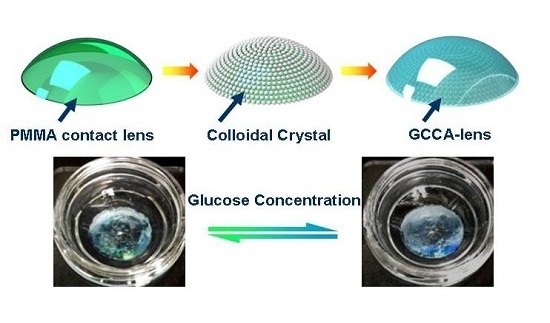
A Gelated Colloidal Crystal Attached Lens for Noninvasive Continuous Monitoring of Tear Glucose
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Self-Assembly of PS Colloids on Contact Lens
2.3. Gelation of CCA-Lens by Glucose-Responsive PVA
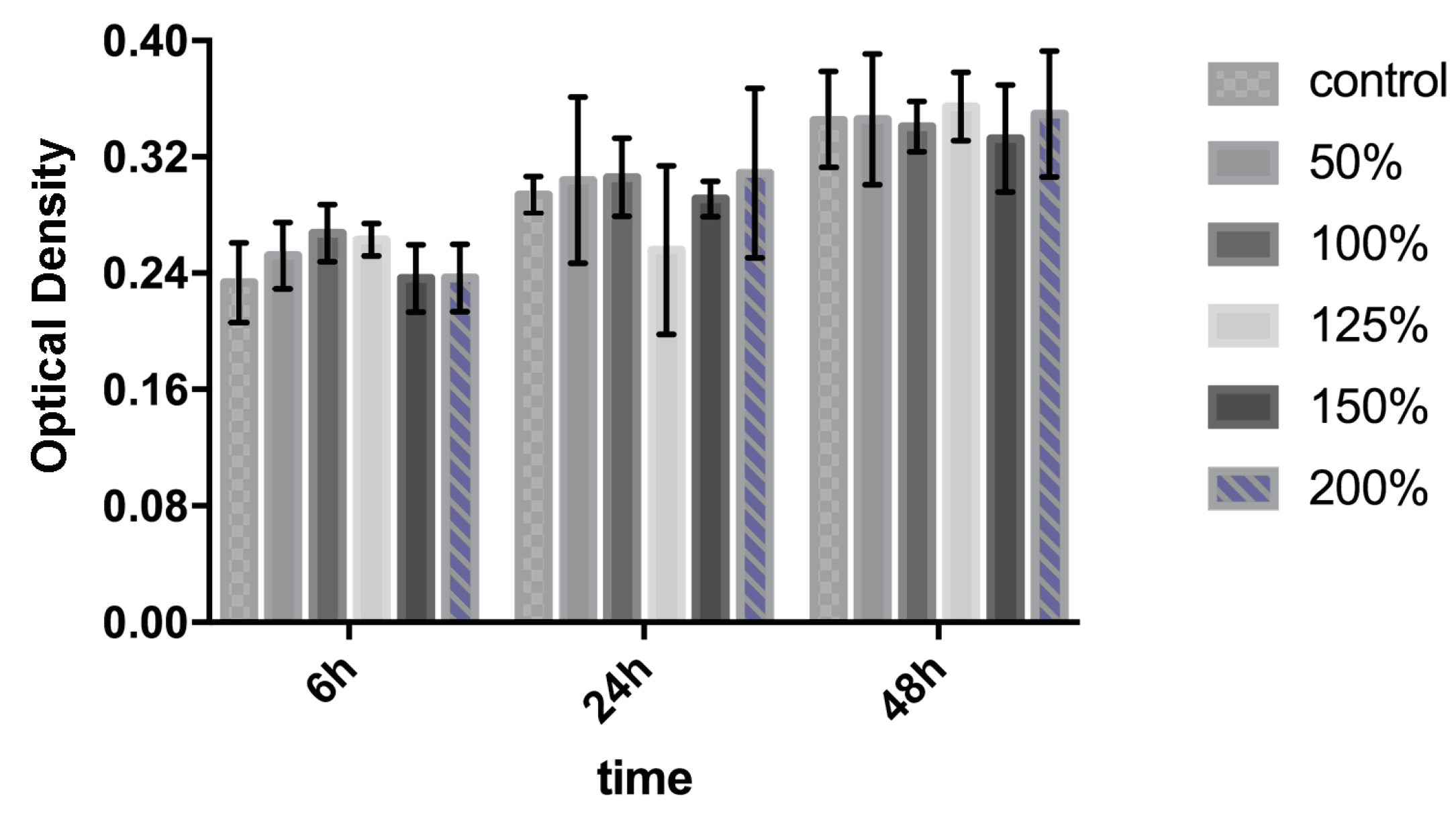
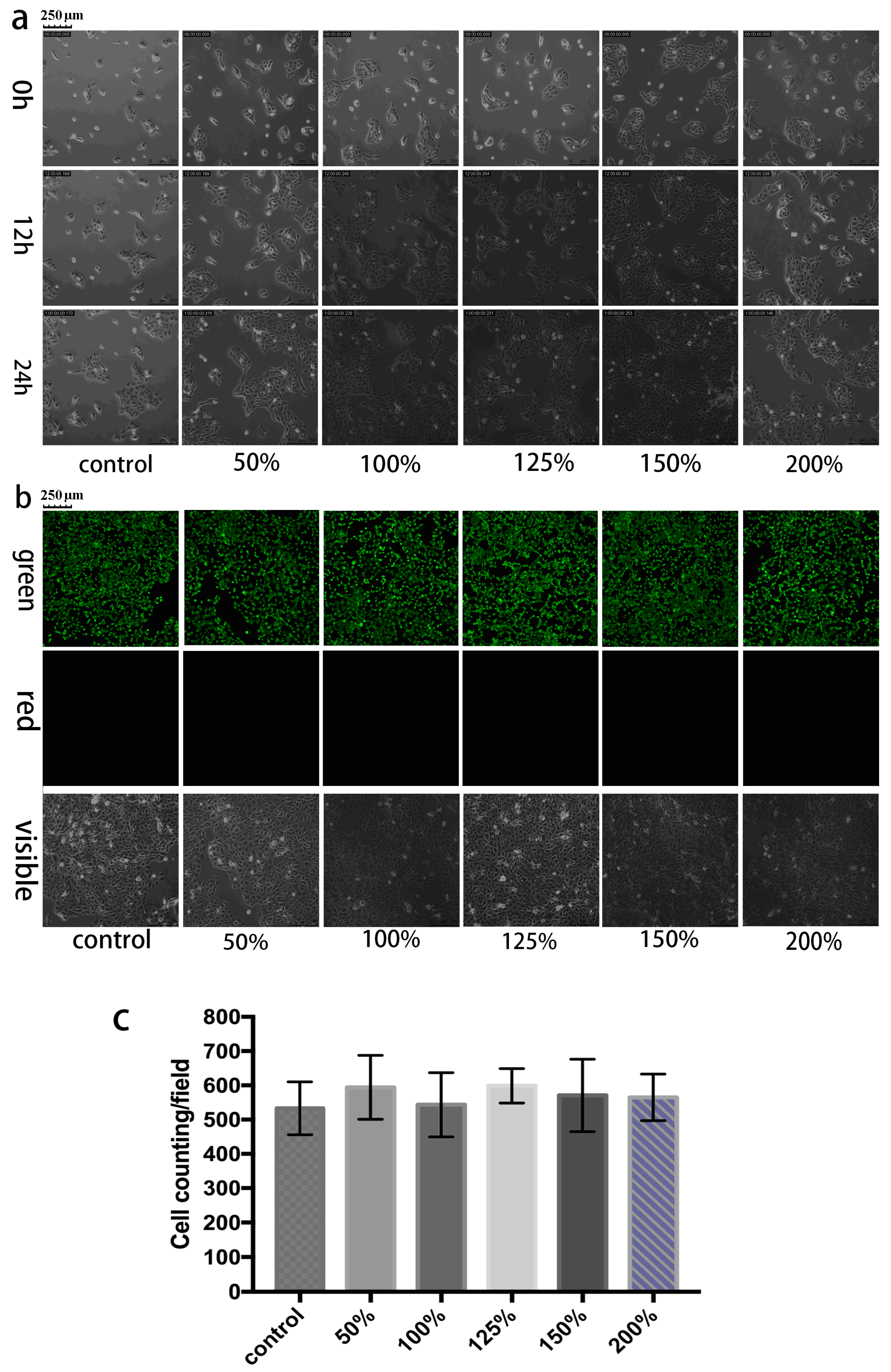
2.4. Cell Culture and Cytotoxicity Test
2.5. Characterizations of the CCA Embedded Hydrogel
2.6. Reflection Measurement of the GCCA-Lens
2.7. Statistical Analysis
3. Results and Discussion
3.1. Biocompatibility Characterization of GCCA-Lens
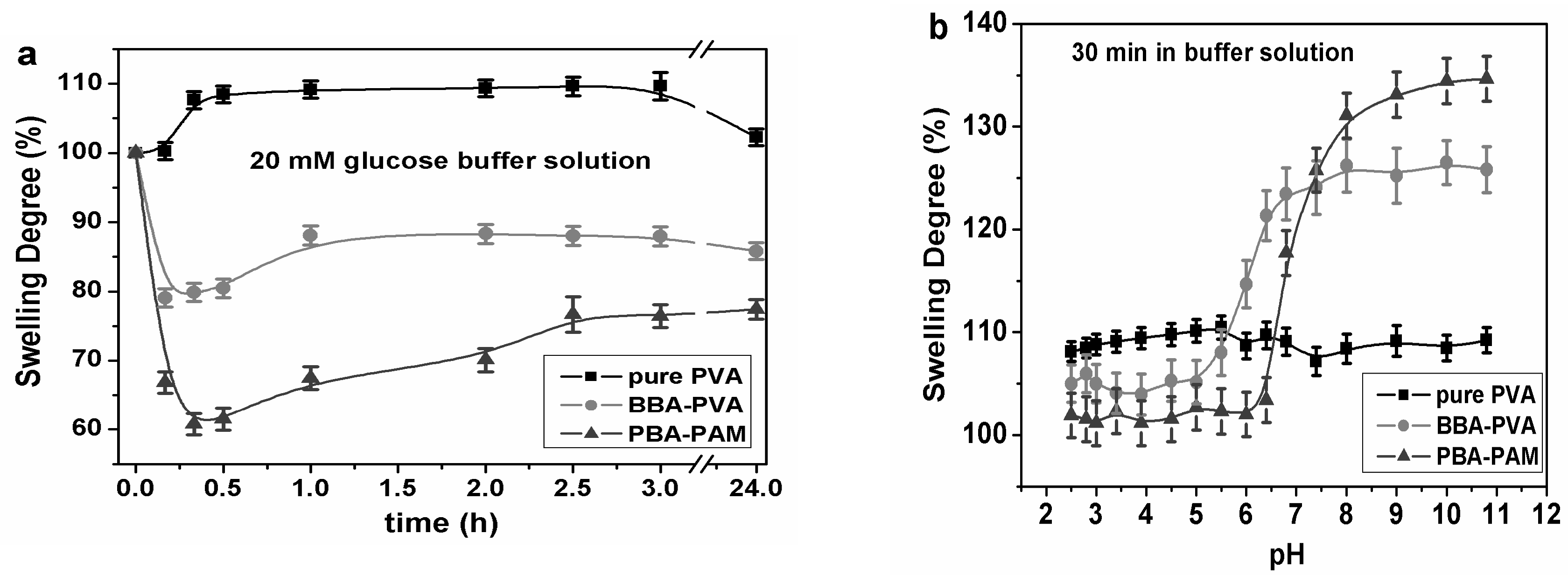
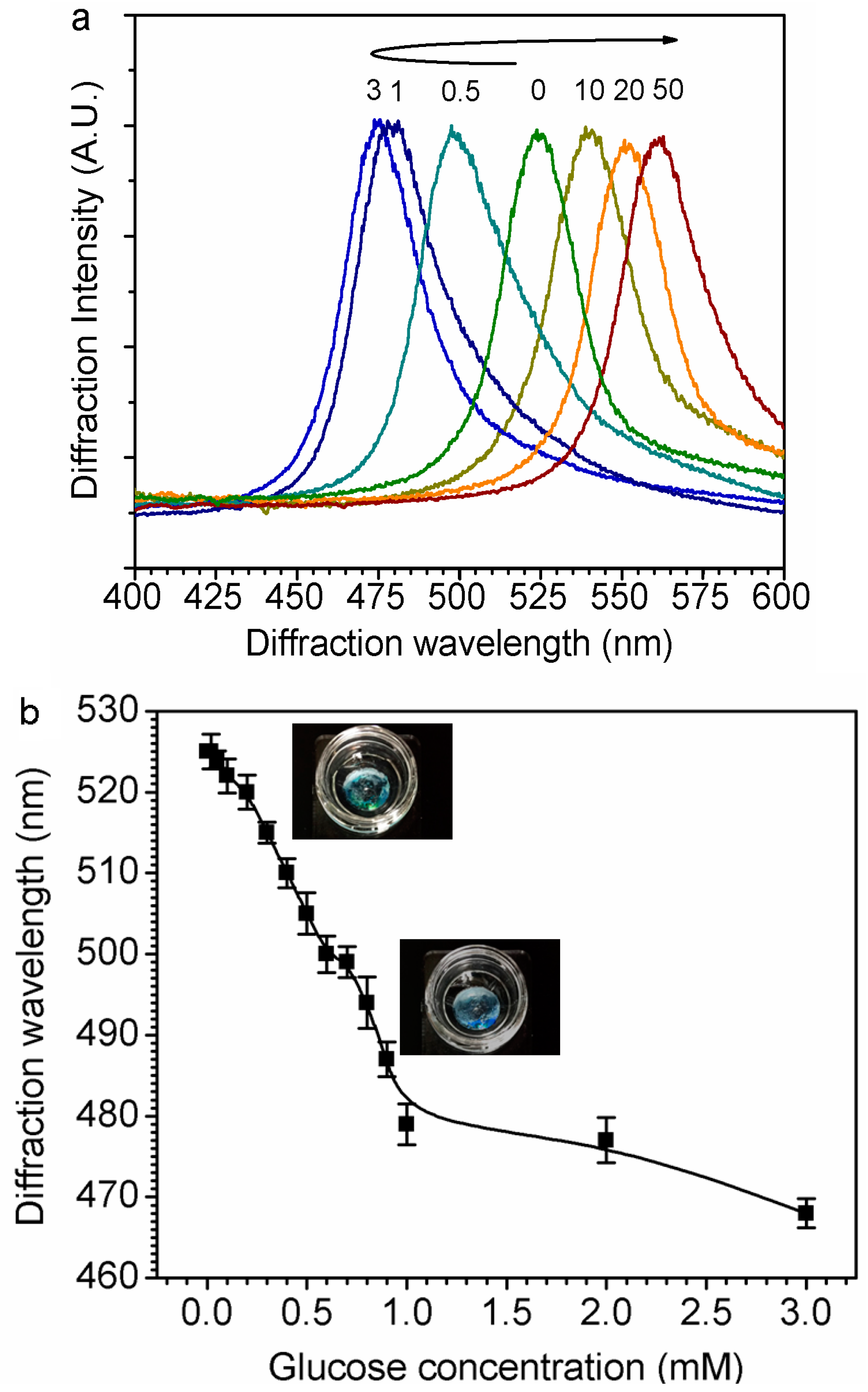
3.2. Swelling Ability of Hydrogel and Sensing Mechanism of GCCA-Lens
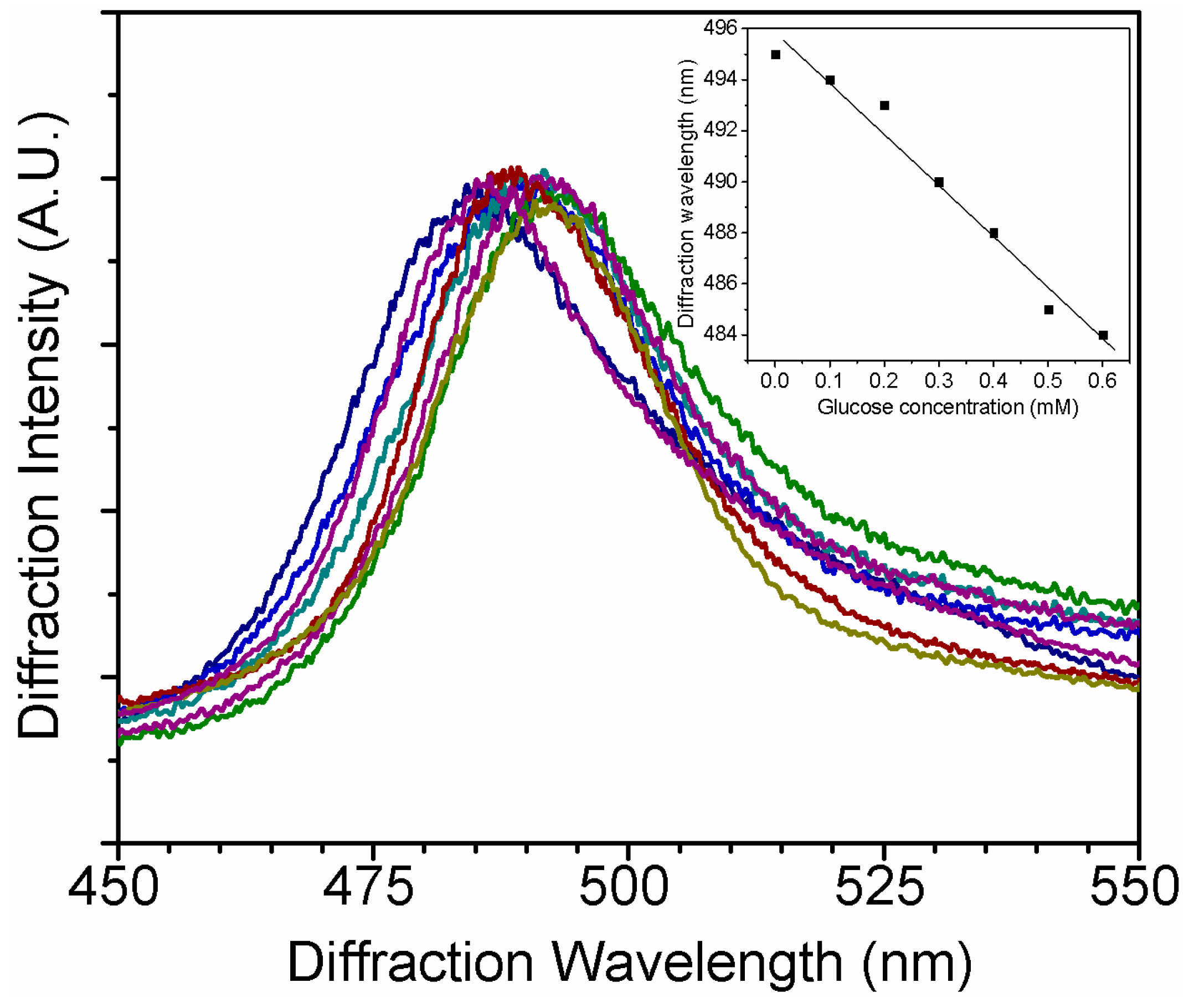
3.3. Glucose Sensing in Glucose Solution and STF
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benkhadra, K.; Alahdab, F.; Tamhane, S.; Wang, Z.; Prokop, L.J.; Hirsch, I.B.; Raccah, D.; Riveline, J.P.; Kordonouri, O.; Murad, M.H. Real time continuous glucose monitoring in type 1 diabetes: A systematic review and individual patient data meta-analysis. Clin. Endocrinol. Oxf. 2017, 86, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.M.; Varghese, T.; Hein, R.E.; Dhindsa, D.S.; Mahlof, E.N.; Cai, H.C.; Southmayd, G.; Sandesaraa, P.B.; Eapen, D.J.; Sperling, L.S. Natural approaches in diabetes management: A review of diet, exercise, and natural products. Curr. Pharm. Des. 2016. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and emerging technology for continuous glucose monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xie, L.; Lin, X.; Su, B. Detection of metoprolol in human biofluids and pharmaceuticals via ion-transfer voltammetry at the nanoscopic liquid/liquid interface array. Anal. Chem. 2017, 89, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, H.; Schultz, Z.D.; Camden, J.P. Sensing glucose in urine and serum and hydrogen peroxide in living cells by use of a novel boronate nanoprobe based on surface-enhanced raman spectroscopy. Anal. Chem. 2016, 88, 7191–7197. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xue, M.; He, Q.; Lu, W.; Meng, Z.; Yan, D.; Qiu, L.; Zhou, L.; Yu, Y. A non-enzymatic urine glucose sensor with 2-d photonic crystal hydrogel. Anal. Bioanal. Chem. 2016, 408, 8317–8323. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, C. A novel screen-printed microfluidic paper-based electrochemical device for detection of glucose and uric acid in urine. Biomed. Microdevices 2016, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.O.; Ulyanova, Y.V.; Figueroa-Teran, R.; Bhatt, K.H.; Singhal, S.; Atanassov, P. Wearable sensor system powered by a biofuel cell for detection of lactate levels in sweat. ECS J. Solid State Sci. Technol. 2016, 5, M3075–M3081. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Trans. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.; Bavarian, B. Facile and scalable disposable sensor based on laser engraved graphene for electrochemical detection of glucose. Sci. Rep. 2016, 6, 27975. [Google Scholar] [CrossRef] [PubMed]
- Ascaso, F.J.; Huerva, V. Noninvasive continuous monitoring of tear glucose using glucose-sensing contact lenses. Optom. Vis. Sci. 2016, 93, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Baca, J.T.; Finegold, D.N.; Asher, S.A. Tear glucose analysis for the noninvasive detection and monitoring of diabetes mellitus. Ocul. Surf. 2007, 5, 280–293. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Ophthalmic glucose monitoring using disposable contact lenses—A review. J. Fluoresc. 2004, 14, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. A glucose-sensing contact lens: From bench top to patient. Curr. Opin. Biotechnol. 2005, 16, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hodge, W.; Hutnick, C.; Wang, X. Noninvasive diagnostic devices for diabetes through measuring tear glucose. J. Diabetes Sci. Technol. 2011, 5, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.R.; De, S.; Datta, H.; Chatterjee, S.; Biswas, M.C.; Sarkar, K.; Mandal, L.K. Estimation of tear glucose level and its role as a prompt indicator of blood sugar level. J. Indian Med. Assoc. 2003, 101, 481–483. [Google Scholar] [PubMed]
- Chen, R.; Jin, Z.; Colon, L.A. Analysis of tear fluid by ce/lif: A noninvasive approach for glucose monitoring. J. Capill. Electrophor. 1996, 3, 243–248. [Google Scholar]
- Das, B.N.; Sengupta, S.; Das, B.K.; Goswami, N.R. Tear glucose estimation—An alternative to blood glucose estimation. J. Indian Med. Assoc. 1995, 93, 127–128. [Google Scholar] [PubMed]
- Gupta, N.; Gupta, M.C. Tear glucose estimation—An alternative to blood glucose estimation. J. Indian Med. Assoc. 1996, 94, 391. [Google Scholar] [PubMed]
- Lane, J.D.; Krumholz, D.M.; Sack, R.A.; Morris, C. Tear glucose dynamics in diabetes mellitus. Curr. Eye Res. 2006, 31, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.G.; Garcia-Gancedo, L.; Chen, C.; Zhu, X.R.; Xie, H.Q.; Flewitt, A.J.; Milne, W.I. Enzyme-free glucose biosensor based on low density CNT forest grown directly on a Si/SiO2 substrate. Sens. Actuators B Chem. 2013, 178, 586–592. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.Q.; Moussy, F.; Milne, W.I. A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and grapheme. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, S.; Kudo, H.; Saito, T.; Ogawa, M.; Saito, H.; Otsuka, K.; Funakubo, A.; Mitsubayashi, K. A flexible and wearable biosensor for tear glucose measurement. Biomed. Microdevices 2007, 9, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Sawada, T.; Kazawa, E.; Yoshida, H.; Iwasaki, Y.; Mitsubayashi, K. A flexible and wearable glucose sensor based on functional polymers with soft-mems techniques. Biosens. Bioelectron. 2006, 22, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Lu, J.; Balijepalli, A.S.; Major, T.C.; Cohan, B.E.; Meyerhoff, M.E. Evaluation of enzyme-based tear glucose electrochemical sensors over a wide range of blood glucose concentrations. Biosens. Bioelectron. 2013, 49, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Taormina, C.R.; Baca, J.T.; Finegold, D.N.; Asher, S.A.; Grabowski, J.J. Analysis of tear glucose concentration with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Fluorescence sensors for monosaccharides based on the 6-methylquinolinium nucleus and boronic acid moiety: Potential application to ophthalmic diagnostics. Talanta 2005, 65, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tse, W.H.; Chen, Y.; McDonald, M.W.; Melling, J.; Zhang, J. Nanostructured biosensor for detecting glucose in tear by applying fluorescence resonance energy transfer quenching mechanism. Biosens. Bioelectron. 2016, 91, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, A.Y.; Wheeler, D.A.; Bond, T.C.; Gu, C.; Li, Y. Direct molecule-specific glucose detection by raman spectroscopy based on photonic crystal fiber. Anal. Bioanal. Chem. 2012, 402, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, V.L.; Das, S.; Finegold, D.N.; Asher, S.A. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem. 2004, 50, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Baca, J.T.; Taormina, C.R.; Feingold, E.; Finegold, D.N.; Grabowski, J.J.; Asher, S.A. Mass spectral determination of fasting tear glucose concentrations in nondiabetic volunteers. Clin. Chem. 2007, 53, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Ben-Moshe, M.; Alexeev, V.L.; Asher, S.A. Fast responsive crystalline colloidal array photonic crystal glucose sensors. Anal. Chem. 2006, 78, 5149–5157. [Google Scholar] [CrossRef] [PubMed]
- Muscatello, M.M.W.; Asher, S.A. Poly(vinyl alcohol) rehydratable photonic crystal sensor materials. Adv. Funct. Mater. 2008, 18, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Guan, Y. New polymerized crystalline colloidal array for glucose sensing. Chem. Commun. (Camb.) 2009, 14, 1867–1869. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Tighe, B. Contact lens interactions with the tear film. Exp. Eye Res. 2013, 117, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control. Release 2016, 226, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S. It’s all in the eyes: The eyes as a window to the body and brain may not be a brand-new idea—But it is a newly revitalized one, thanks to improved technologies. IEEE Pulse 2015, 6, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sheng, Y.; Sun, Y.; Feng, J.; Wang, S.; Zhang, J.; Xu, J.; Jiang, D. A glucose oxidase-coupled dnazyme sensor for glucose detection in tears and saliva. Biosens. Bioelectron. 2015, 70, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, Z.G.; Shih, W.H.; Ge, Q.Q.; Liu, M.J.; Zhu, X.R. Facile preparation and self-assembly of monodisperse polystyrene nanospheres for photonic crystals. J. Nanosci. Nanotechnol. 2015, 15, 3239–3243. [Google Scholar] [CrossRef] [PubMed]
- Ayyub, O.B.; Ibrahim, M.B.; Briber, R.M.; Kofinas, P. Self-assembled block copolymer photonic crystal for selective fructose detection. Biosens. Bioelectron. 2013, 46, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Nozawa, K.; Sugimoto, Y.; Shioya, M. Extraordinarily large swelling energy of iodine-treated poly(vinyl alcohol) demonstrated by jump of a film. J. Polym. Sci. B Polym. Phys. 2014, 52, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.K.; Cho, P.; Boost, M.V. Cytotoxicity and effects on metabolism of contact lens care solutions on human corneal epithelium cells. Clin. Exp. Optom. 2012, 95, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Neo, P.Y.; Shi, P.; Goh, J.C.; Toh, S.L. Characterization and mechanical performance study of silk/pva cryogels: Towards nucleus pulposus tissue engineering. Biomed. Mater. 2014, 9, 065002. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W. Synthesis, characterization and applications of n-quaternized chitosan/poly(vinyl alcohol) hydrogels. Int. J. Biol. Macromol. 2015, 80, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, Y.H.; Bao, H.; Yang, X.L.; Li, C.Z. Physically controlled cross-linking in gelated crystalline colloidal array photonic crystals. ACS Appl. Mater. Interfaces 2010, 2, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, Y.H.; Bao, H.; Zhao, P.; Jiang, H.L.; Peng, L.M.; Yang, X.L.; Li, C.Z. Solvent-assisted poly(vinyl alcohol) gelated crystalline colloidal array photonic crystals. Soft Matter 2011, 7, 915–921. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.H.; Bao, H.; Shen, J.H.; Jiang, H.L.; Peng, L.M.; Yang, X.L.; Li, C.Z.; Chen, G.R. Ethanol-assisted multi-sensitive poly(vinyl alcohol) photonic crystal sensor. Chem. Commun. 2011, 47, 5530–5532. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, J.-L.; Chen, C.; Shen, J.-H.; Zhao, X.-L.; Qian, S.-H.; Zhu, Z.-G. A Gelated Colloidal Crystal Attached Lens for Noninvasive Continuous Monitoring of Tear Glucose. Polymers 2017, 9, 125. https://doi.org/10.3390/polym9040125
Ruan J-L, Chen C, Shen J-H, Zhao X-L, Qian S-H, Zhu Z-G. A Gelated Colloidal Crystal Attached Lens for Noninvasive Continuous Monitoring of Tear Glucose. Polymers. 2017; 9(4):125. https://doi.org/10.3390/polym9040125
Chicago/Turabian StyleRuan, Jia-Li, Cheng Chen, Jian-Hua Shen, Xue-Ling Zhao, Shao-Hong Qian, and Zhi-Gang Zhu. 2017. "A Gelated Colloidal Crystal Attached Lens for Noninvasive Continuous Monitoring of Tear Glucose" Polymers 9, no. 4: 125. https://doi.org/10.3390/polym9040125