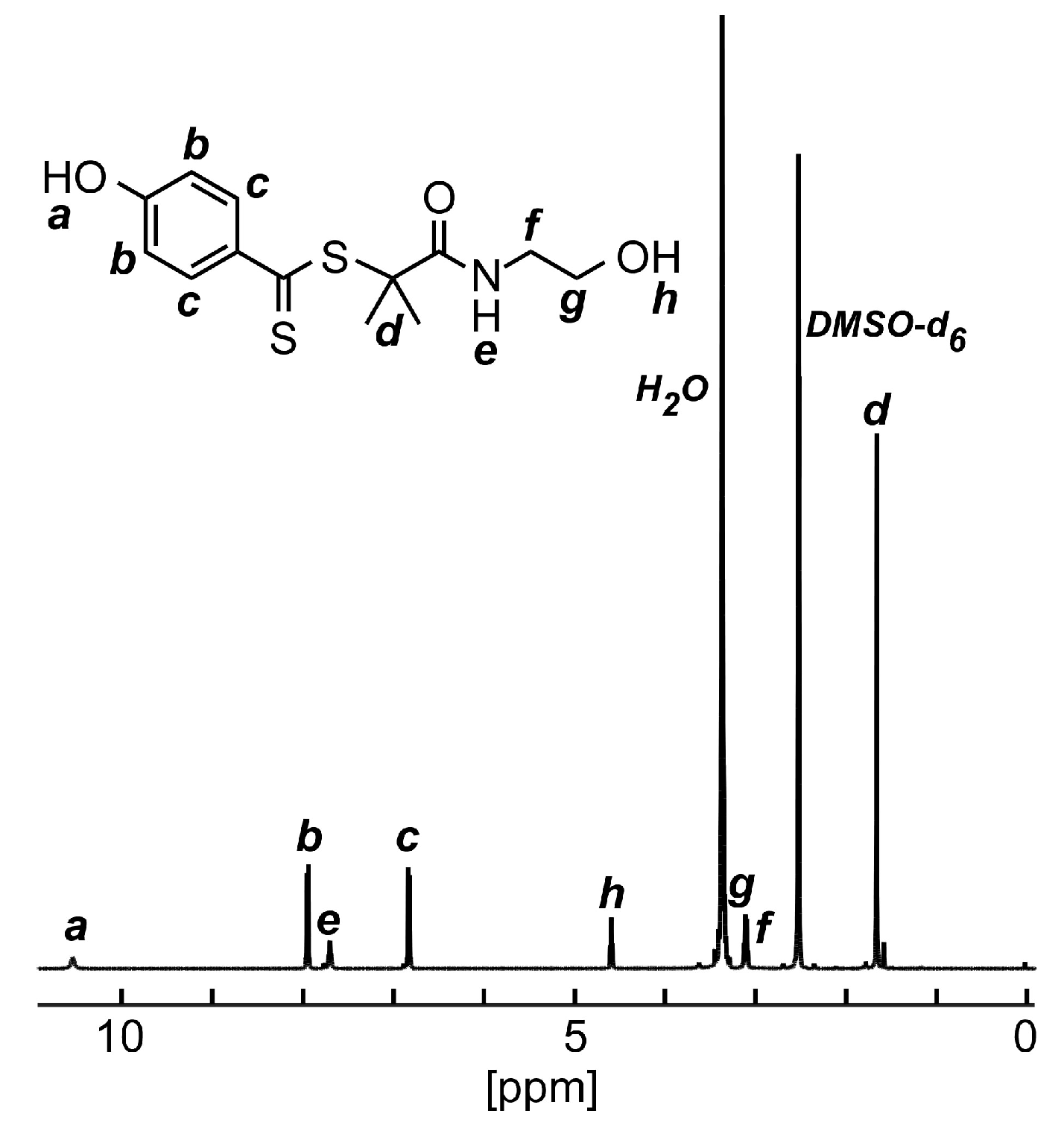
2.4. Synthesis of “Dithiobenzoate”-Type CTA, 2-[N-(2-hydroxyethyl)carbamoyl]prop-2-yl 4-hydroxydithiobenzoate (HECPHD), Bearing Hydroxyl Groups at Both Terminals
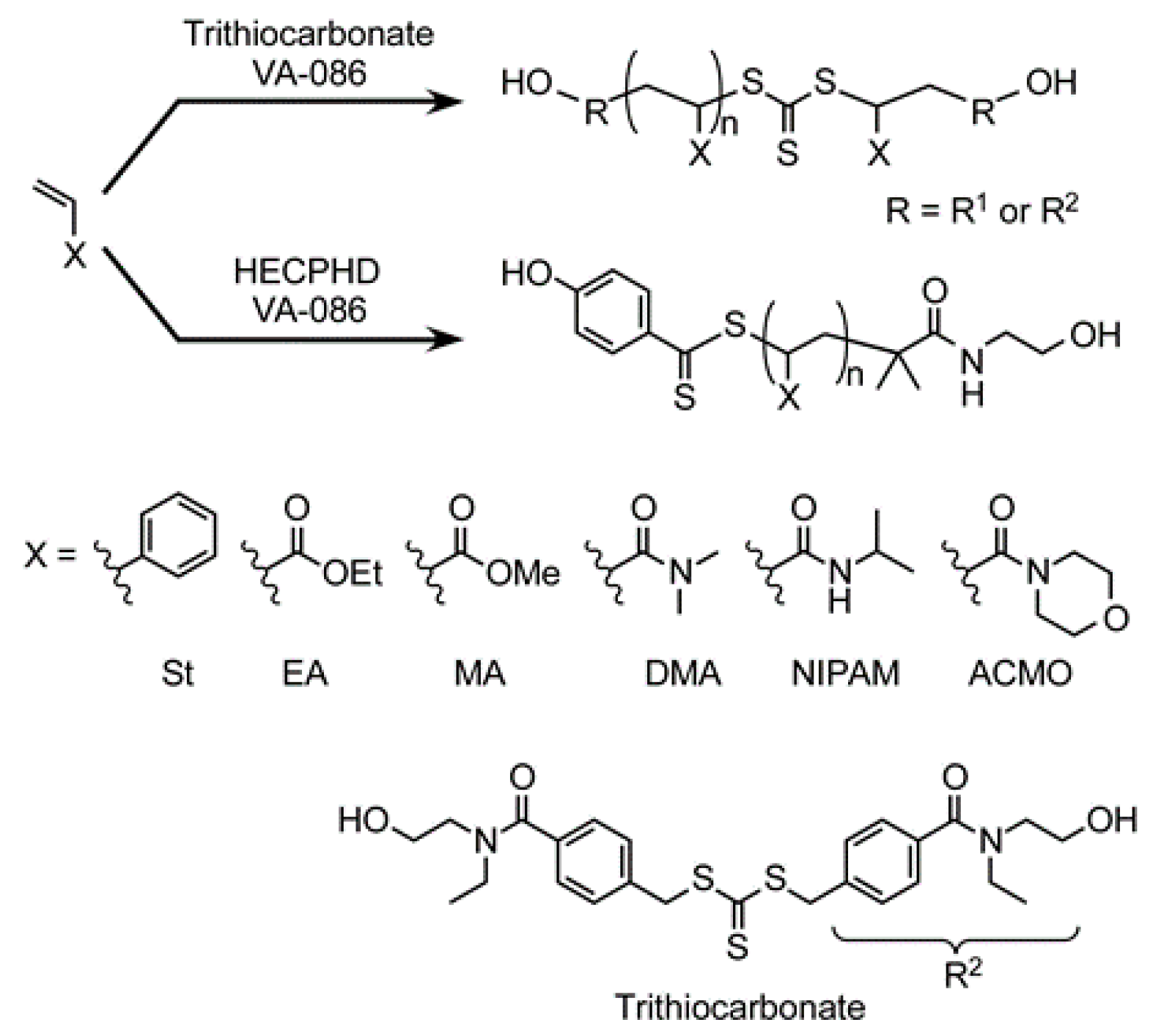
2.5. RAFT Polymerization of Various Monomers Using CTAs
3. Results and Discussion
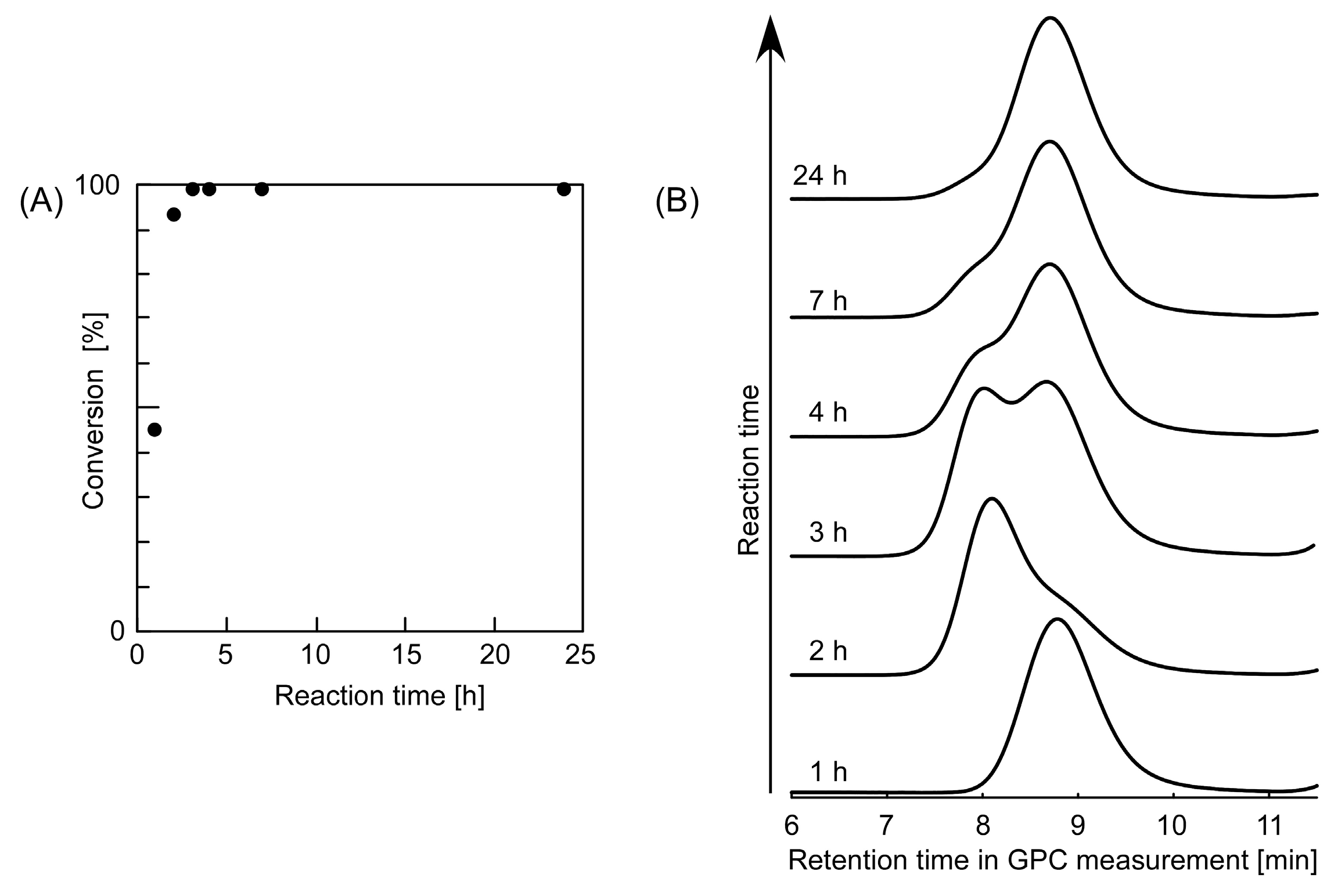
3.1. Synthesis of Telechelic Diol Polymers by Means of a Trithiocarbonate-Type CTA Bearing Hydroxyl Groups at Both Terminals
3.2. Synthesis of HECPHD
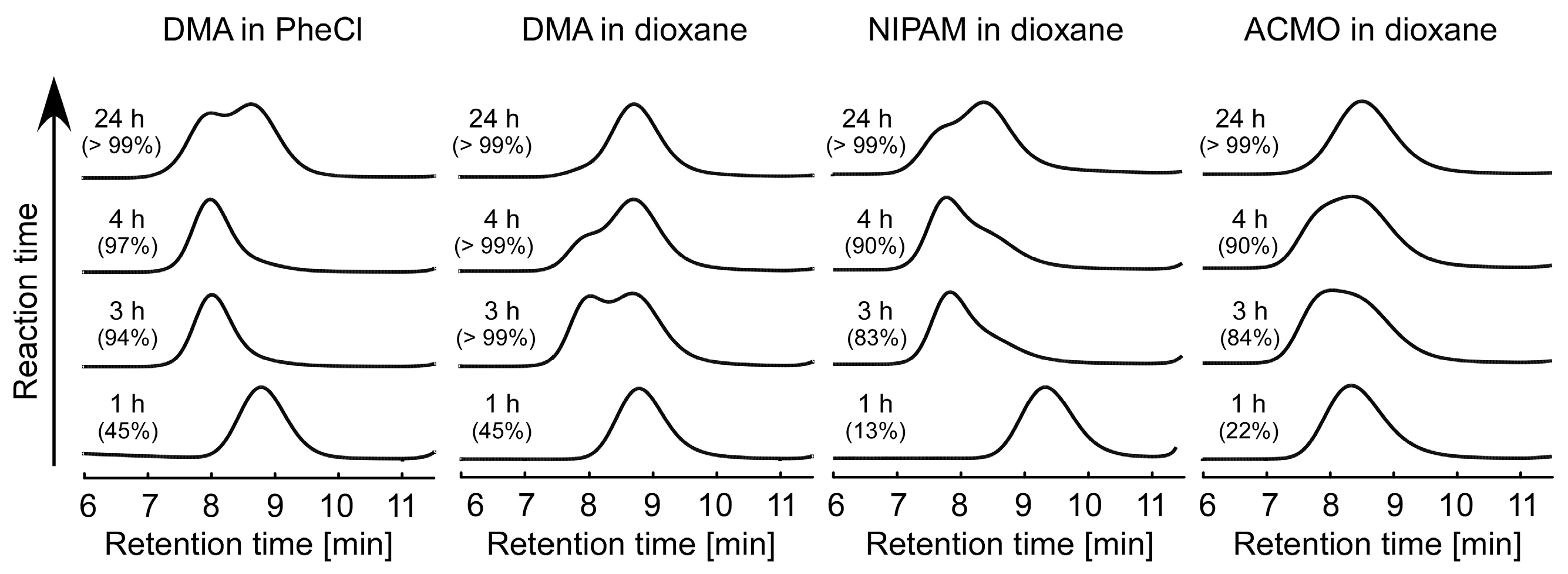
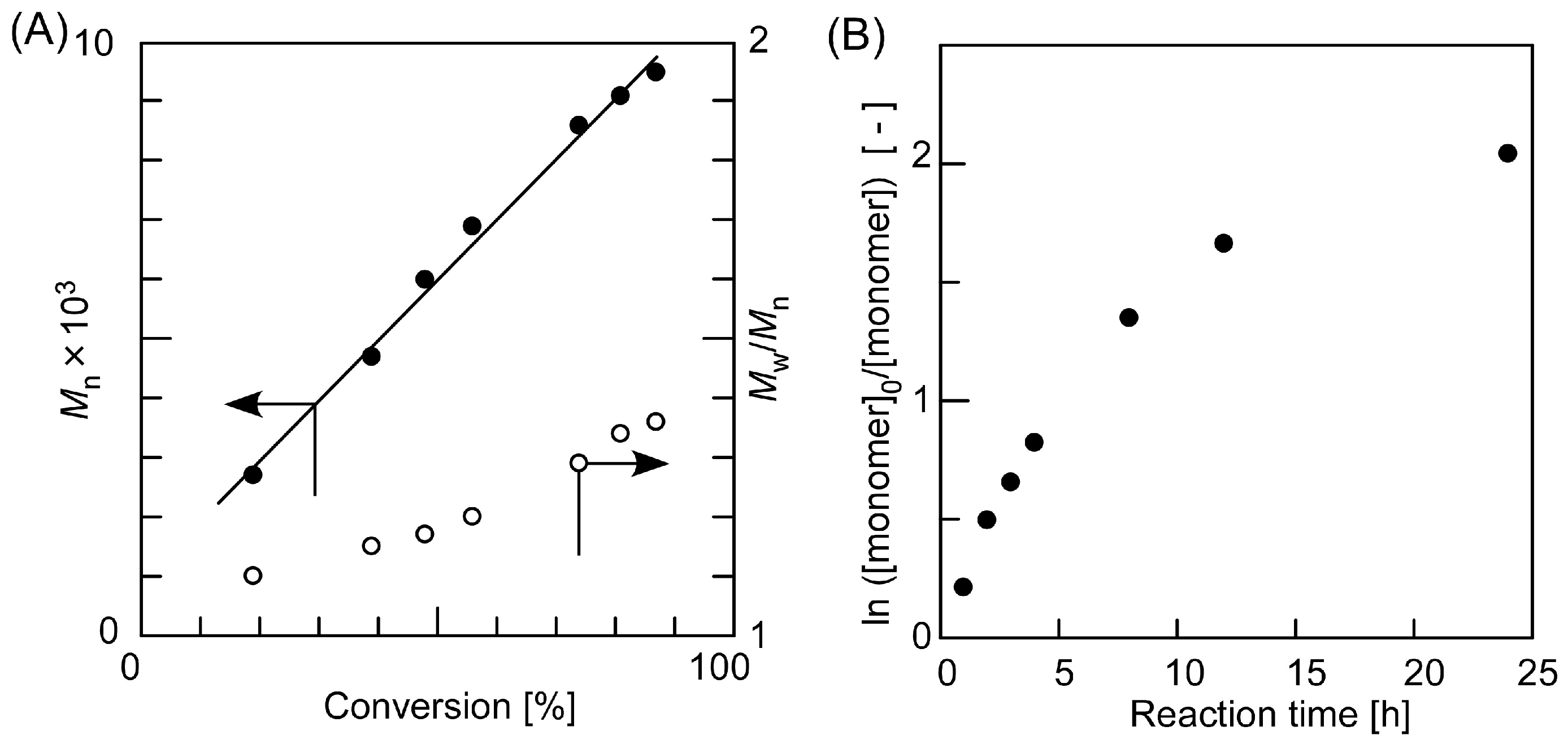
3.3. RAFT Polymerization of Various Monomers with HECPHD
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Tasdelen, M.A.; Kahveci, M.U.; Yagci, Y. Telechelic polymers by living and controlled/living polymerization methods. Prog. Polym. Sci. 2011, 36, 455–567. [Google Scholar] [CrossRef]
- Guillaume, S.M. Recent advances in ring-opening polymerization strategies toward α,ω-hydroxy telechelic polyesters and resulting copolymers. Eur. Polym. J. 2013, 49, 768–779. [Google Scholar] [CrossRef]
- Sarbu, T.; Lin, K.-Y.; Spanswick, J.; Gil, R.R.; Siegwart, D.J.; Matyjaszewski, K. Synthesis of hydroxy-telechelic poly(methyl acrylate) and polystyrene by atom transfer radical coupling. Macromolecules 2004, 37, 9694–9700. [Google Scholar] [CrossRef]
- Chessa, G.; Scrivanti, A.; Matteoli, U.; Castelvetro, V. Synthesis of three- and six-arms polystyrene via living/controlled free radical polymerization. Polymer 2001, 42, 9347–9353. [Google Scholar] [CrossRef]
- Radhakrishnan, B.; Balerdi, G.; Cloutet, E.; Cramail, H. Hydroxy and di-hydroxy end-capped polybutadiene as reactive steric stabilizers for the synthesis of polyurethane particles in organic dispersant media. Macromol. Symp. 2005, 229, 56–65. [Google Scholar] [CrossRef]
- Debuigne, A.; Schoumacher, M.; Willet, N.; Riva, R.; Zhu, X.; Rutten, S.; Jeromeme, C.; Detrembleur, C. New functional poly(N-vinylpyrrolidone) based (co)polymers via photoinitiated cobalt-mediated radical polymerization. Chem. Commun. 2011, 47, 12703–12705. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Matyjaszewski, K. Controlled/“living” radical polymerization. Halogen atom transfer radical polymerization promoted by a Cu(I)/Cu(II) redox process. Macromolecules 1995, 28, 7901–7910. [Google Scholar] [CrossRef]
- Xia, J.; Matyjaszewski, K. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process—A second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Benoit, D.; Chaplinski, V.; Braslau, R.; Hawker, C.J. Development of a universal alkoxyamine for “living” free radical polymerizations. J. Am. Chem. Soc. 1999, 121, 3904–3920. [Google Scholar] [CrossRef]
- Lee, N.S.; Li, Y.; Ruda, C.M.; Wooley, K.L. Aqueous-only, pH-induced nanoassembly of dual pKa-driven contraphilic block copolymers. Chem. Commun. 2008, 41, 5339–5341. [Google Scholar] [CrossRef] [PubMed]
- Sudo, A.; Hamaguchi, T.; Aoyagi, N.; Endo, T. RAFT-approach to well-defined telechelic vinyl polymers with hydroxyl terminals as polymeric diol-type building blocks for polyurethanes. J. Polym. Sci. A Polym. Chem. 2013, 51, 318–326. [Google Scholar] [CrossRef]
- You, Y.; Hong, C.; Wang, W.; Lu, W.; Pan, C. Preparation and characterization of thermally responsive and biodegradable block copolymer comprised of PNIPAAM and PLA by combination of ROP and RAFT methods. Macromolecules 2004, 37, 9761–9767. [Google Scholar] [CrossRef]
- Thomas, D.B.; Convertine, A.J.; Hester, R.D.; Lowe, A.B.; McCormick, C.L. Hydrolytic susceptibility of dithioester chain transfer agents and implications in aqueous RAFT polymerizations. Macromolecules 2004, 37, 1735–1741. [Google Scholar] [CrossRef]
- Patton, D.L.; Mullings, M.; Fulghum, T.; Advincula, R.C. A facile synthesis route to thiol-functionalized α,ω-telechelic polymers via reversible addition fragmentation chain transfer polymerization. Macromolecules 2005, 38, 8597–8602. [Google Scholar] [CrossRef]
- Legge, T.M.; Slark, A.T.; Perrier, S. Thermal stability of reversible addition–fragmentation chain transfer/macromolecular architecture design by interchange of xanthates chain-transfer agents. J. Polym. Sci. A 2006, 44, 6980–6987. [Google Scholar] [CrossRef]
- Zhou, Y.; He, J.; Li, C.; Hong, L.; Yang, Y. Dependence of thermal stability on molecular structure of RAFT/MADIX agents: A kinetic and mechanistic study. Macromolecules 2011, 44, 8446–8457. [Google Scholar] [CrossRef]
- Abel, B.A.; McCormick, C.L. Mechanistic insights into temperature-dependent trithiocarbonate chain-end degradation during the RAFT polymerization of N-arylmethacrylamides. Macromolecules 2016, 49, 465–474. [Google Scholar] [CrossRef]



| Monomers | Solvents | Concentrations [M] | Conversion [%] b | Mn,theo c | Mn | Mw/Mn | |
|---|---|---|---|---|---|---|---|
| Monomers | VA-086 | ||||||
| St a | PhCl | 0.5 | 0.005 | 82 | 5000 | 4400 | 1.14 |
| EA a | PhCl | 0.5 | 0.005 | 97 | 5000 | 5500 | 1.15 |
| DMA | PhCl | 1 | 0.0025 | >99 | 10,000 | - d | - d |
| DMA | dioxane | 1 | 0.0025 | >99 | 10,000 | 5400 | 1.26 |
| NIPAM | dioxane | 1 | 0.0025 | >99 | 11,300 | 6000 | 1.29 |
| ACMO | dioxane | 1 | 0.0025 | >99 | 12,600 | 6000 | 1.28 |
| Monomers | Conversion [%] b | Mn,theo c | Mn | Mw/Mn |
|---|---|---|---|---|
| St | 29 | 3000 | 3000 | 1.12 |
| St d | 48 | 4800 | 4500 | 1.13 |
| EA | 69 | 6900 | 4800 | 1.33 |
| MA | 72 | 6200 | 4700 | 1.24 |
| DMA | 90 | 9000 | 7200 | 1.29 |
| NIPAM | 87 | 9800 | 9500 | 1.36 |
| ACMO | 95 | 14,000 | 8300 | 1.29 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, K.; Mori, Y.; Takami, T.; Uchida, Y.; Murakami, Y. Design of Dithiobenzoate RAFT Agent Bearing Hydroxyl Groups and Its Application in RAFT Polymerization for Telechelic Diol Polymers. Polymers 2017, 9, 44. https://doi.org/10.3390/polym9020044
Kinoshita K, Mori Y, Takami T, Uchida Y, Murakami Y. Design of Dithiobenzoate RAFT Agent Bearing Hydroxyl Groups and Its Application in RAFT Polymerization for Telechelic Diol Polymers. Polymers. 2017; 9(2):44. https://doi.org/10.3390/polym9020044
Chicago/Turabian StyleKinoshita, Keita, Yuta Mori, Taku Takami, Yusuke Uchida, and Yoshihiko Murakami. 2017. "Design of Dithiobenzoate RAFT Agent Bearing Hydroxyl Groups and Its Application in RAFT Polymerization for Telechelic Diol Polymers" Polymers 9, no. 2: 44. https://doi.org/10.3390/polym9020044




