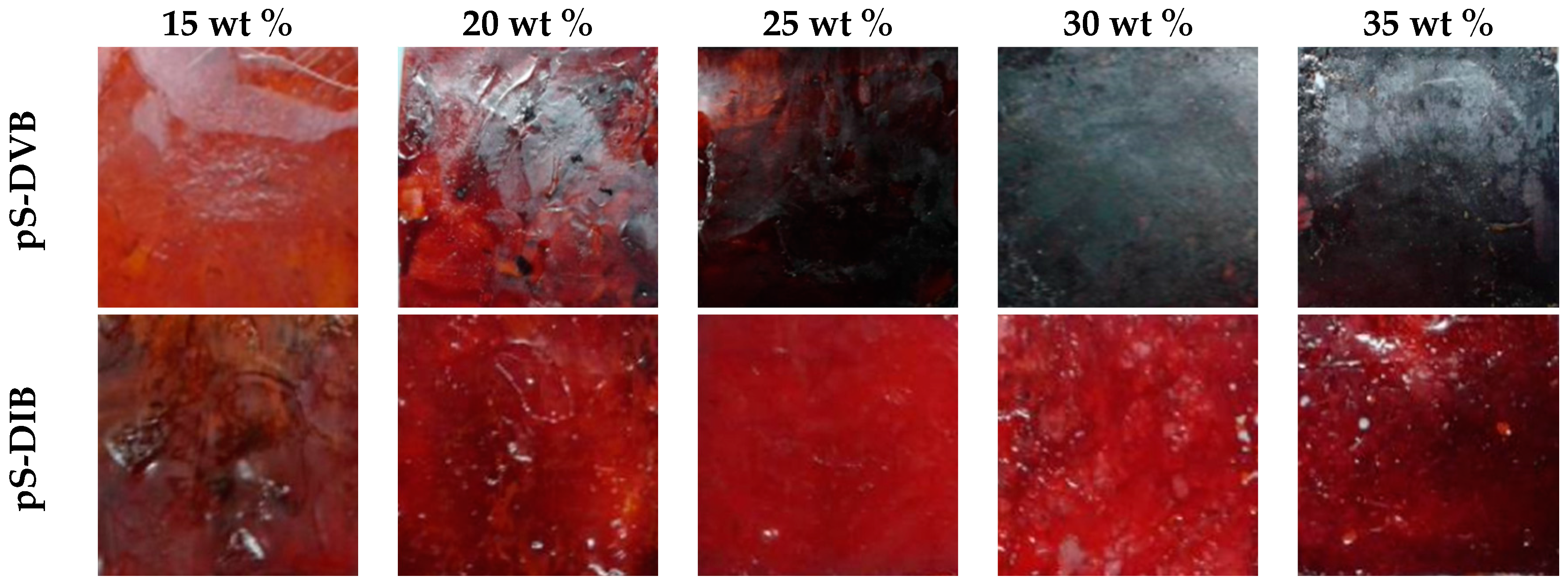
Depending on the comonomer type or feed, hyper-branched sulfur-containing polymers are obtained which are predominantly thermoplastics with more or less good shape retention. There are, however, influencing factors, such as the gel effect (chain reduction), yielding highly viscous plastomers of the sulfur copolymer with divinylbenzene (pS-DVB), or the impact of intensive heat treatment by the hot press (post cross-linking) leading to brittle thermosettings (pS-DIB). Only high comonomer contents of 30%–35% are affected by this. pS-DIB can form at high temperatures above 100 °C, a highly viscous melt that enables self-healing properties, while pS-DVB exhibits a permanent network, and it can only soften on the surface and is more difficult to process.
3.2. Structure and Surface
Sulfur copolymers are completely amorphous materials. Nevertheless, the polymer networks can show slight crystalline reflections in the PXRD. The detected attenuated crystalline reflections are in agreement with the powder X-ray diffractogram of crystalline elemental sulfur. In the case of low residual elemental sulfur in all sulfur copolymers, the respective monomers have not been converted completely and the depolymerization occurs with a temporal dependence. The small quantities of sulfur have no influence on thermal or mechanical properties. S8 can be qualitatively detected in the first heating phase of the DSC measurement and, in very small amounts, in the SEM images and PXRD diffractograms.
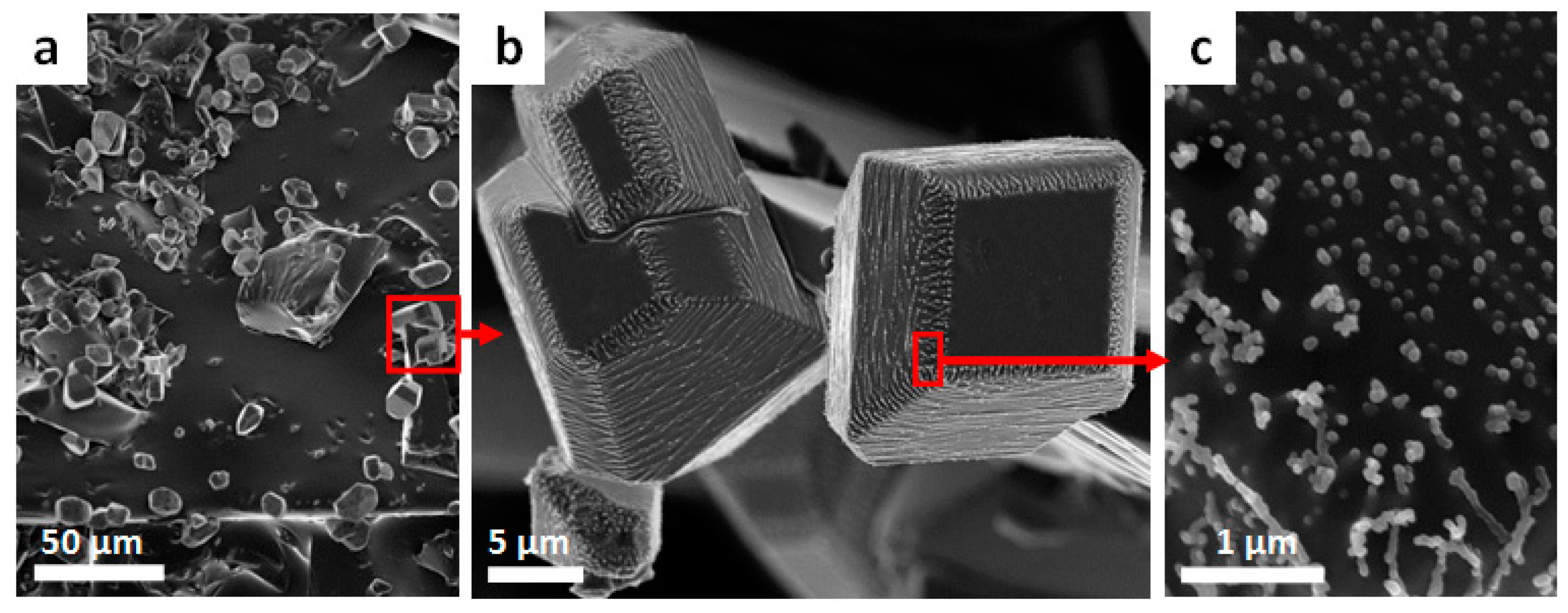
SEM-images of sulfur copolymers show sulfur microcrystals (
Figure 3b). These are characterized by an angular shape (
Figure 3b) with a plain surface showing little spherical dots (
Figure 3c). The microcrystals form agglomerates (
Figure 3a). The image shows exemplarily a section with an S
8 that remains on the polymer and is not distributed over the entire surface of the copolymers. This observation is confirmed by Salman et al. [
28].
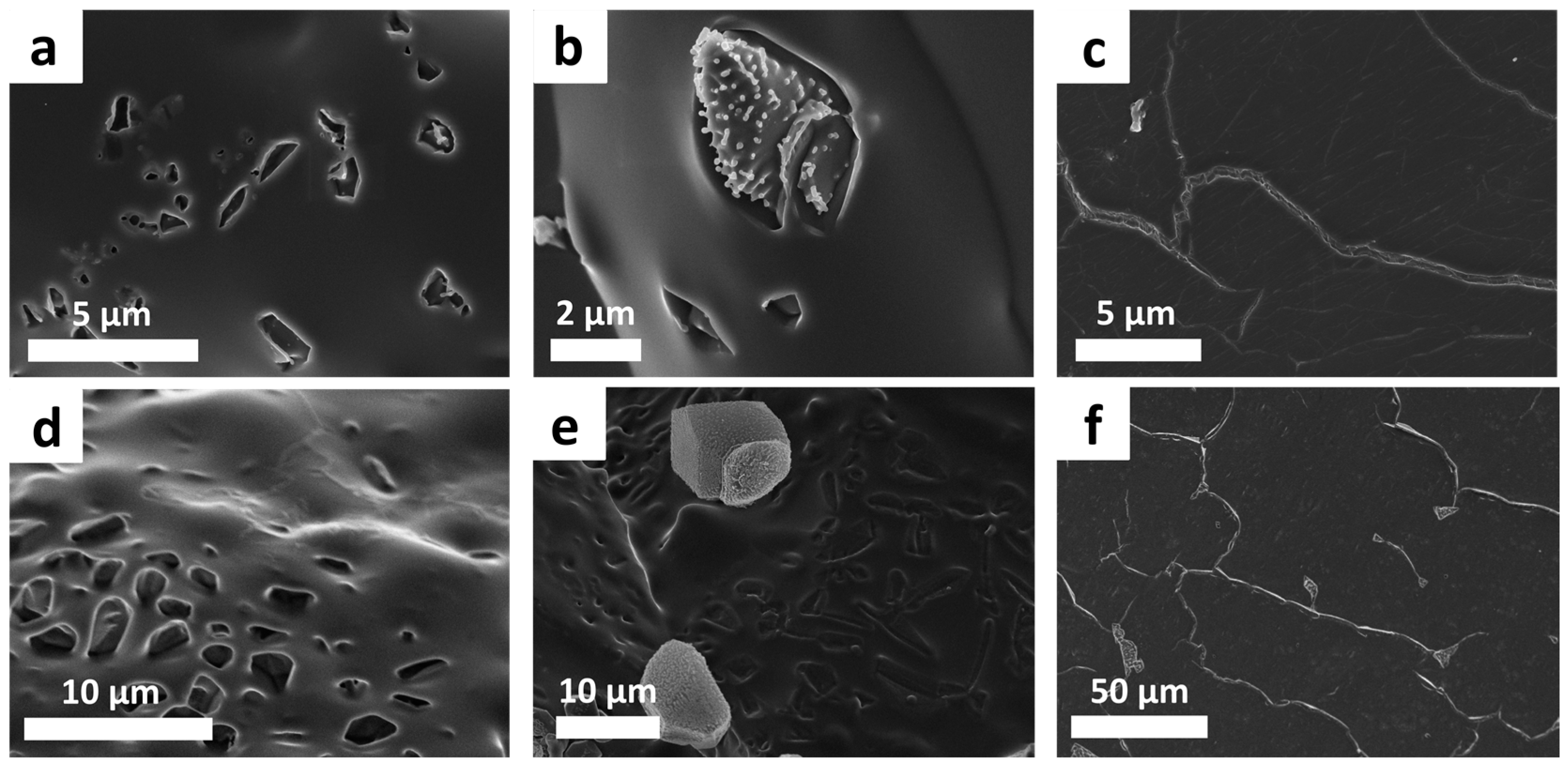
The sulfur copolymer films exhibit a plain surface, partly with unevenly distributed holes to rod-shaped notches (
Figure 4a,d). The holes are probably the remainders of sulfur-occupied gaps (
Figure 4b,e). After usage of the hot press, elemental sulfur almost disappears, probably by post cross-linking (virtually full conversion of sulfur). The sulfur copolymers show thin cracks when using the hot press (
Figure 4c,f).
Consequently, the structure appears slightly inhomogeneous due to residual elemental sulfur, holes, pores and cracks. The linking agents DVB and DIB seem to be incorporated completely into the polymer network because no residual monomer is detected in the gas chromatography. However, low double bonds are available (solid-state NMR), which show the presence of a few non-converted double bonds, so probably only one functional group of the divinyl monomers is affected. This observation of incomplete conversion of double bonds of the vinyl monomers agrees with the investigations of Pyun using larger reaction scales [
10]. Despite the same conditions for each reaction and thus similar conversion values of double bonds, other effects (gel effect, post cross-linking) have an additional influence on the properties, possibly with no linear correlation.
3.3. Thermal Properties
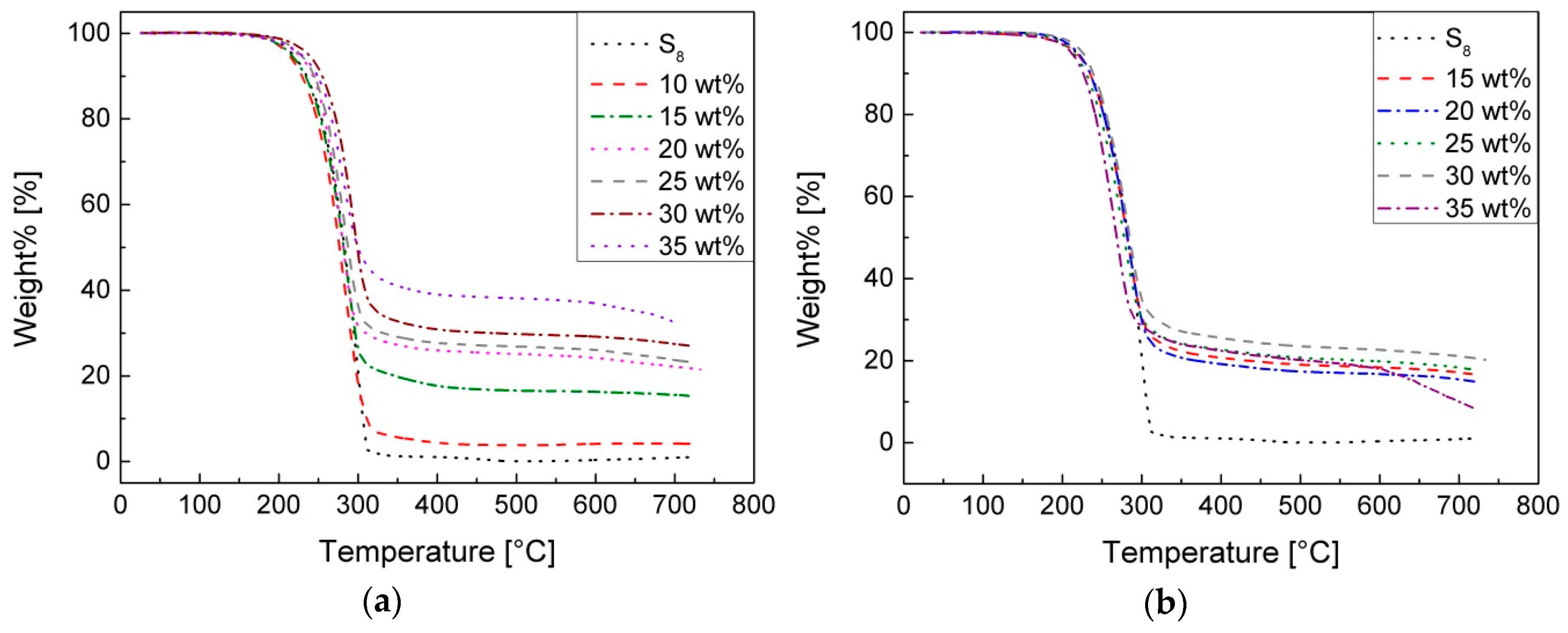
The thermogravimetric analysis (
Figure 5) shows thermal resistance up to 220 °C of all types of synthesized sulfur copolymers. Between 200–300 °C, there is a steep, massive weight% loss which is related to the decomposition of the sulfur. By increasing the temperature, no further mass loss occurs. The residues of the pS-DVBs correlate with the respective incorporated monomer content. The residue of pS-DIBs seems to be independent of the incorporated monomer content. The mass residue has a constant value of 17% ± 2%. The high residue after the decomposition indicates that presumably a cross-linking network is present.
In comparison with the results of Pyun, the decomposition curve of pS-DVB is shifted to a higher temperature (+20 °C), which indicates a higher temperature stability [
11]. The course of the decomposition curve of the pS-DIB is neither comparable nor the uniform residue explainable without any dependence of the monomer content. The decomposition temperature corresponds to 5% weight loss of the initial mass, when the decomposition starts. This value occurred in all samples at an average temperature of 222 ± 9 °C (
Table 1). Different publications report on similar results above 200 °C of the decomposition temperature [
8,
11,
14].
Bear et al. have analyzed the leaching out in the annealing process between 300–400 °C by means of EDS (Energy-dispersive X-ray spectroscopy) and have attributed it to the decrease of sulfur. Independent of the DIB content, the decay contains approximately 90 wt % sulfur. By means of the supported quantitative XPS analysis (X-ray photoelectron spectroscopy), it was determined that the remaining sulfur does not alter significantly after annealing. In the remaining compound, organic sulfate groups (168.0 eV) were identified, including such species such as thioethers or disulfides (164.0 eV), C–S (163.98 eV), SOx and R–SOx–R′ (167.88 eV) as well as C=S (161.5 eV, low intensity). With 80% of the sulfur signals, the C–S bonds have the highest quantity, even though they represent only an average of 8% of the total remaining structure. This indicates an “inverse vulcanization” architecture of the copolymer by retention after the annealing process. Independent of the sample, there is a critical amount of sulfur between 7.4%–14.1%, which remains after annealing. However, the carbon composition fluctuates very strongly. Probably, more stable mono- or disulfide linkages are formed, which stabilize the remaining sulfur content, and a higher initial sulfur concentration preferentially forms longer polysulfide chains (S–Sn–S) between the –C–S linkages. The longer chains containing S–S bonds tend to decompose at high temperatures under sublimation.
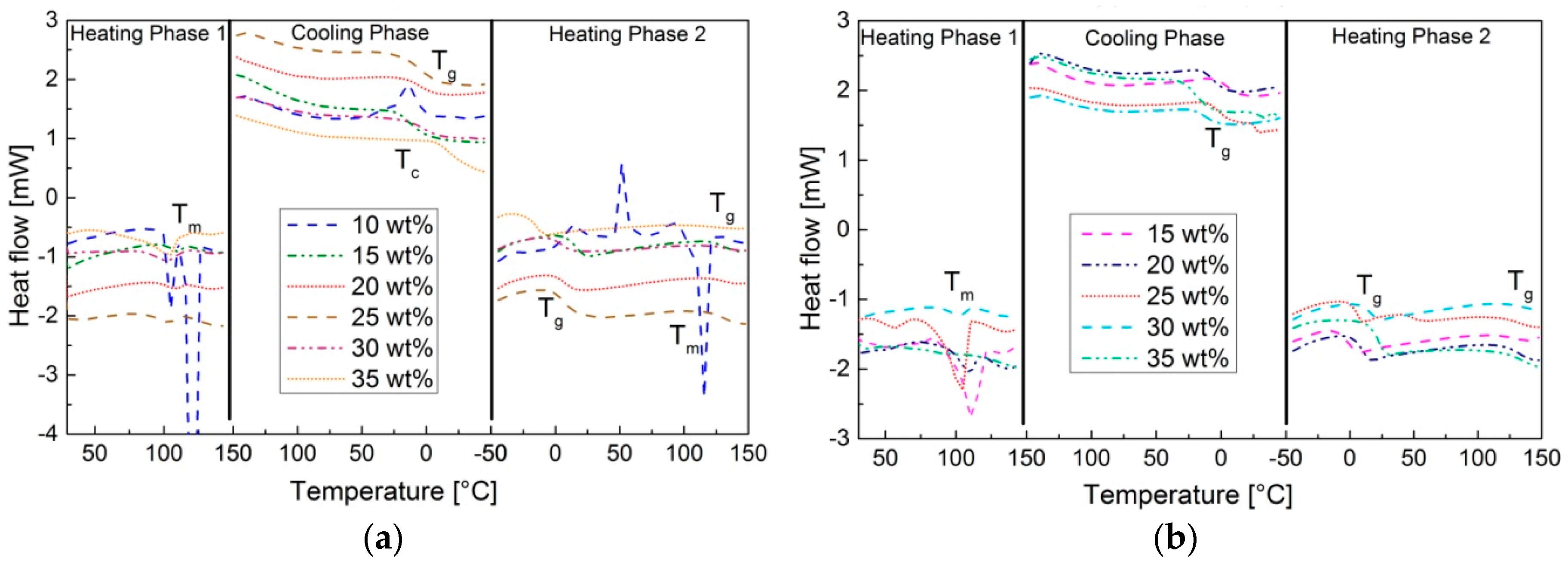
The observed melting point in the first heating step in the DSC-thermogram of pS-DVB (<15 wt % DVB,
Figure 6a) and pS-DIB (15–25 wt % DIB,
Figure 6b) is caused by small amounts of non- converted elemental sulfur. This residue does not crystallize in the cooling step, so neither
Tc nor
Tm appear afterwards, which shows that a complete S
8-conversion has taken place after the thermal treatment.
Tg and other transitions are determined for all sulfur copolymers in the second heating step (
Figure 6).
The more pronounced first step, the
Tg, shows the transition from glass to plastic in the pS-DVB and from brittle to thermoplastic in the pS-DIB. The
Tg increases by higher cross-linking density or molecular weight as shown in pS-DIB (
Figure 7b). pS-DVB (
Figure 7a) does not behave accordingly. From an amount of 17.5 wt % DVB, the
Tg tends to decrease with an increase of added DVB content. At a lower scale, an increase in
Tg with increasing monomer content [
11] can be observed in the pS-DVB. This divergence, which occurs in comparison to a 200 g scale, can be explained by the chain reduction of pS-DVB (≥30 wt % DVB) caused by exceeding a temperature of 200 °C (gel effect) [
3]. Likewise, in the case of a higher DIB content, the
Tg is higher at a lower scale [
8,
10].
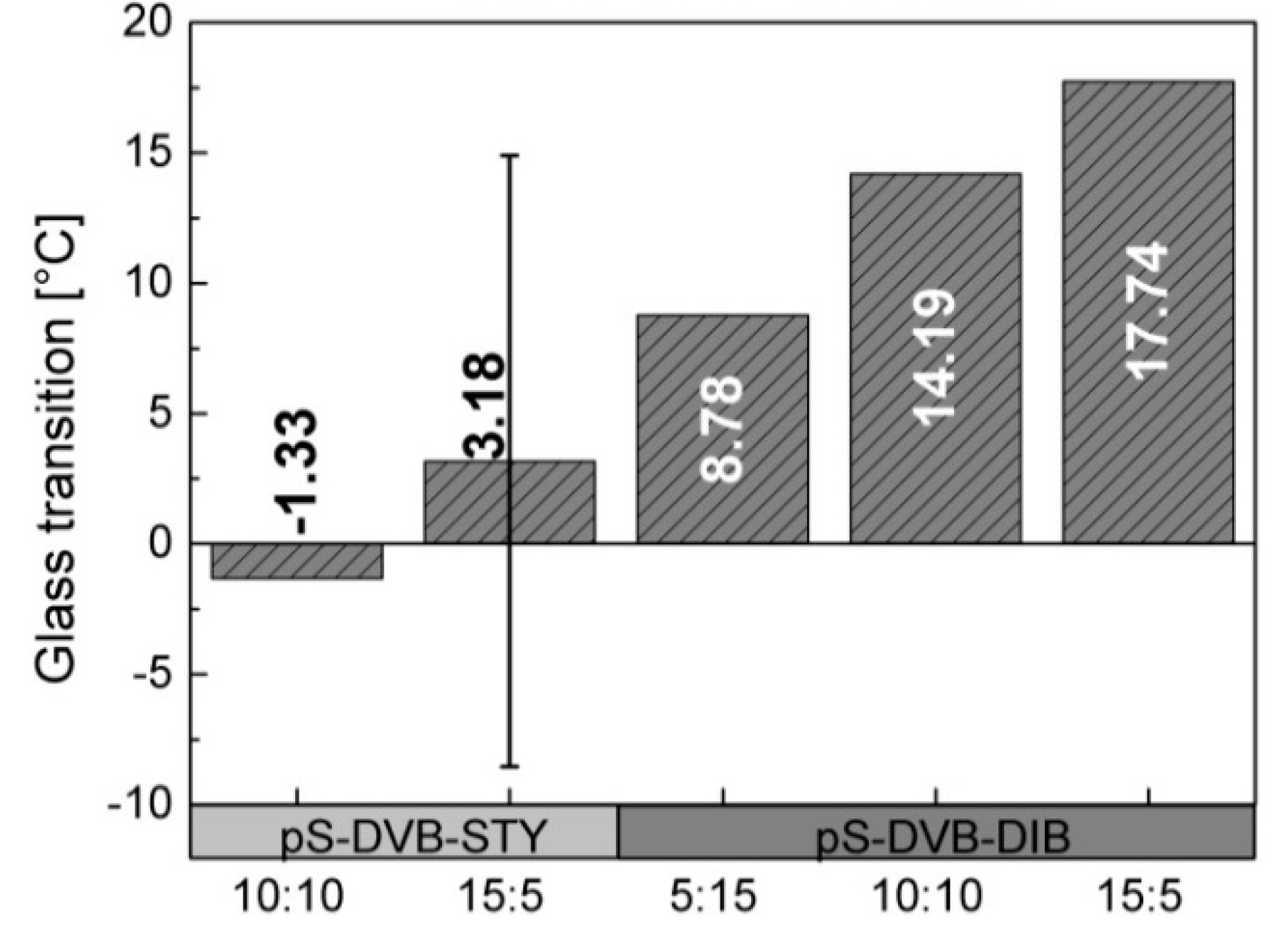
The
Tg of the terpolymer tends to increase when the added styrene content is decreased. The decrease of the DIB content in the DVB-based terpolymers leads to an increase of
Tg due to higher cross-linking abilities of DVB in comparison with DIB monomers (
Figure 8). Consequently, a higher cross-linking density leads to a higher
Tg [
29]. The work of Parker confirmed that styrene reduces the
Tg (similar to DIB) due to its relatively low
Tg and viscous, flexible ductility. Consequently, it shows properties of a plasticizer [
13].
The second lower transition could not be assigned, but its presence can be confirmed by the thermogram in the Dynamic Mechanical Analysis (DMA). This deformation transition occurs in pS-DVB as a softening point and in pS-DIB as a transition to a viscous melt. This occurs for all polymers at a temperature of approx. 131.5 ± 1 °C.
To become a viscous melt, despite the cross-linked structure, allows pS-DIB to act as a self-healing material. This behavior occurs due to the lower dissociation energy of S–S bonds (dynamic covalent bonds) in longer S−S chains (33 kcal∙mol
−1) [
30]. Upon thermal activation, the microstructure of the sulfur copolymer backbone cleaves and reorganizes itself into a different macromolecular framework. pS-DVBs exhibit only a slight softening point at high temperatures (>130 °C) without a molten state. Thus, pS-DVBs provide excellent properties in terms of temperature resistance and shape retention. The properties range from thermosetting regarding the cross-linking to plastic regarding the ductility.
3.4. Electrical Properties
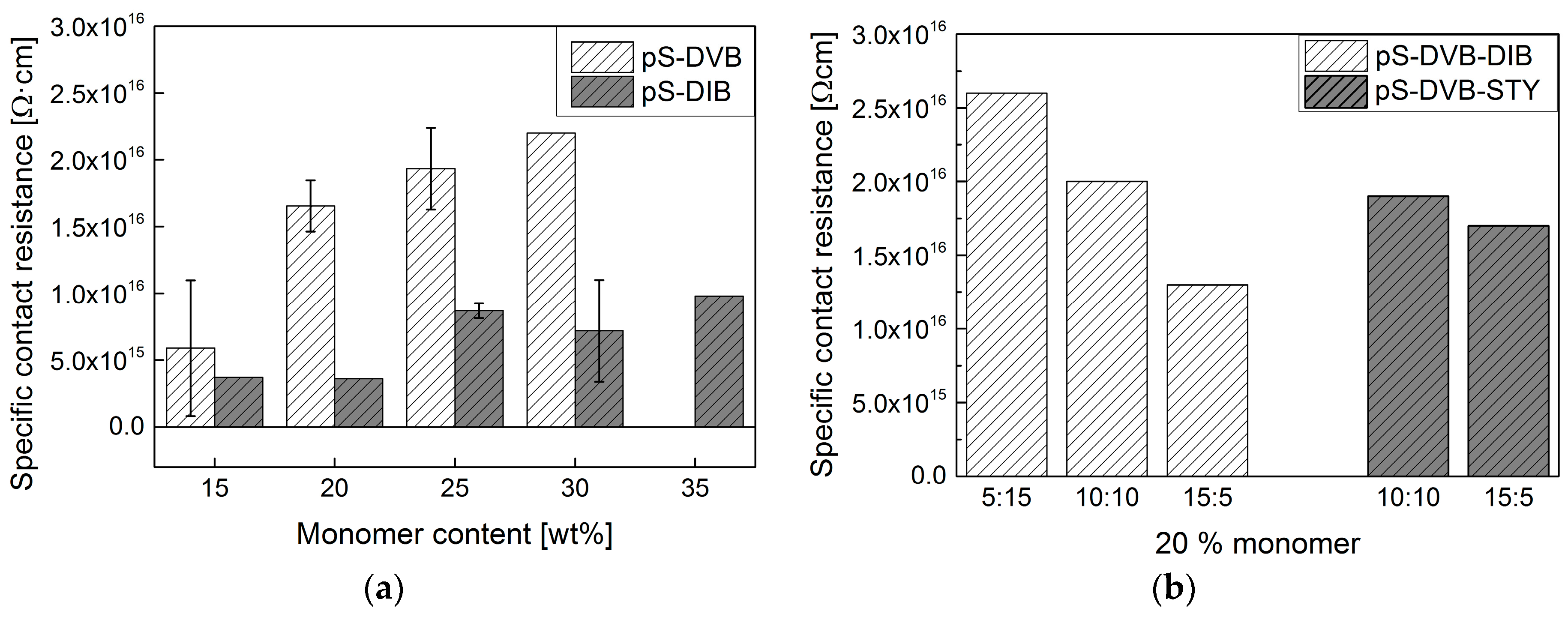
All synthesized types of sulfur copolymers have exceptionally good insulating properties. The specific contact resistance ranges from 10
15–10
16 Ω∙cm (
Figure 9). Thereby, they provide similarly good properties as previously known insulating materials, such as conventional hydrocarbons like PMMA with 10
15 Ω∙cm or PE, PP and PB with 10
16 Ω∙cm [
31,
32]. The specific contact resistance of sulfur copolymers is one order of magnitude higher than that of elemental sulfur with 10
15 Ω∙cm. Furthermore, sulfur copolymer materials have a much higher resistivity than the brittle elemental sulfur that does not have any ductility. Thus, sulfur copolymers would be a good alternative for insulating materials with high amounts of inexpensive elemental sulfur. Only materials such as PTFE have a higher specific contact resistance of 10
17 Ω∙cm [
33].
The specific contact resistance of pS-DVB increases with increasing cross-linker DVB (
Figure 9a). Thus, a higher DVB content increases the insulating properties. The pS-DIB does not show any correlation with added DIB content and lies in the same order of magnitude (
Figure 9a). In this connection, the terpolymer pS-DVB-DIB elucidates the correlation of the increasing specific contact resistance with an increasing DVB content (
Figure 9b). In contrast to this, the terpolymer pS-DVB-STY (
Figure 9b) refutes the insight that there is an influence on the insulating properties. Certainly, its value is similar and also the influence of the styrene (STY) could be higher than that of DVB.
The resistance of insulating sulfur copolymers against high voltage or dielectric strength is very low. The value of dielectric strength of all test specimens is about 9 kV∙mm
−1, similar to the value of glass (10 kV∙mm
−1). Conventional polymers (hydrocarbons) have a dielectric strength of one to two orders of magnitude higher than sulfur copolymers [
31]. This test is very sensitive towards air inclusions and inhomogeneity. Whether these low values are due to the material itself or caused by the imperfections of the test specimens could not be resolved definitively. It indicates that the presence of elemental sulfur in the copolymer strongly affects its stability and the reproducibility of the results obtained by different analytical methods. If these negative side effects could be eliminated, the sulfur copolymers with STY and DVB contents in particular would have the potential to be well insulating materials.
3.5. Mechanical Properties
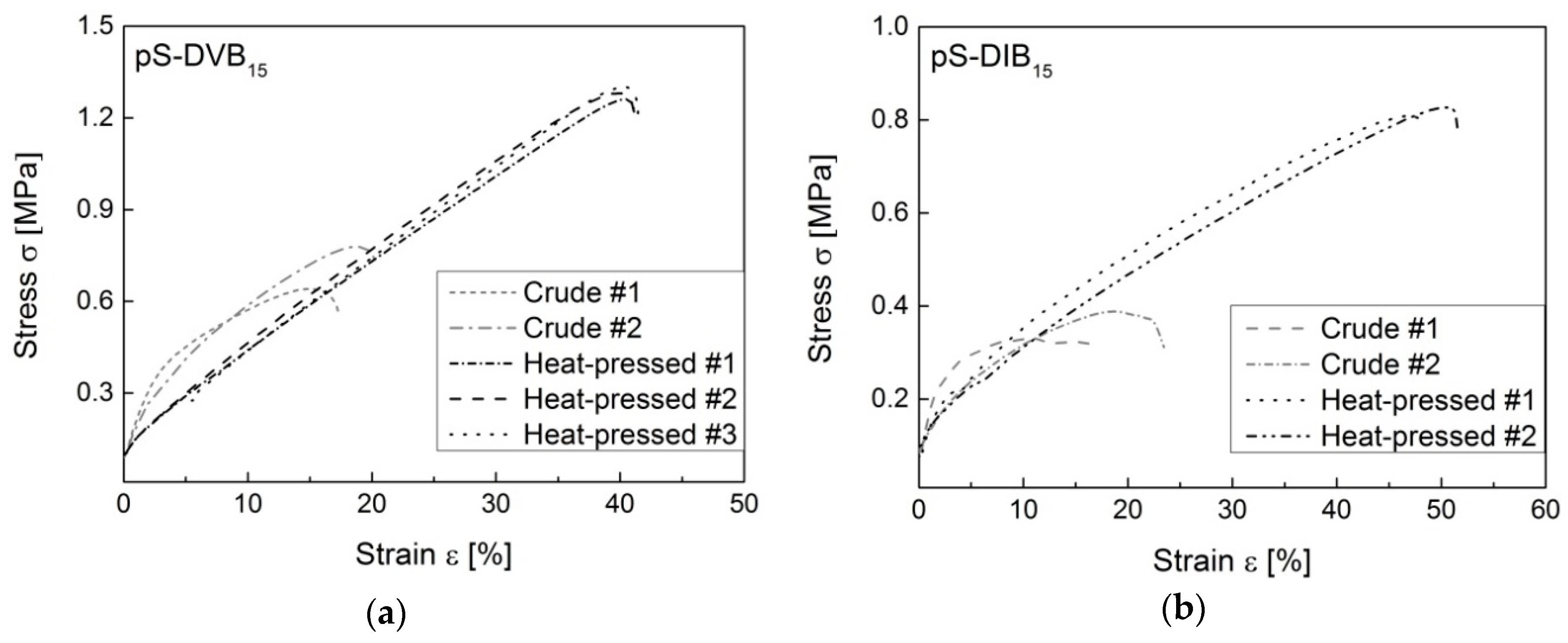
The mechanical tests are very important to characterize the virtually unexplored ductile properties of the products synthesized by inverse vulcanization. Consequently, it is possible to tune these materials for specific applications. All mechanical measurements were done with hot-pressed sulfur copolymers because it is often nearly impossible to reprocess the crude polymers into test specimens. Ductile experiments indicate that hot-pressed sulfur copolymers have better ductile properties than crude copolymers (
Figure 10). They show a two to three times higher elongation. Hence, the remolded material has a higher stability for different applications. Attention should be paid to the heating time to prevent the formation of thermosets due to post cross-linking.
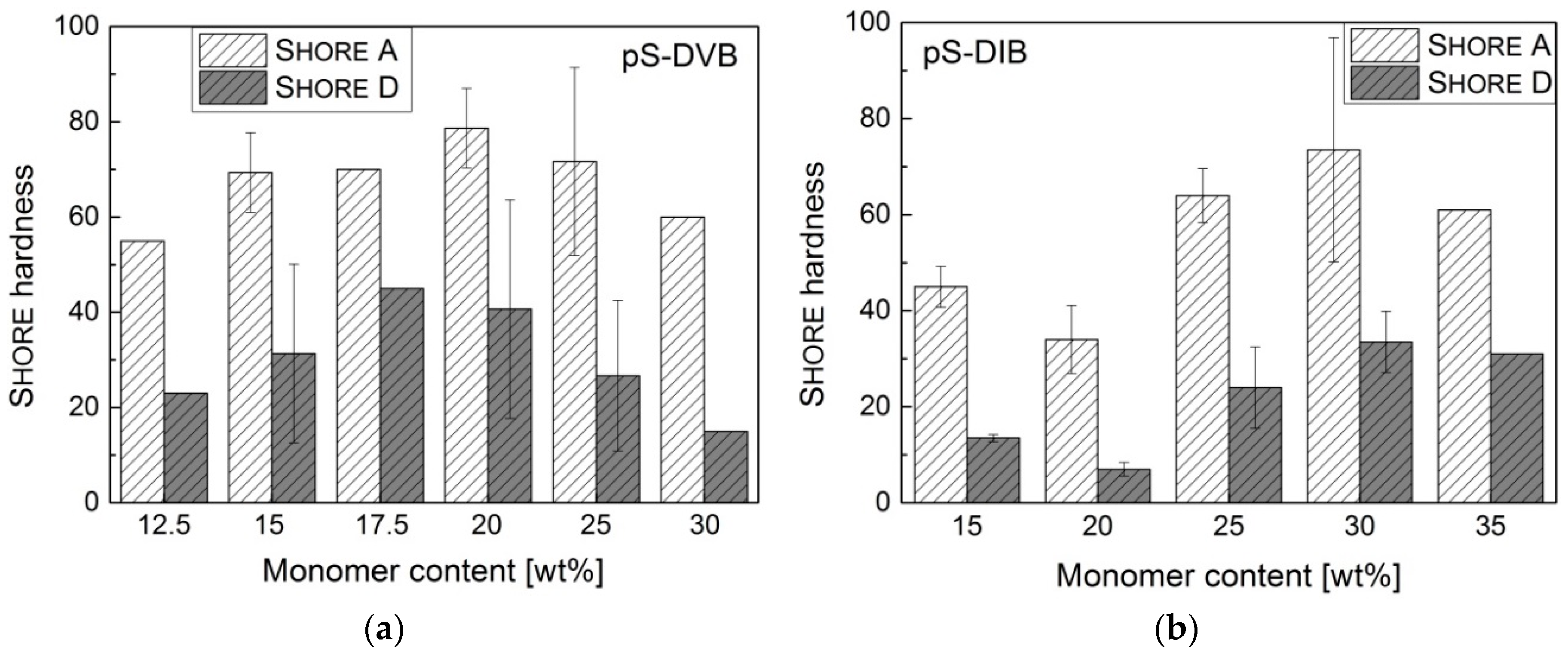
The Shore hardness of all sulfur copolymers (reprocessed with a hot press) has no clear correlation with the incorporated monomer content or cannot be detected due to the large standard deviations. The deviations of measurements are caused by inhomogeneities of the sulfur copolymers through air inclusions or the unsuccessful uniform reprocessing with the hot press. The values of the Shore hardness correspond to those of soft plastics or elastomers (
Figure 11). Films of pS-DIB are relatively softer than those of pS-DVB (S
hore D = 13–67) below an incorporated monomer content of 30 wt %. Regarding the Shore hardness, the sulfur copolymer materials are softer than conventional hydrocarbon polymers like polystyrene (
D = 80), polymethylmethacrylate (PMMA) (
D = 87–88), polycarbonate (
D = 82–85), polypropylene (
D = 65–75), etc. [
34].
Depending on the amount of styrene and DIB added to the DVB-based copolymer to form a terpolymer, a softer material is produced in contrast to conventional pS-DVB. Results of ductility experiments show that test specimens of pS-DVB act as ductile material with properties of amorphous plastomers. The curves of ductility experiments (
Figure 12a) have a small elastic range and a large plastic range with no necking area. The ductile stress to stretch the polymer by the same factor increases with the decrease of the incorporated monomer content of the sulfur copolymer. This implies that a polymer with a lower incorporated monomer content has a higher stability. At a monomer content of 30–35 wt %, the polymers result in a sticky and viscous resin because a higher monomer content implies an autoacceleration (gel effect) that causes a temperature rise above 200 °C. The released exothermic energy decreases the viscosity by reducing the chain length when the temperature limit is exceeded to a value above 200 °C [
3]. These materials cannot be processed into test specimens to carry out mechanical measurements.
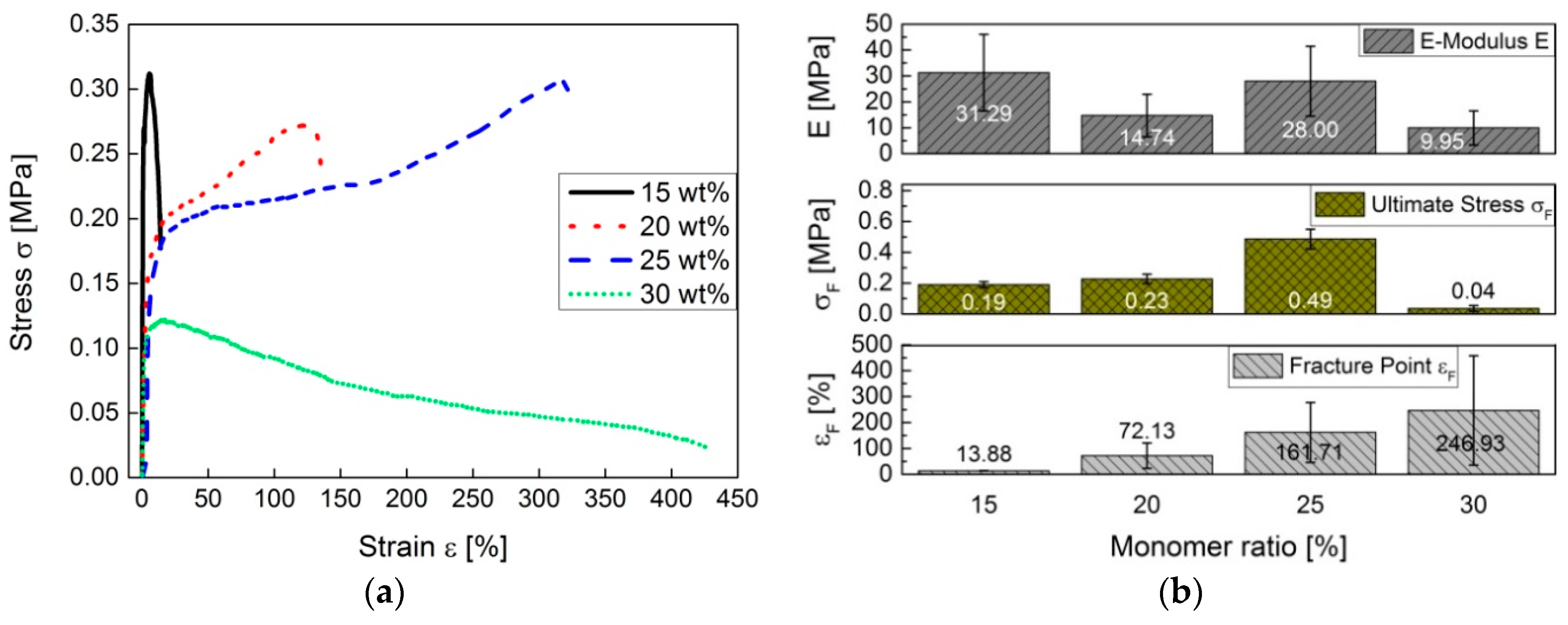
The
E-modulus tends to decrease with an increase of added monomer content. The hardness of the exceptional copolymer with 15 wt % monomer content cannot be explained. Consequently, at a monomer content of 12.5 wt %, the hardness is highest in the plastic material. The stretching area of pS-DVBs extends up to 75%. Apparently, the fracture point decreases first with an increase of the incorporated monomer content, and then increases again at an incorporated monomer content of 25 wt %, when the viscosity of the products decreases (
Figure 12b).
The material of pS-DIB acts as amorphous plastomers with thermoplastic to duromeric properties, depending on the incorporated monomer content. The stress–strain diagram of pS-DIB shows a plastic region with a necking area (
Figure 13a). The material has no elastic properties, since there is no linear elastic range in the stress–strain curve. The elongation increases with an increase of the monomer content added to the sulfur copolymer (
Figure 13b). At an incorporated monomer content of 30–35 wt %, most polymers become brittle thermosets (post cross-linking at the hot press), which break under mechanical stress, for instance, during the process of making specimens. The stretching area of pS-DIB extends up to 432%. The fracture point increases continuously with an increase of incorporated monomer content until thermosetting conditions are reached.
Pyun et al. performed tensile tests based on pS-DIB with 20 and 30 wt % monomer content. If their results are compared to the results obtained in this work, the required stress is two orders of magnitude higher and the
E-modulus is one order of magnitude higher than in the tensile experiments of pS-DIB in this work (
Figure 13). These values indicate a material with higher stiffness, and, consequently, a harder material. A monomer content of 20 wt % shows a comparable elongation (100%–250%). However, it is worth mentioning that the elongation of pS-DIB in this work is two orders of magnitude higher for a monomer content of 30 wt % [
35] in the event that it is not cross-linked to a thermoset by the processing with the hot press. The curve shape of pS-DIB with 20 wt % corresponds to thermoplastics and the copolymer with 30 wt % DIB corresponds to thermosets [
35].
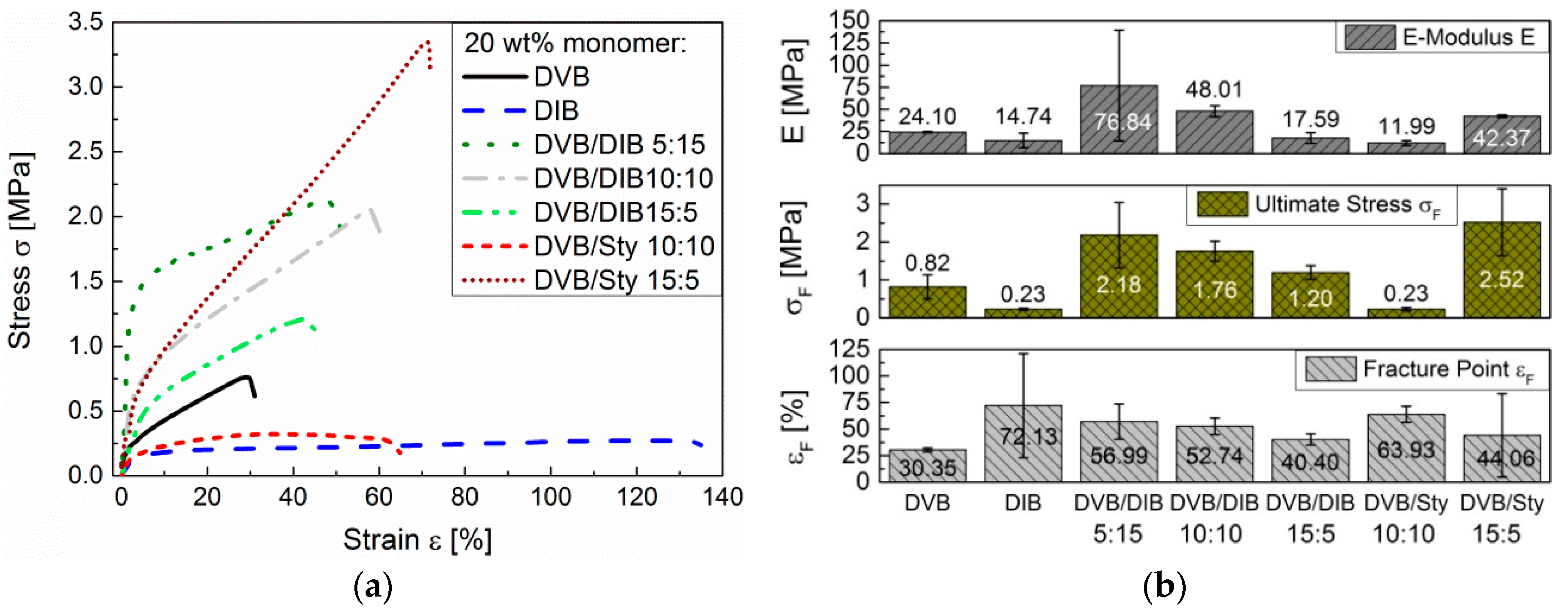
In comparison, the pS-DIB is more ductile than the pS-DVB, since DIB-based copolymers require 10 times less ductility stress for the same elongation than DVB-based copolymers. An increase of the DIB content in the poly(S-
r-DVB-
r-DIB) terpolymer (pS-DVB-DIB) results in an increase of the elongation and of the ultimate stress as well as of the
E-modulus (
Figure 14b). STY acts as a plasticizer (like DIB) and increases the elongation (
Figure 14a) when added to the DVB-based terpolymer. The terpolymers contain a total of 20 wt % of incorporated vinyl comonomers with varying ratios.
By the incorporation of a divinylbenzene network, the mechanical strength values are significantly increased and the ductility is decreased. Regarding the mechanical properties, sulfur copolymer materials are softer than conventional polymers. They are characteristic for low toughness and strength and high ductility [
31].
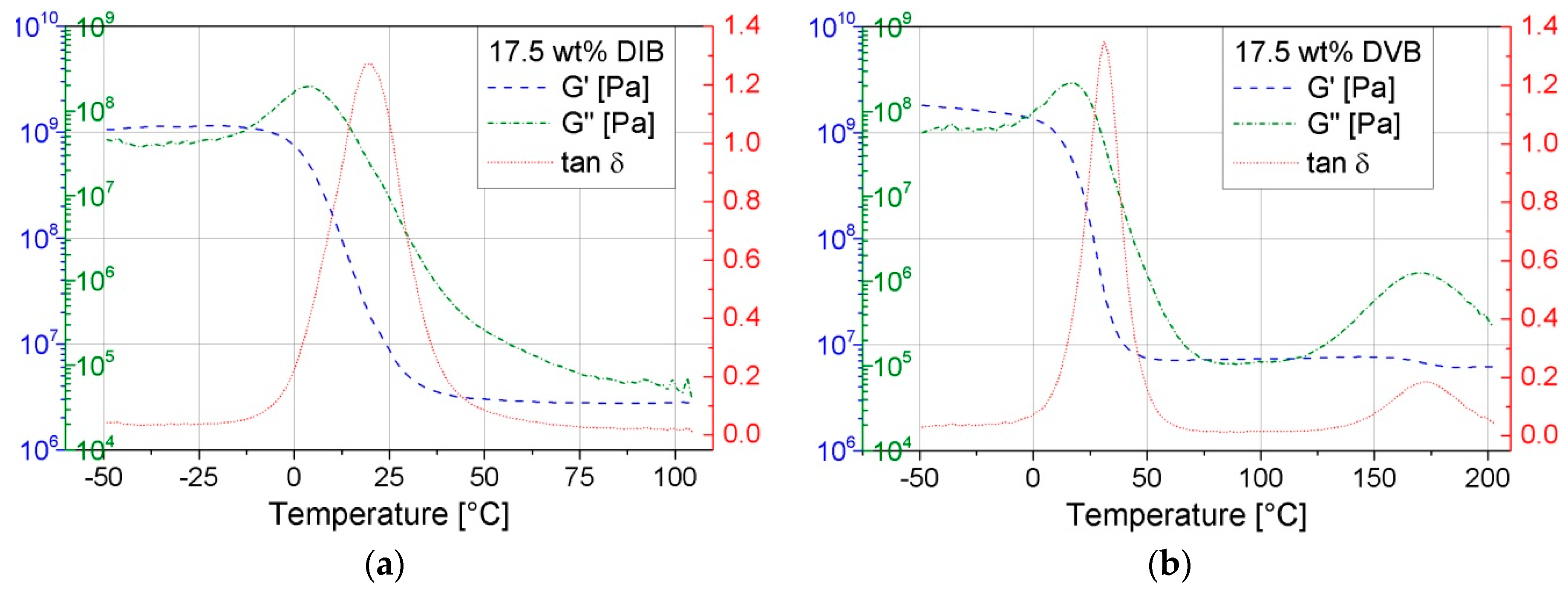
The DMA plots of pS-DVB and pS-DIB (
Figure 15) hardly differ in a sulfur copolymer. Only the three respective graphs are shifted in the respective direction (lower or higher temperature), depending on the
Tg. Moreover, the terminal plateau of G’ increases with higher incorporated comonomer content, which implies a higher cross-linking degree in the form of hyper-branching.
Above a temperature of approximately −10 °C, the glassy state of the materials ends and the glass transition range begins with a sharp increase of the loss modulus G”. Here, the copolymer is increasingly losing its elastic properties and instead a greater conversion of mechanical energy into heat energy takes place. This results in a simultaneous decrease in the storage modulus G’. The loss modulus reaches its maximum in the glass transition range. This maximum results from the required energy to increase the chain mobility (molecular friction processes), which is irreversible.
The maxima of G” can be used as reference points to determine relaxations. This applies also to the maximum of tan δ (loss factor). Thus, the first maximum of G” is exhibited in the Tg, which corresponds to the results of the DSC. The second weak maximum of G” deviates approximately by 30–40 °C from the secondary transition (β-relaxation) in the DSC thermogram. However, the β-relaxation (G”) of the pS-DIBs cannot be determined due to impending softening of the material (viscous polymer melt). The results obtained by DSC are in agreement with those of the DMA regarding the Tg. G’ is steadily decreasing (sigmoidal decrease) throughout the test period and has a turning point in the glass transition range. The course of G’ in the terminal plateau suggests a low altitude, and, consequently, a low cross-linking polymer network, and is confirmed by the results of the stress–strain experiments.
Finally, the sulfur copolymers can be subdivided into different classes regarding their thermomechanical behavior. The most noticeable behavior can be observed above an incorporated monomer content of 30 wt %, in which case a dramatic change of the mechanical properties of both sulfur materials occurs (
Figure 16). In the pS-DVB, the high exothermicity, which is released at a high monomer content (≥30 wt %), is responsible for the property change. In the pS-DIB, however, cross-linking continues upon thermal treatment in the hot press, whereupon a glassy thermosetting occurs.