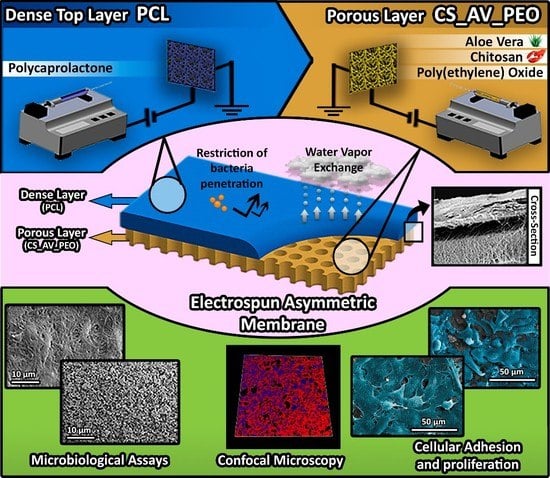
Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Extraction of the AV Gel
2.2.2. Deacetylation of Chitosan
2.2.3. Production of the Electrospun Asymmetric Membranes
2.2.4. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy Analysis
2.2.5. Characterization of the Mechanical Properties of the Membranes
2.2.6. Evaluation of the Porosity of the Produced Membranes
2.2.7. Determination of Contact Angle at the Surface of the Produced Membranes
2.2.8. Water Vapor Transmission Rate (WVTR)
2.2.9. Swelling and Enzymatic Degradation
2.2.10. Protein Adsorption
2.3. Characterization of the Biological Properties of the Produced Membranes
2.3.1. Characterization of Cell Viability and Proliferation in Contact with the Membranes
2.3.2. dsDNA Quantification
2.3.3. Characterization of the Antimicrobial Properties of the Membranes
Analysis of Bacterial Penetration through the Top Layer of the Produced Membranes
Characterization of Bactericidal Activity of the Bottom Layers (CS_PEO and CS_AV_PEO) Membranes
2.3.4. Characterization of the Morphologic Features and Biological Performance of the Electrospun Membranes by SEM Analysis
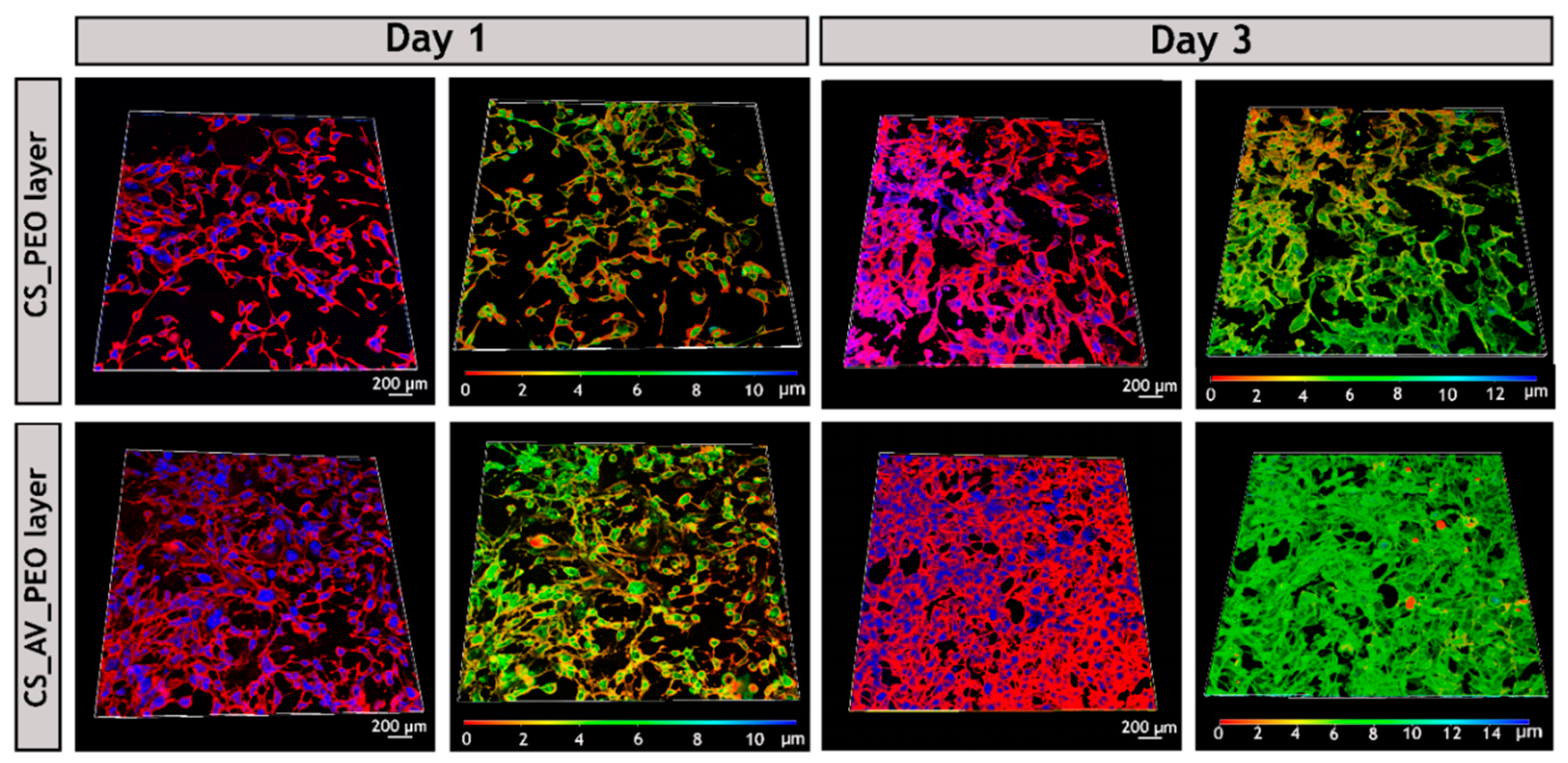
2.3.5. Confocal Microscopy Analysis
2.4. Statistical Analysis
3. Results and Discussion
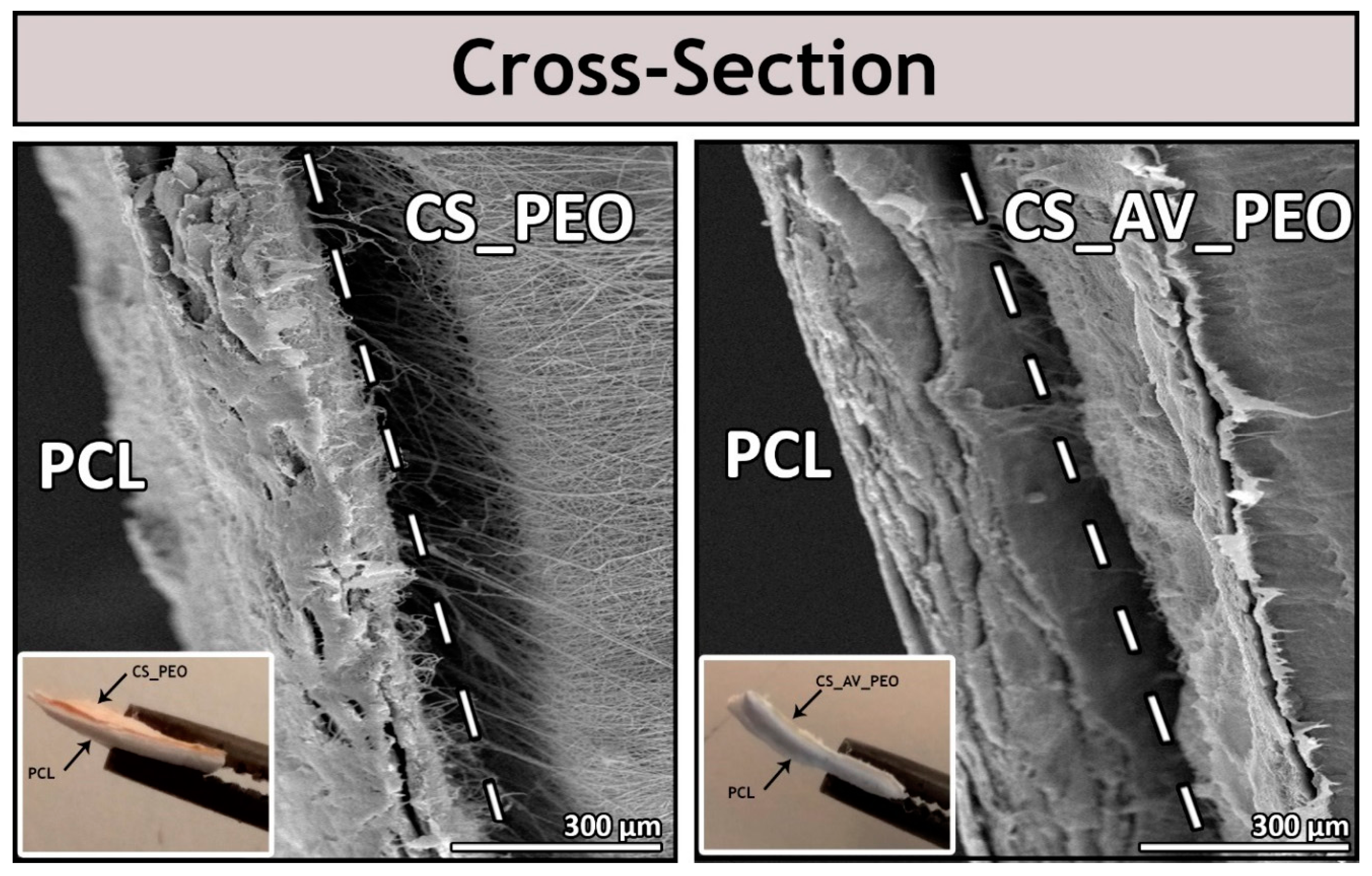
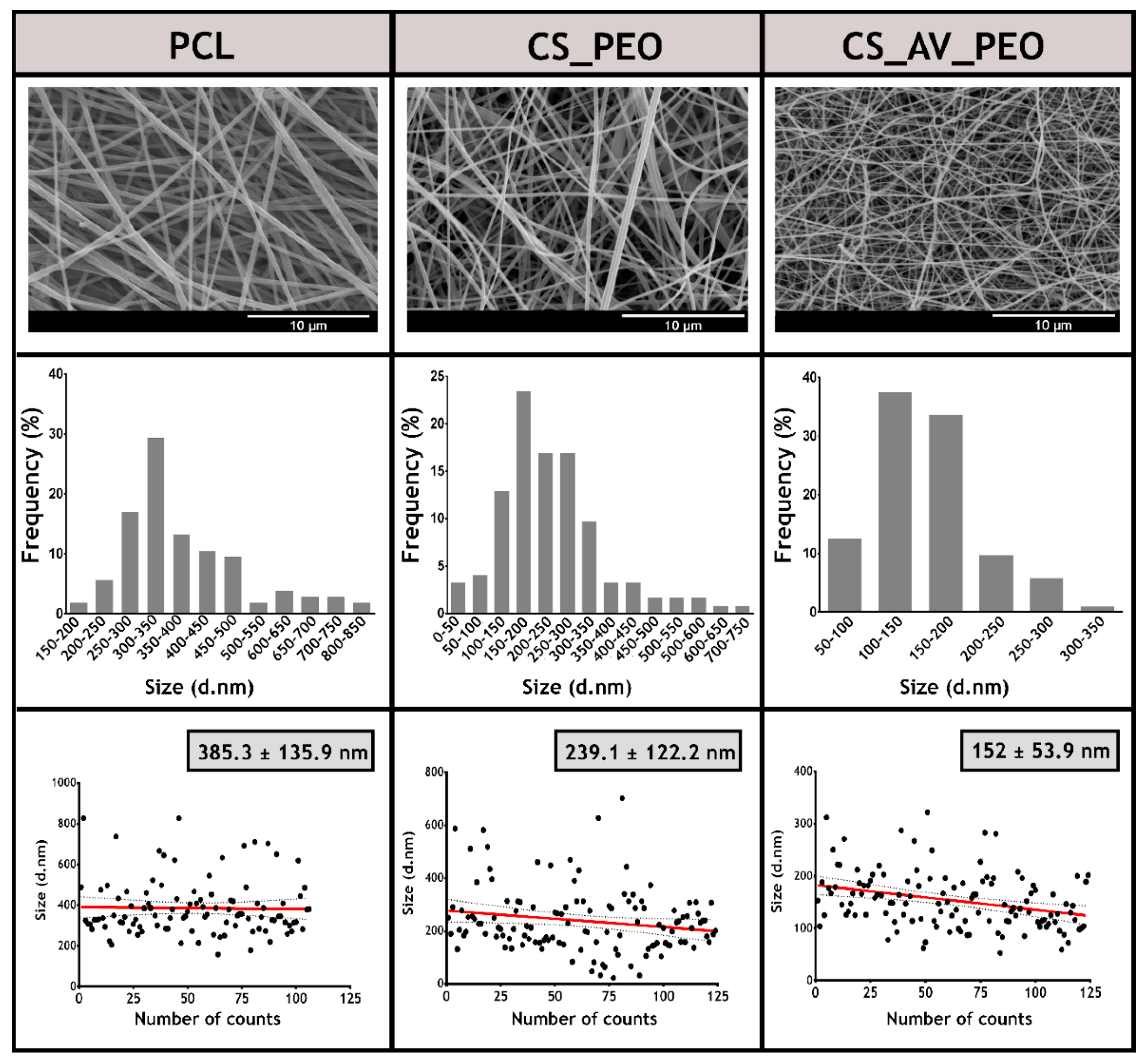
3.1. Characterization of the Morphology of the Membranes
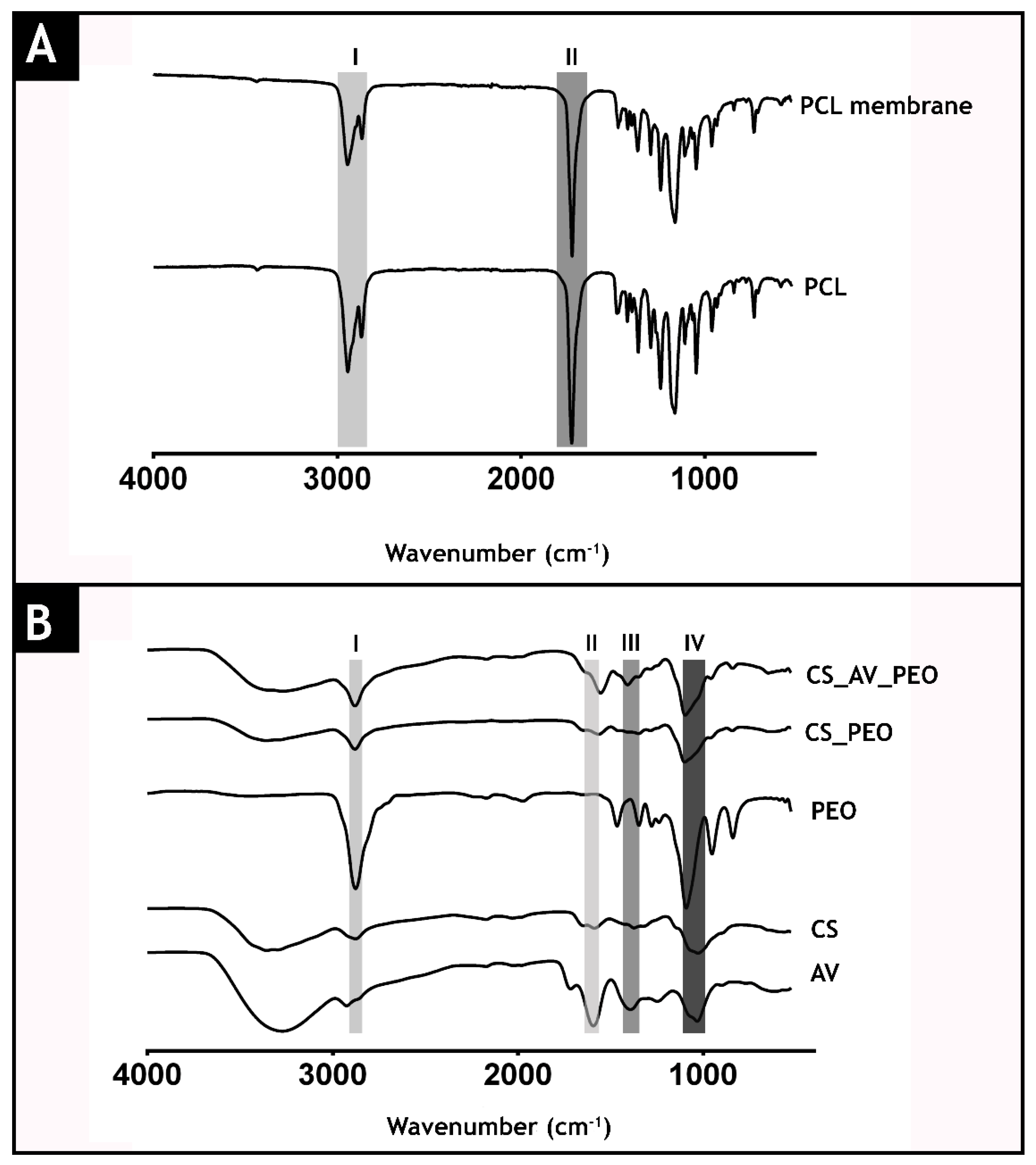
3.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopic Analysis
3.3. Characterization of Mechanical Properties of the Membranes
3.4. Characterization of Membrane Porosity
3.5. Membrane Surface Wettability
3.6. Water Vapor Transmission Rate
3.7. Characterization of the Membranes’ Swelling Profile
3.8. Characterization of Degradation Profile of the Membranes
3.9. Evaluation of Protein Adsorption on the Membranes’ Surface
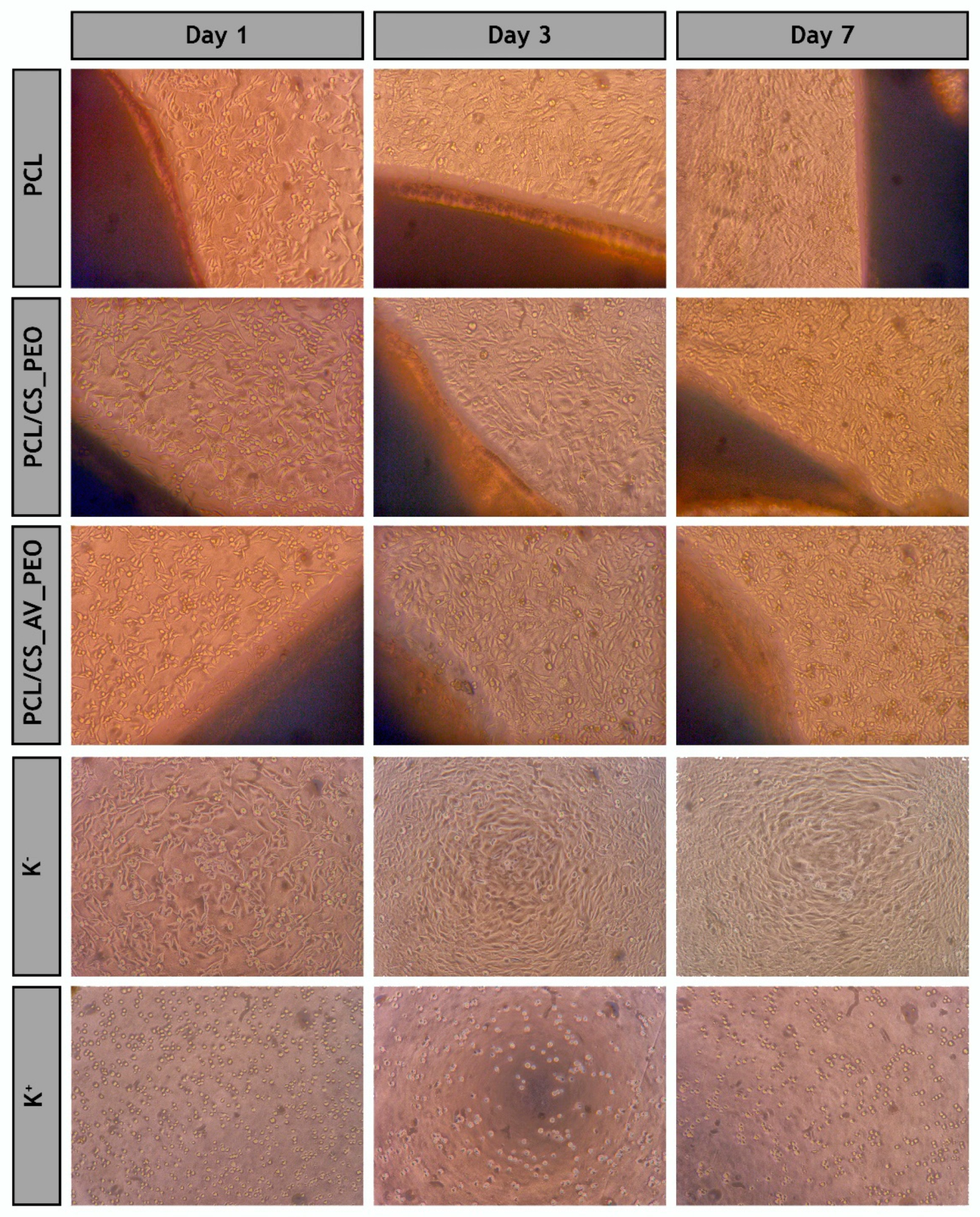
3.10. Evaluation of Cell Viability and Proliferation in the Presence of Membranes
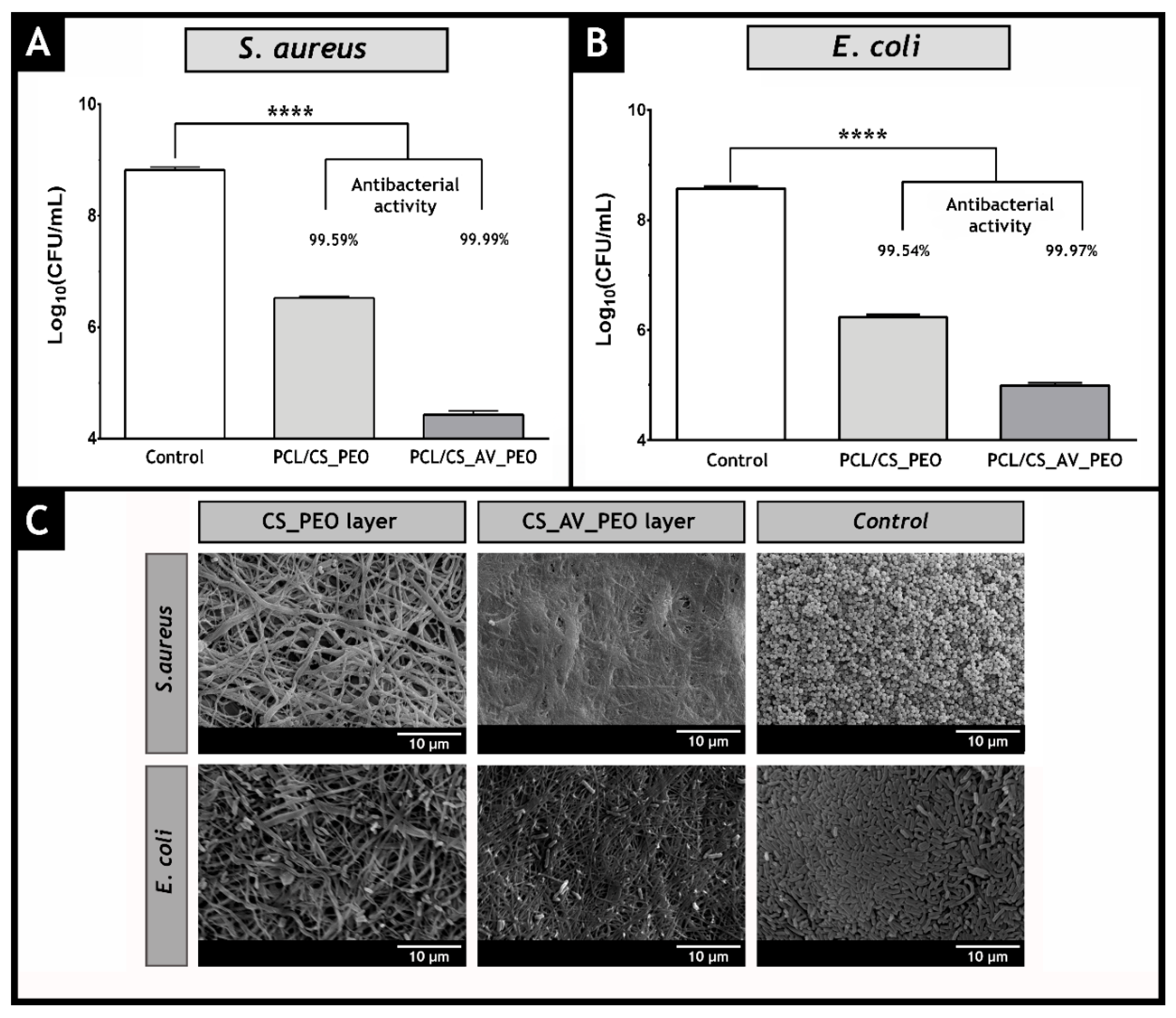
3.11. Characterization of the Antimicrobial Properties of the Produced Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- MacNeil, S. Biomaterials for tissue engineering of skin. Mater. Today 2008, 11, 26–35. [Google Scholar] [CrossRef]
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.D. Epidemiology of burns throughout the world. Part II: Intentional burns in adults. Burns 2012, 38, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2015, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing—A review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef]
- Nyame, T.T.; Chiang, H.A.; Orgill, D.P. Clinical applications of skin substitutes. Surg. Clin. N. Am. 2014, 94, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Greaves, N.S.; Iqbal, S.A.; Baguneid, M.; Bayat, A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen. 2013, 21, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Catalano, E.; Cochis, A.; Varoni, E.; Rimondini, L.; Azzimonti, B. Tissue-engineered skin substitutes: An overview. J. Artif. Organs 2013, 16, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J. Membr. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Xu, H.; Chang, J.; Chen, Y.; Fan, H.; Shi, B. Asymmetric polyurethane membrane with inflammation-responsive antibacterial activity for potential wound dressing application. J. Mater. Sci. 2013, 48, 6625–6639. [Google Scholar] [CrossRef]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel asymmetric wettable agnps/chitosan wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.-H.; Kim, C.S. Electrospun bioactive poly (ɛ-caprolactone)–cellulose acetate–dextran antibacterial composite mats for wound dressing applications. Colloids Surf. A 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Hinrichs, W.; Lommen, E.; Wildevuur, C.R.; Feijen, J. Fabrication and characterization of an asymmetric polyurethane membrane for use as a wound dressing. J. Appl. Biomater. 1992, 3, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar-Ricardo, A. Poly (vinyl alcohol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Membr. Sci. 2014, 469, 262–271. [Google Scholar] [CrossRef]
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone-hyaluronic acid/chitosan-zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, T.; Xin, Y.; Zhang, Z.; Ren, Z.; Lei, J.; Chu, B.; Wang, Y.; Tang, S. Nanofibrous asymmetric membranes self-organized from chemically heterogeneous electrospun mats for skin tissue engineering. Biomed. Mater. 2016, 11, 035019. [Google Scholar] [CrossRef] [PubMed]
- Rim, N.G.; Shin, C.S.; Shin, H. Current approaches to electrospun nanofibers for tissue engineering. Biomed. Mater. 2013, 8, 014102. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Estellés, J.M.; Vidaurre, A.; Duenas, J.M.M.; Cortázar, I.C. Physical characterization of polycaprolactone scaffolds. J. Mater. Sci. 2008, 19, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Wu, Y.B.; Shyu, S.S.; Schoung, J.Y.; Huang, Y.B.; Tsai, Y.H.; Hao, J.Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002, 59, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, S.; Onishi, H.; Machida, Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int. J. Pharm. 2007, 330, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-P.; Chang, G.-Y.; Chen, J.-K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf. A 2008, 313, 183–188. [Google Scholar] [CrossRef]
- Antunes, B.; Moreira, A.; Gaspar, V.; Correia, I. Chitosan/arginine–chitosan polymer blends for assembly of nanofibrous membranes for wound regeneration. Carbohydr. Polym. 2015, 130, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, H.; Zhang, J.; Zhao, Y.; Yuan, X. Preparation and antibacterial activity of electrospun chitosan/poly (ethylene oxide) membranes containing silver nanoparticles. Colloid Polym. Sci. 2009, 287, 1425–1434. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Duan, B.; Wu, L.; Li, S.; Yuan, X. Preparation of electrospun chitosan/poly (vinyl alcohol) membranes. Colloid Polym. Sci. 2007, 285, 855–863. [Google Scholar] [CrossRef]
- Duan, B.; Yuan, X.; Zhu, Y.; Zhang, Y.; Li, X.; Zhang, Y.; Yao, K. A nanofibrous composite membrane of plga–chitosan/pva prepared by electrospinning. Eur. Polym. J. 2006, 42, 2013–2022. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Eshun, K.; He, Q. Aloe vera: A valuable ingredient for the food, pharmaceutical and cosmetic industries—A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Maenthaisong, R.; Chaiyakunapruk, N.; Niruntraporn, S.; Kongkaew, C. The efficacy of aloe vera used for burn wound healing: A systematic review. Burns 2007, 33, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Caridade, S.; Mano, J.; Reis, R. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr. Polym. 2013, 98, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.; Marques, A.; Caridade, S.; Teixeira, P.; Sousa, C.; Mano, J.; Reis, R. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef] [PubMed]
- Suganya, S.; Venugopal, J.; Mary, S.A.; Ramakrishna, S.; Lakshmi, B.; Dev, V.G. Aloe vera incorporated biomimetic nanofibrous scaffold: A regenerative approach for skin tissue engineering. Iran. Polym. J. 2014, 23, 237–248. [Google Scholar] [CrossRef]
- Suganya, S.; Venugopal, J.; Ramakrishna, S.; Lakshmi, B.; Dev, V.G. Naturally derived biofunctional nanofibrous scaffold for skin tissue regeneration. Int. J. Biol. Macromol. 2014, 68, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.; Rocchetti, R. Determination of the degree of acetylation of chitosans by first derivative ultraviolet spectrophotometry. Carbohydr. Polym. 1985, 5, 461–472. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/pcl composite membrane for gtr/gbr application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.-N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Levard, C.; Mitra, S.; Yang, T.; Jew, A.D.; Badireddy, A.R.; Lowry, G.V.; Brown, G.E., Jr. Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli. Environ. Sci. Technol. 2013, 47, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-H. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Wu, Y.-B.; Shyu, S.-S.; Chao, A.-C.; Lai, J.-Y.; Su, C.-C. Asymmetric chitosan membranes prepared by dry/wet phase separation: A new type of wound dressing for controlled antibacterial release. J. Membr. Sci. 2003, 212, 237–254. [Google Scholar] [CrossRef]
- Reis, T.C.; Correia, I.J.; Aguiar-Ricardo, A. Electrodynamic tailoring of self-assembled three-dimensional electrospun constructs. Nanoscale 2013, 5, 7528–7536. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Phan, T.; Lim, I.; Zhang, Y.; Bay, B.; Ramakrishna, S.; Lim, C. Evaluation of electrospun pcl/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-loaded poly (ε-caprolactone) nanofibres: Diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Zhang, Y.; Ramakrishna, S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif. Organs 2006, 30, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Venugopal, J.; Huang, Z.-M.; Lim, C.; Ramakrishna, S. Characterization of the surface biocompatibility of the electrospun pcl-collagen nanofibers using fibroblasts. Biomacromolecules 2005, 6, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Karuppuswamy, P.; Venugopal, J.R.; Navaneethan, B.; Laiva, A.L.; Sridhar, S.; Ramakrishna, S. Functionalized hybrid nanofibers to mimic native ecm for tissue engineering applications. Appl. Surf. Sci. 2014, 322, 162–168. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Polymeric nanofibers as novel carriers for the delivery of therapeutic molecules. J. Nanosci. Nanotechnol. 2006, 6, 2591–2607. [Google Scholar] [CrossRef] [PubMed]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Catledge, S.; Clem, W.; Shrikishen, N.; Chowdhury, S.; Stanishevsky, A.; Koopman, M.; Vohra, Y. An electrospun triphasic nanofibrous scaffold for bone tissue engineering. Biomed. Mater. 2007, 2, 142. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.-H.; Ramakrishna, S. Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Inpanya, P.; Faikrua, A.; Ounaroon, A.; Sittichokechaiwut, A.; Viyoch, J. Effects of the blended fibroin/aloe gel film on wound healing in streptozotocin-induced diabetic rats. Biomed. Mater. 2012, 7, 035008. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Chen, B.; Feng, Y. Water-soluble polysaccharides isolated from skin juice, gel juice and flower of aloe vera miller. J. Taiwan Inst. Chem. Eng. 2011, 42, 197–203. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Elsner, J.J.; Zilberman, M. Novel antibiotic-eluting wound dressings: An in vitro study and engineering aspects in the dressing’s design. J. Tissue Viability 2010, 19, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Elsner, J.J.; Shefy-Peleg, A.; Zilberman, M. Novel biodegradable composite wound dressings with controlled release of antibiotics: Microstructure, mechanical and physical properties. J. Biomed. Mater. Res. Part B 2010, 93, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen loaded pva/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Zahouani, H.; Pailler-Mattei, C.; Sohm, B.; Vargiolu, R.; Cenizo, V.; Debret, R. Characterization of the mechanical properties of a dermal equivalent compared with human skin in vivo by indentation and static friction tests. Skin Res. Technol. 2009, 15, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.Y.; Hung, L.H.; Chu, I.; Ko, C.S.; Lee, Y.D. The application of type II collagen and chondroitin sulfate grafted pcl porous scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2010, 92, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Feng, B.; Zheng, R.; Lu, Y.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, Y.; Zhang, W.J. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials 2013, 34, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Fradique, R.; Correia, T.; Miguel, S.; de Sá, K.; Figueira, D.; Mendonça, A.; Correia, I. Production of new 3d scaffolds for bone tissue regeneration by rapid prototyping. J. Mater. Sci. 2016, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Freyman, T.; Yannas, I.; Gibson, L. Cellular materials as porous scaffolds for tissue engineering. Prog. Mater. Sci. 2001, 46, 273–282. [Google Scholar] [CrossRef]
- Dagalakis, N.; Flink, J.; Stasikelis, P.; Burke, J.; Yannas, I. Design of an artificial skin. Part III. Control of pore structure. J. Biomed. Mater. Res. 1980, 14, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold design for tissue engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Griffith, L.G. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann. N.Y. Acad. Sci. 2002, 961, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Choi, J.-W. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2014, 28, 843–863. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Zhou, S.; Weng, J. Degradation patterns and surface wettability of electrospun fibrous mats. Polym. Degrad. Stab. 2008, 93, 731–738. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Khang, G.; Lee, S.J.; Lee, J.H.; Kim, Y.S.; Lee, H.B. Interaction of fibroblast cells on poly (lactide-co-glycolide) surface with wettability chemogradient. Bio-Med. Mater. Eng. 1999, 9, 179–187. [Google Scholar]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly (lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/pcl composite fibrous scaffolds. J. Biomed. Mater. Res. Part B 2005, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly (ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Agnes Mary, S.; Giri Dev, V. In vivo bioactivity of herbal-drug-incorporated nanofibrous matrixes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Jithendra, P.; Rajam, A.M.; Kalaivani, T.; Mandal, A.B.; Rose, C. Preparation and characterization of aloe vera blended collagen-chitosan composite scaffold for tissue engineering applications. ACS Appl. Mater. Interfaces 2013, 5, 7291–7298. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Ioannovich, J.; Al-Amm, C.A.; El-Musa, K.A. Management of acute and chronic open wounds: The importance of moist environment in optimal wound healing. Curr. Pharm. Biotechnol. 2002, 3, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Rolstad, B. Hypergranulation tissue: A nontraumatic method of management. Ostomy Wound Manag. 1994, 40, 20–22, 24, 26–30. [Google Scholar]
- Gu, S.-Y.; Wang, Z.-M.; Ren, J.; Zhang, C.-Y. Electrospinning of gelatin and gelatin/poly (l-lactide) blend and its characteristics for wound dressing. Mater. Sci. Eng. C 2009, 29, 1822–1828. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, L.; Yuan, T.; Zhang, Q.; Fan, H. Asymmetric polyurethane membrane with in situ-generated nano-tio2 as wound dressing. J. Appl. Polym. Sci. 2011, 119, 1532–1541. [Google Scholar] [CrossRef]
- Lamke, L.-O.; Nilsson, G.; Reithner, H. The evaporative water loss from burns and the water-vapour permeability of grafts and artificial membranes used in the treatment of burns. Burns 1977, 3, 159–165. [Google Scholar] [CrossRef]
- Queen, D.; Gaylor, J.; Evans, J.; Courtney, J.; Reid, W. The preclinical evaluation of the water vapour transmission rate through burn wound dressings. Biomaterials 1987, 8, 367–371. [Google Scholar] [CrossRef]
- Jonkman, M.F.; Molenaar, I.; Nieuwenhuis, P.; Bruin, P.; Pennings, A.J. New method to assess the water vapour permeance of wound coverings. Biomaterials 1988, 9, 263–267. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, Y.; Lim, C. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. 2010, 2, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Feng, B.; Guo, X.; Wang, J.; Zhao, L.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, W.J. Engineering of epidermis skin grafts using electrospun nanofibrous gelatin/polycaprolactone membranes. Int. J. Nanomed. 2013, 8, 2077–2084. [Google Scholar]
- Henry, J.B.; AuBuchon, J.P. Clinical diagnosis and management by laboratory methods. Arch. Pathol. Lab. Med. 1997, 121, 1016. [Google Scholar]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of n-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Zonari, A.; Martins, T.M.; Paula, A.C.C.; Boeloni, J.N.; Novikoff, S.; Marques, A.P.; Correlo, V.M.; Reis, R.L.; Goes, A.M. Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomater. 2015, 17, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas co 2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Mignatti, P.; Rifkin, D.B.; Welgus, H.G.; Parks, W.C. Proteinases and tissue remodeling. In The Molecular and Cellular Biology of Wound Repair; Springer: New York, NY, USA, 1988; pp. 427–474. [Google Scholar]
- Ponche, A.; Ploux, L.; Anselme, K. Protein/material interfaces: Investigation on model surfaces. J. Adhes. Sci. Technol. 2010, 24, 2141–2164. [Google Scholar] [CrossRef]
- Brown, R.A.; Phillips, J.B. Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int. Rev. Cytol. 2007, 262, 75–150. [Google Scholar] [PubMed]
- Cai, K.; Bossert, J.; Jandt, K.D. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B 2006, 49, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lee, C.; Teoh, S. Nanofibrous modification on ultra-thin poly (e-caprolactone) membrane via electrospinning. Mater. Sci. Eng. C 2007, 27, 325–332. [Google Scholar] [CrossRef]
- Mattanavee, W.; Suwantong, O.; Puthong, S.; Bunaprasert, T.; Hoven, V.P.; Supaphol, P. Immobilization of biomolecules on the surface of electrospun polycaprolactone fibrous scaffolds for tissue engineering. ACS Appl. Mater. Interfaces 2009, 1, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Haipeng, G.; Yinghui, Z.; Jianchun, L.; Yandao, G.; Nanming, Z.; Xiufang, Z. Studies on nerve cell affinity of chitosan-derived materials. J. Biomed. Mater. Res. 2000, 52, 285–295. [Google Scholar] [CrossRef]
- Prasitsilp, M.; Jenwithisuk, R.; Kongsuwan, K.; Damrongchai, N.; Watts, P. Cellular responses to chitosan in vitro: The importance of deacetylation. J. Mater. Sci. 2000, 11, 773–778. [Google Scholar]
- Bumgardner, J.; Wiser, R.; Elder, S.; Jouett, R.; Yang, Y.; Ong, J. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J. Biomater. Sci. Polym. Ed. 2003, 14, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.; Sorg, H. Wound repair and regeneration. Eur. Surgical Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, R.G.; Athanasiou, K.A. Extracellular matrix cell adhesion peptides: Functional applications in orthopedic materials. Tissue Eng. 2000, 6, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Chithra, P.; Sajithlal, G.; Chandrakasan, G. Influence of aloe vera on the healing of dermal wounds in diabetic rats. J. Ethnopharmacol. 1998, 59, 195–201. [Google Scholar] [CrossRef]
- Hutter, J.A.; Salman, M.; Stavinoha, W.B.; Satsangi, N.; Williams, R.F.; Streeper, R.T.; Weintraub, S.T. Antiinflammatory c-glucosyl chromone from aloe barbadensis. J. Nat. Prod. 1996, 59, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Danhof, I.E.; McAnalley, B. Stabilized aloe vera-effect on human-skin cells. Drug Cosmet. Ind. 1983, 133, 52. [Google Scholar]
- Jettanacheawchankit, S.; Sasithanasate, S.; Sangvanich, P.; Banlunara, W.; Thunyakitpisal, P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type i collagen; and wound healing. J. Pharmacol. Sci. 2009, 109, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Romanov, A.; Rooney, J.; Chen, W. Non-cytotoxic, in situ gelable hydrogels composed of n-carboxyethyl chitosan and oxidized dextran. Biomaterials 2008, 29, 3905–3913. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.; Duerden, B.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Pandey, R.; Mishra, A. Antibacterial activities of crude extract of aloe barbadensis to clinically isolated bacterial pathogens. Appl. Biochem. Biotechnol. 2010, 160, 1356–1361. [Google Scholar] [CrossRef] [PubMed]




| Sample | Nominal DD a (%) | Determined DD b (%) |
|---|---|---|
| (1) Comercial Chitosan | 75–85 | 82.02 ± 1.87 |
| (2) Purified Chitosan | — | 97.88 ± 1.19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers 2017, 9, 183. https://doi.org/10.3390/polym9050183
Miguel SP, Ribeiro MP, Coutinho P, Correia IJ. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers. 2017; 9(5):183. https://doi.org/10.3390/polym9050183
Chicago/Turabian StyleMiguel, Sónia P., Maximiano P. Ribeiro, Paula Coutinho, and Ilídio J. Correia. 2017. "Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications" Polymers 9, no. 5: 183. https://doi.org/10.3390/polym9050183