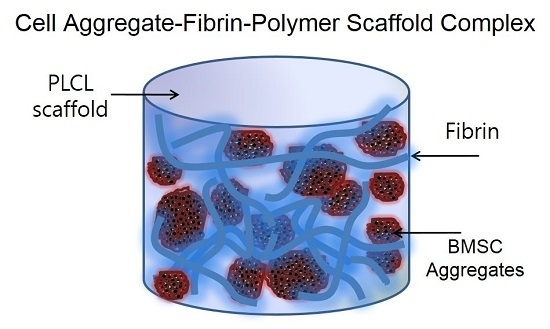
Enhanced Cartilaginous Tissue Formation with a Cell Aggregate-Fibrin-Polymer Scaffold Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the PLCL Scaffolds
2.2. Bone Marrow Stromal Cell Isolation and Culture
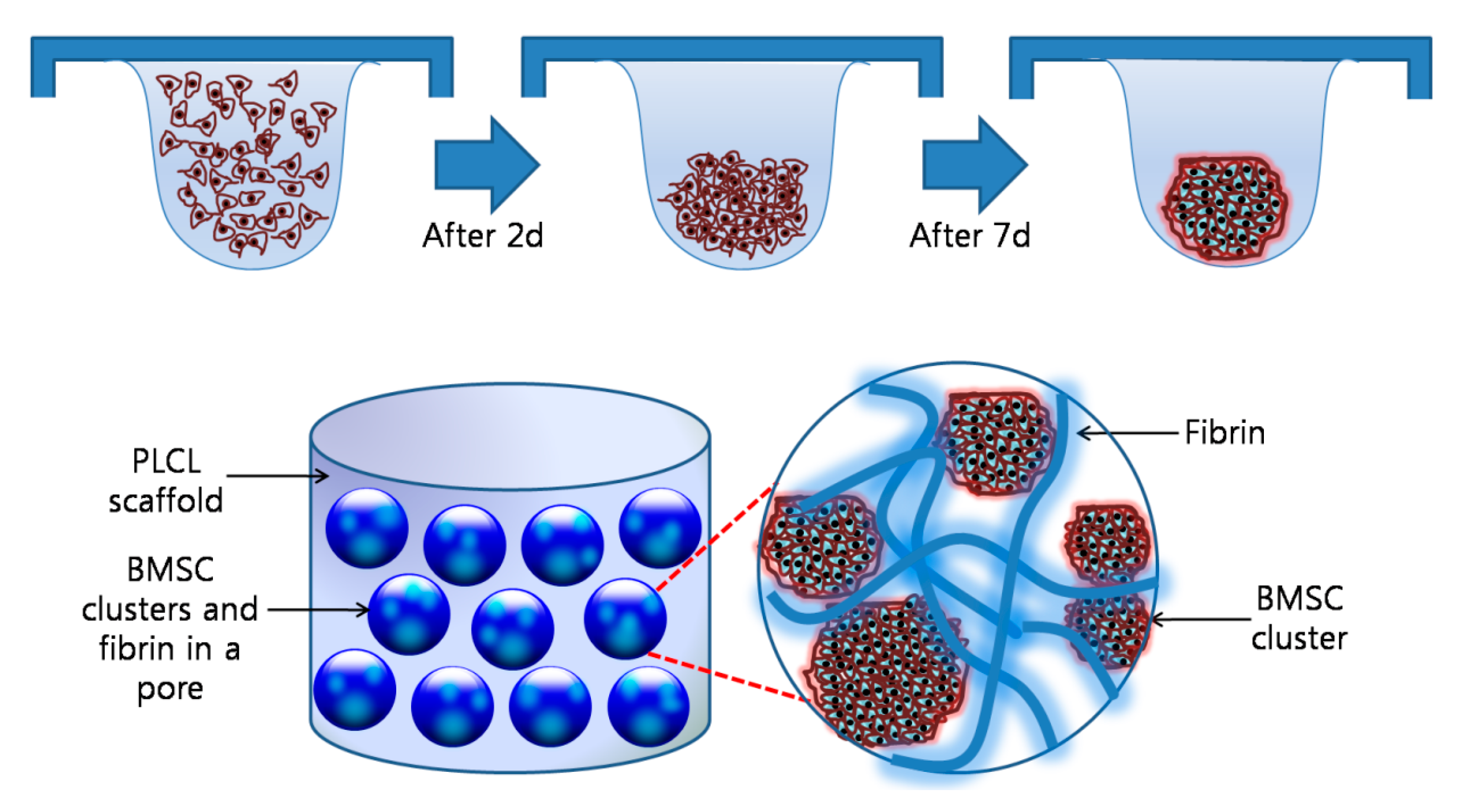
2.3. Preparation of the Cell Aggregates with the Hanging Drop Method
2.4. In Vitro and In Vivo Studies of the Cells-Scaffold Complexes with Fibrin Gels and PLCL Scaffolds
2.5. Evaluation of the Cells-Scaffold Complexes
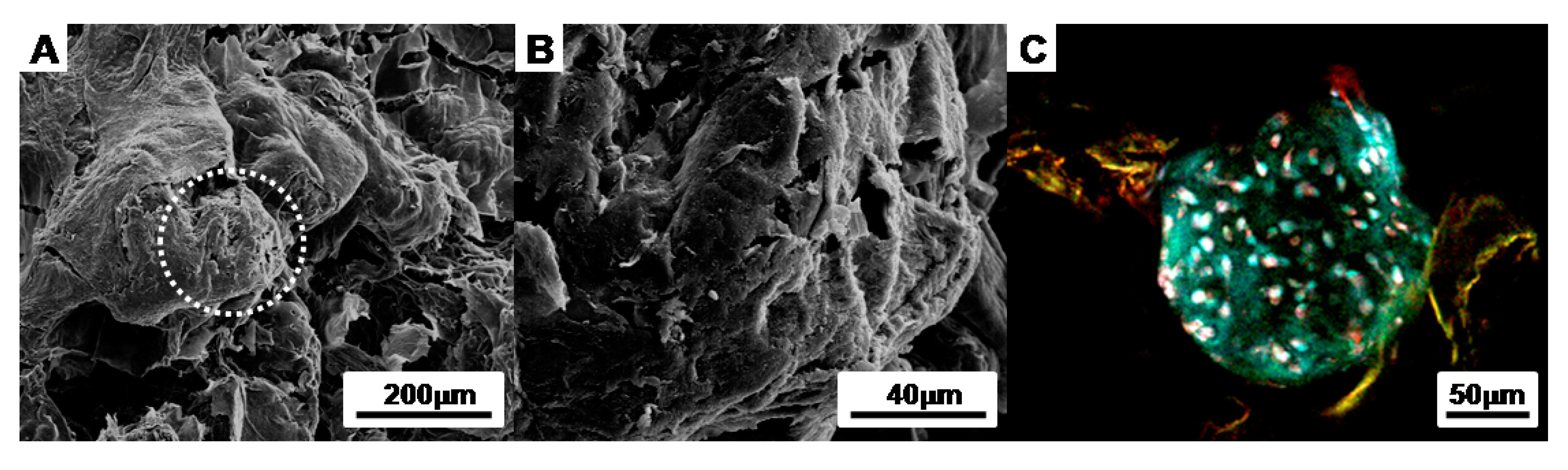
2.5.1. Scanning Electron Microscopy (SEM) Micrographs
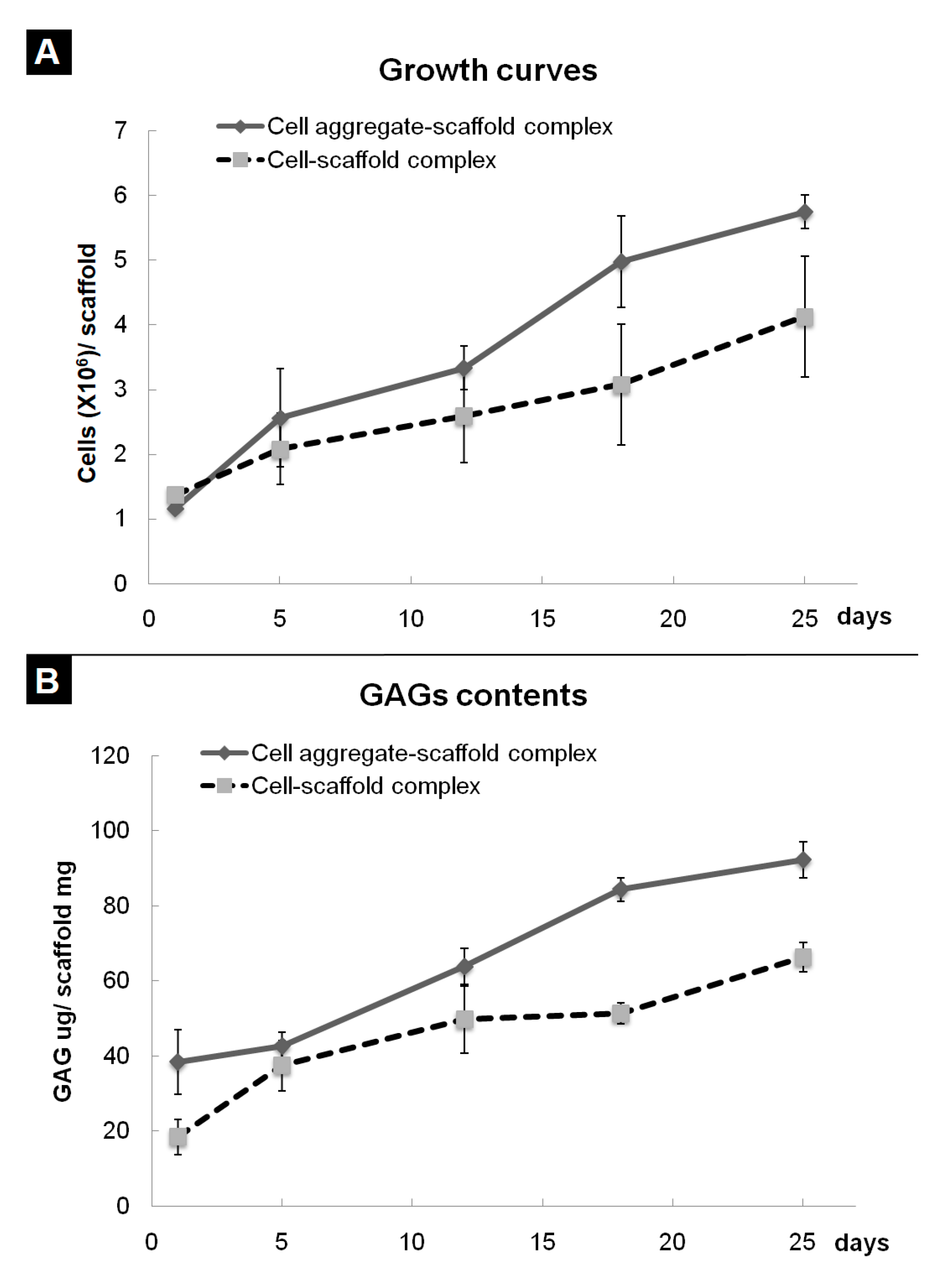
2.5.2. Water Soluble Tetrazolium Salts (WST) Assay
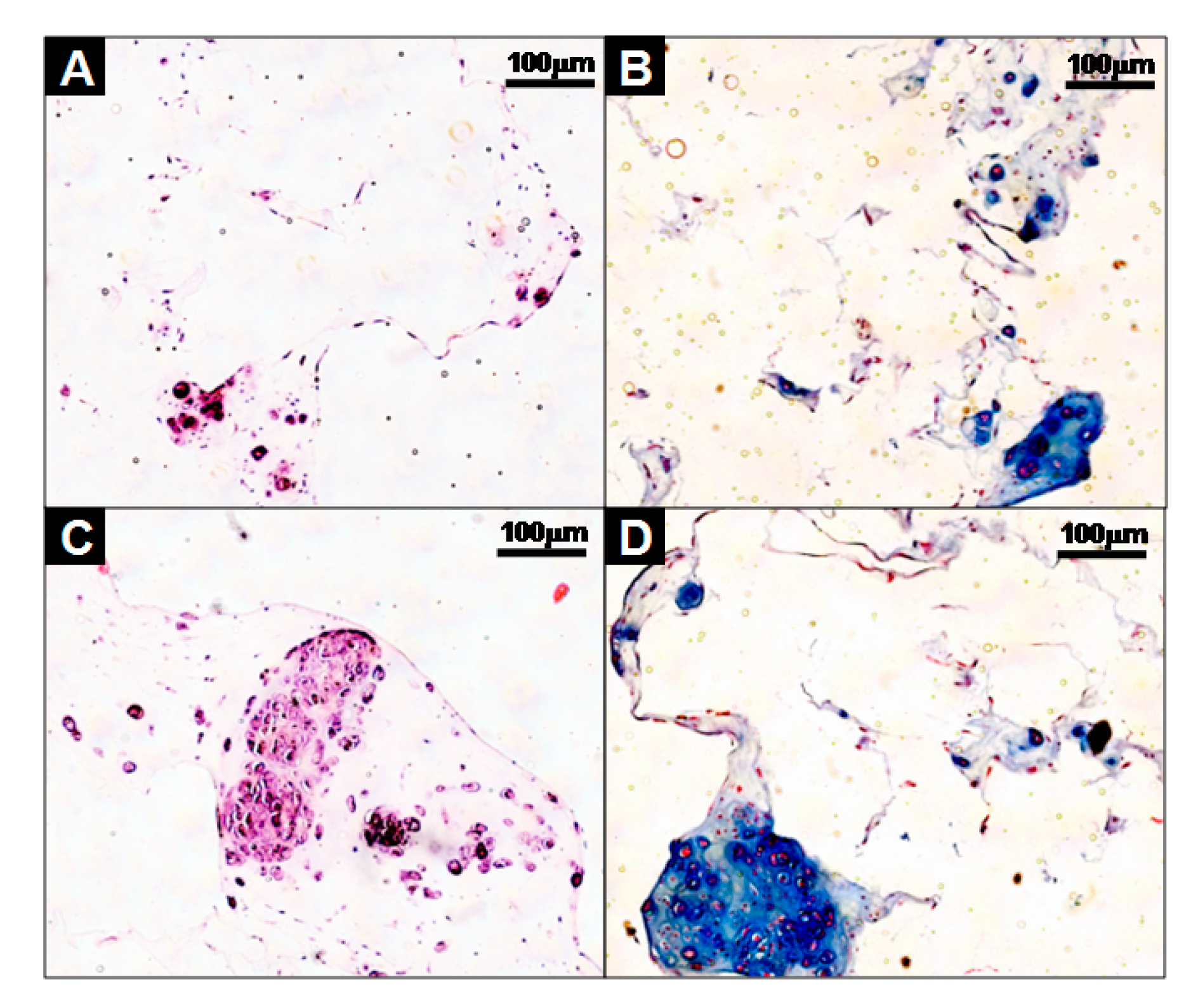
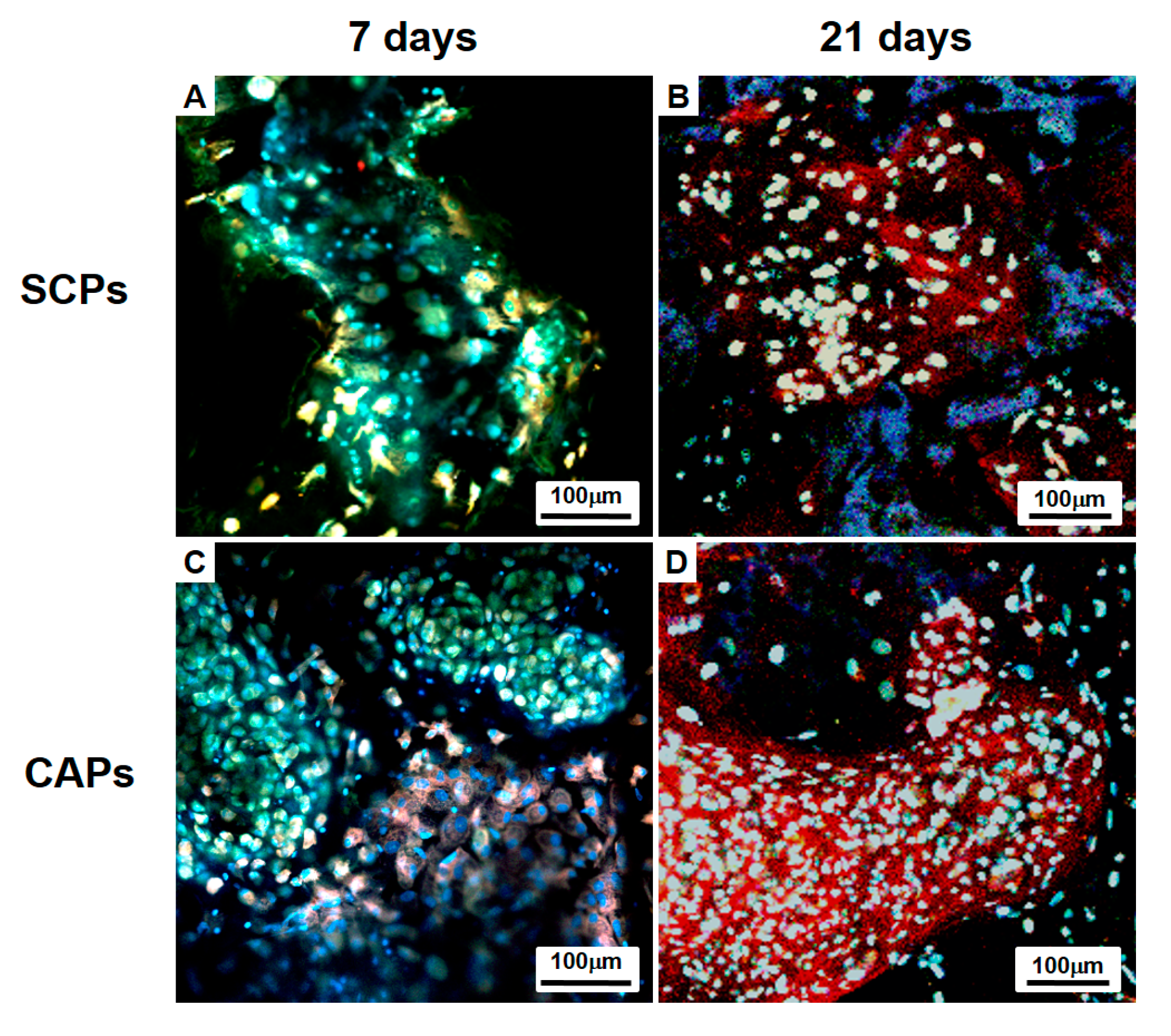
2.5.3. Immunofluorescent and Histological Analysis
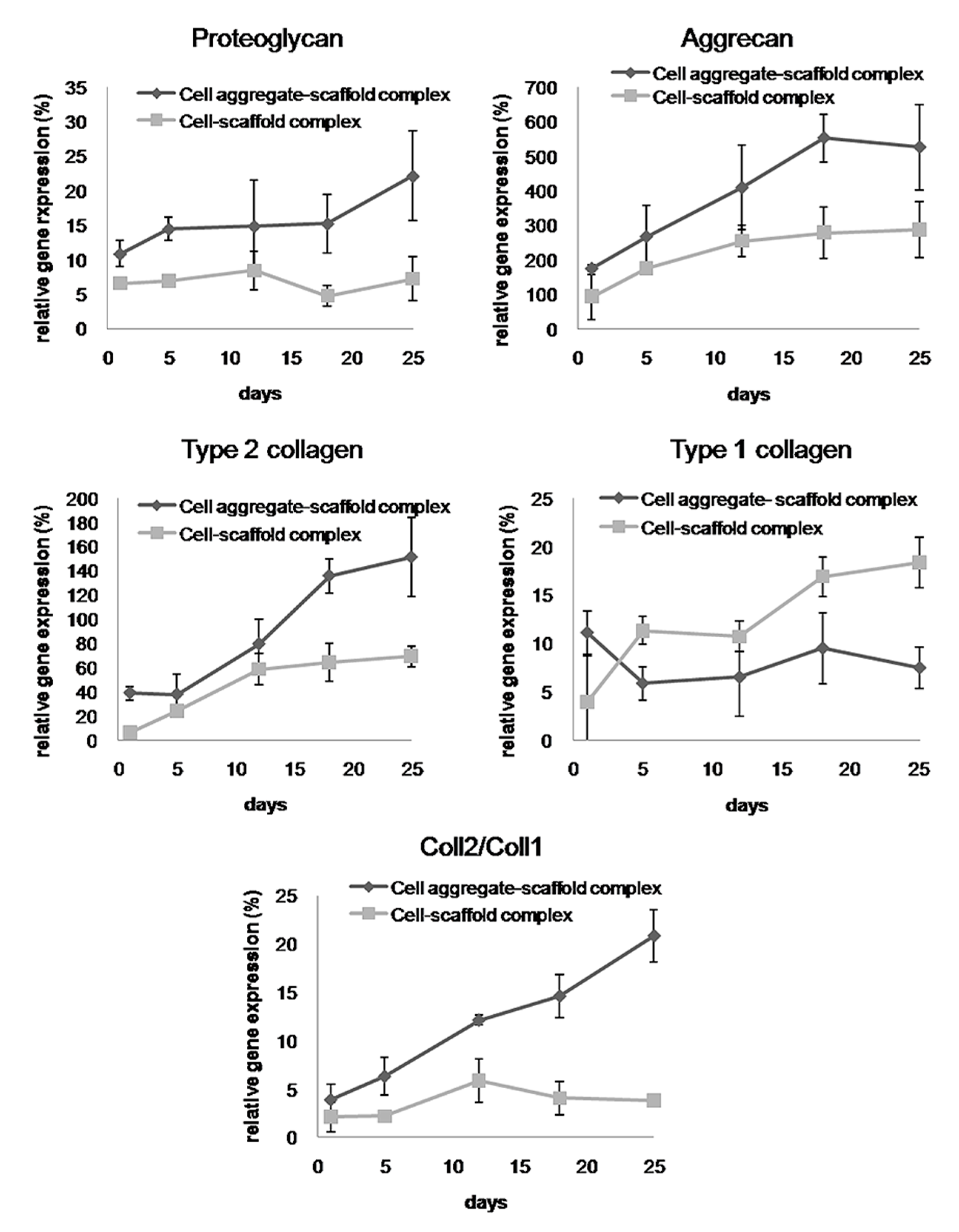
2.5.4. Real-Time Polymerase Chain Reaction (Real-Time PCR)
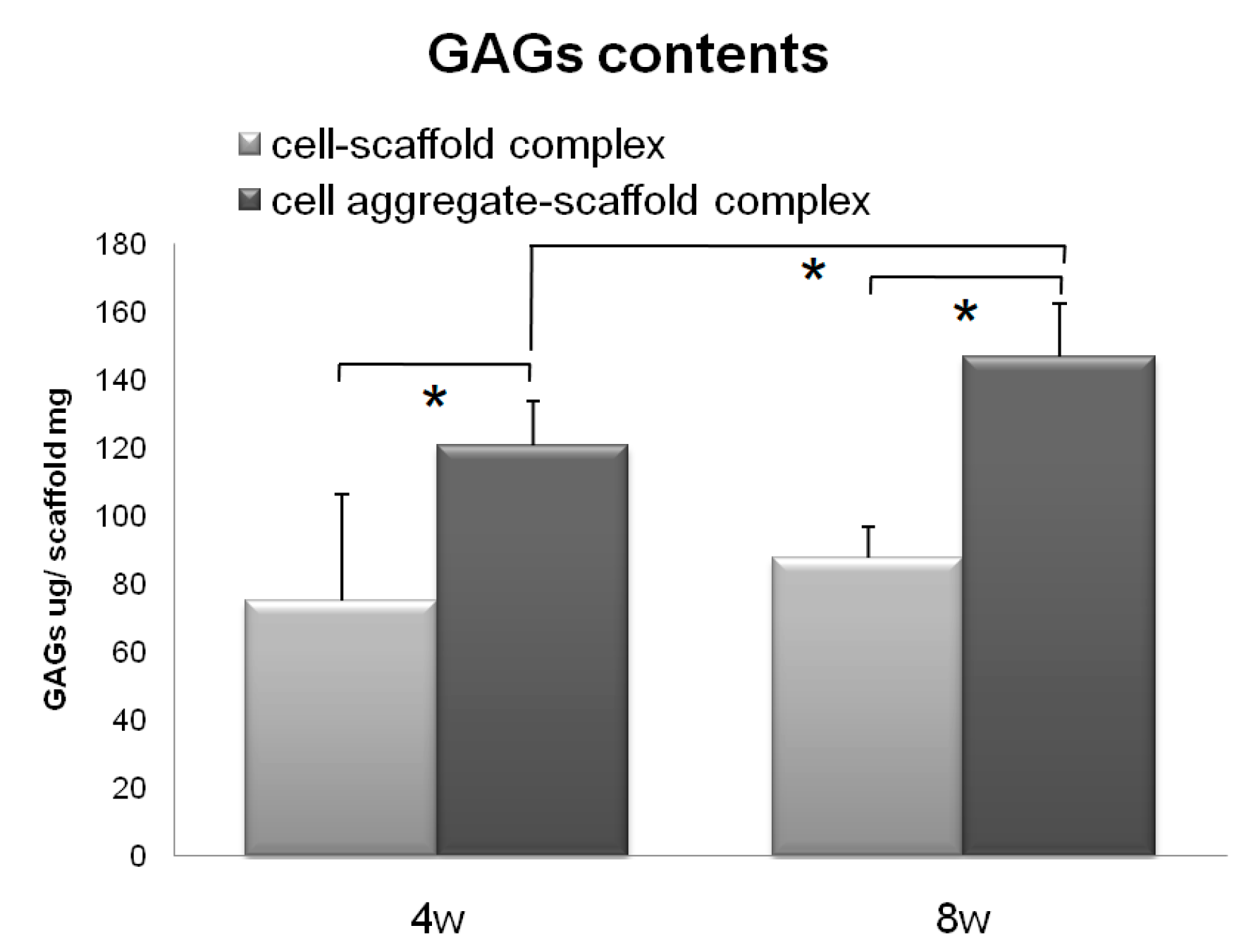
2.5.5. Measurement of the Glycosaminoglycan (GAG) Levels
2.6. Statistical Analysis
3. Results and Discussion
3.1. PLCL Scaffold Characteriztion
3.2. Charaterization of the Cell Aggregates
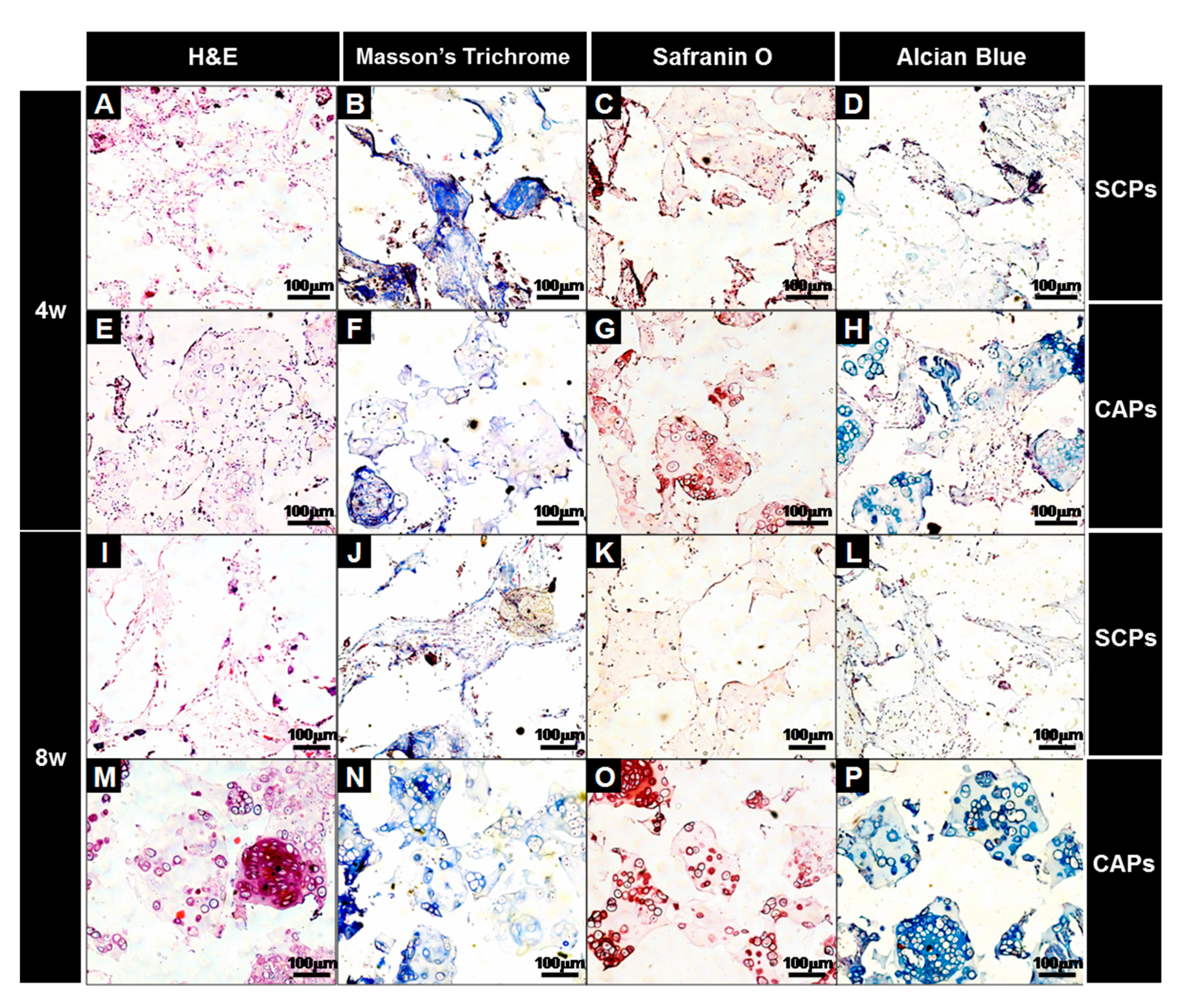
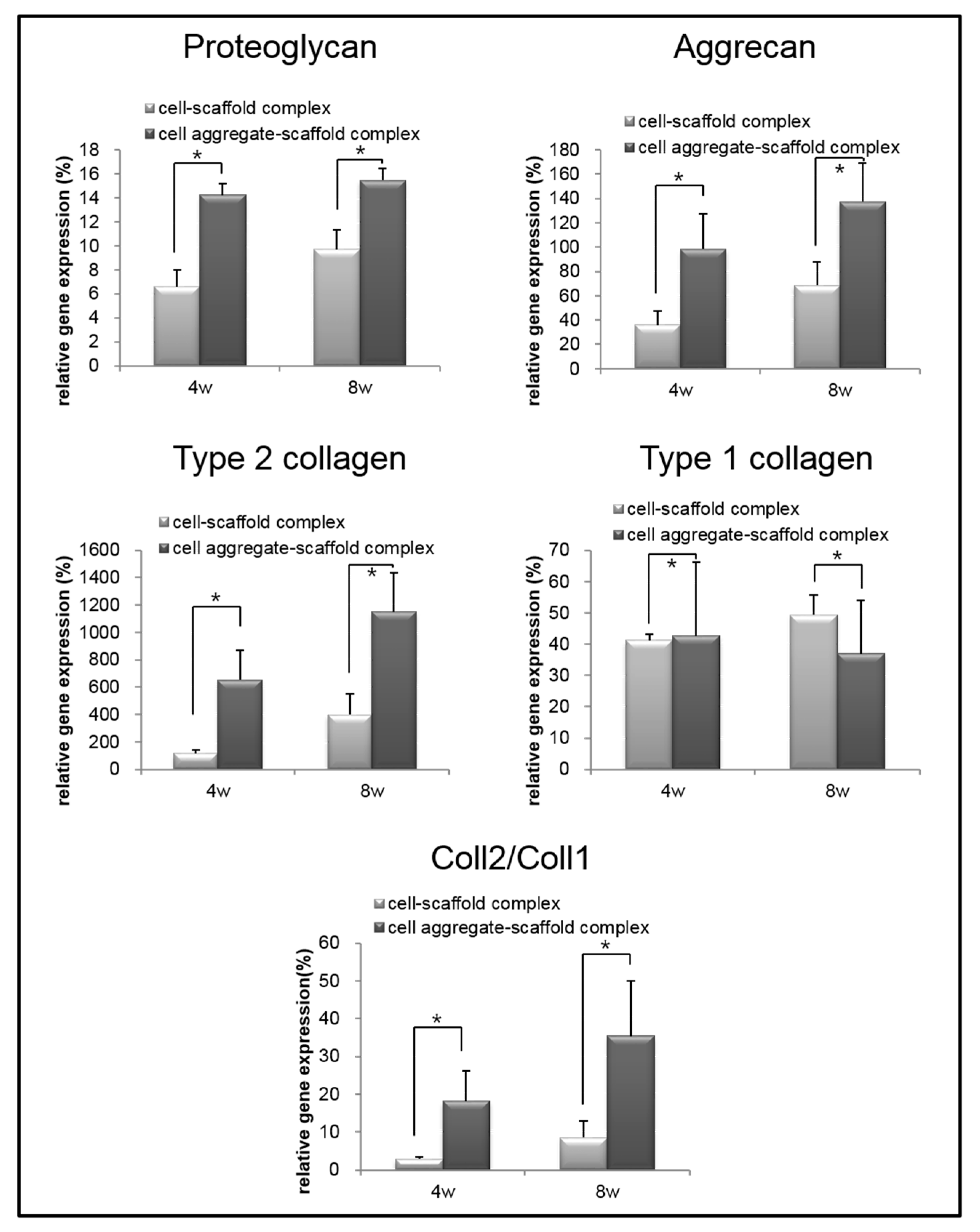
3.3. Evaluation of Cells-Scaffold Complexes: In vitro and In vivo Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buschmann, M.D.; Gluzband, Y.A.; Grodzinsky, A.J.; Hunziker, E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell Sci. 1995, 108, 1497–1508. [Google Scholar] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Almarza, A.J.; Athanasiou, K.A. Design characteristics for the tissue engineering of cartilaginous tissues. Ann. Biomed. Eng. 2004, 32, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Quinn, T.M.; Hauselmann, H.J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr. Cartil. 2002, 10, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Goldberg, V.M.; Caplan, A.I. Cartilage regeneration using principles of tissue engineering. Clin. Orthop. Relat. Res. 2001, 391, S161–S170. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.; Biant, L.C.; Carrington, R.W.; Akmal, M.; Goldberg, A.; Williams, A.M.; Skinner, J.A.; Pringle, J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J. Bone Jt. Surg. 2003, 85, 223–230. [Google Scholar] [CrossRef]
- Fu, F.H.; Zurakowski, D.; Browne, J.E.; Mandelbaum, B.; Erggelet, C.; Moseley, J.B., Jr.; Anderson, A.F.; Micheli, L.J. Autologous chondrocyte implantation versus debridement for treatment of full-thickness chondral defects of the knee: An observational cohort study with 3-year follow-up. Am. J. Sports Med. 2005, 33, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, H.; Hui, J.H.; Feng Choong, E.P.; Tai, B.C.; Lee, E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: An observational cohort study. Am. J. Sports Med. 2010, 38, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, D.Y.; Min, B.H. A new era of cartilage repair using cell therapy and tissue engineering: Turning current clinical limitations into new ideas. Tissue Eng. Regen. Med. 2012, 9, 240–248. [Google Scholar] [CrossRef]
- Hung, C.T.; Lima, E.G.; Mauck, R.L.; Takai, E.; LeRoux, M.A.; Lu, H.H.; Stark, R.G.; Guo, X.E.; Ateshian, G.A. Anatomically shaped osteochondral constructs for articular cartilage repair. J. Biomech. 2003, 36, 1853–1864. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Rosenberg, L.C. Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Jt. Surg. 1996, 78, 721–733. [Google Scholar] [CrossRef]
- Lynn, A.K.; Brooks, R.A.; Bonfield, W.; Rushton, N. Repair of defects in articular joints. Prospects for material-based solutions in tissue engineering. J. Bone Jt. Surg. 2004, 86, 1093–1099. [Google Scholar] [CrossRef]
- Risbud, M.V.; Sittinger, M. Tissue engineering: Advances in in vitro cartilage generation. Trends Biotechnol. 2002, 20, 351–356. [Google Scholar] [CrossRef]
- Swieszkowski, W.; Tuan, B.H.; Kurzydlowski, K.J.; Hutmacher, D.W. Repair and regeneration of osteochondral defects in the articular joints. Biomol. Eng. 2007, 24, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S. A second-generation autologous chondrocyte implantation approach to the treatment of focal articular cartilage defects. Arthritis Res. Ther. 2007, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E.; Vickers, S.M.; Frank, E.; Grodzinsky, A.J.; Spector, M. The effects of glycosaminoglycan content on the compressive modulus of cartilage engineered in type II collagen scaffolds. Osteoarthr. Cartil. 2008, 16, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Minas, T.; Brittberg, M.; Nilsson, A.; Sjogren-Jansson, E.; Lindahl, A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Relat. Res. 2000, 374, 212–234. [Google Scholar] [CrossRef]
- Tung, Y.C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, P.; Xu, C.; Yang, J.; Yu, W.; Huang, D. Chondrogenic differentiation of human mesenchymal stem cells: A comparison between micromass and pellet culture systems. Biotechnol. Lett. 2010, 32, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Ruedel, A.; Hofmeister, S.; Bosserhoff, A.K. Development of a model system to analyze chondrogenic differentiation of mesenchymal stem cells. Int. J. Clin. Exp. Pathol. 2013, 6, 3042–3048. [Google Scholar] [PubMed]
- Jung, Y.; Kim, S.H.; Kim, Y.H.; Kim, S.H. The effects of dynamic and three-dimensional environments on chondrogenic differentiation of bone marrow stromal cells. Biomed. Mater. 2009, 4, 055009. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Uemura, T.; Shirasaki, Y.; Tateishi, T. Promotion of bone formation using highly pure porous β-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials 2002, 23, 4493–4502. [Google Scholar] [CrossRef]
- Cho, S.W.; Park, H.J.; Ryu, J.H.; Kim, S.H.; Kim, Y.H.; Choi, C.Y.; Lee, M.J.; Kim, J.S.; Jang, I.S.; Kim, D.I.; et al. Vascular patches tissue-engineered with autologous bone marrow-derived cells and decellularized tissue matrices. Biomaterials 2005, 26, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Bhonde, R.R. Application of hanging drop technique for stem cell differentiation and cytotoxicity studies. Cytotechnology 2006, 51, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; Balkis, M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 2003, 41, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Ritz, U.; Verrier, S.; Eglin, D.; Alini, M.; Fuchs, S.; Kirkpatrick, C.J.; Rommens, P.M. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials 2008, 29, 4217–4226. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, S.H.; Kim, S.H.; Kim, Y.H.; Xie, J.; Matsuda, T.; Min, B.G. Cartilaginous tissue formation using a mechano-active scaffold and dynamic compressive stimulation. J. Biomater. Sci. Polym. Ed. 2008, 19, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Chung, Y.I.; Kim, S.H.; Tae, G.; Kim, Y.H.; Rhie, J.W.; Kim, S.H.; Kim, S.H. In situ chondrogenic differentiation of human adipose tissue-derived stem cells in a TGF-beta1 loaded fibrin-poly(lactide-caprolactone) nanoparticulate complex. Biomaterials 2009, 30, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, N.; Sato, M.; Ishihara, M.; Mitani, G.; Sakai, H.; Mochida, J. Bioengineered chondrocyte sheets may be potentially useful for the treatment of partial thickness defects of articular cartilage. Biochem. Biophys. Res. Commun. 2006, 349, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Tonomura, H.; Takahashi, K.A.; Mazda, O.; Arai, Y.; Shin-Ya, M.; Inoue, A.; Honjo, K.; Hojo, T.; Imanishi, J.; Kubo, T. Effects of heat stimulation via microwave applicator on cartilage matrix gene and HSP70 expression in the rabbit knee joint. J. Orthop. Res. 2008, 26, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Jeuken, R.M.; Roth, A.K.; Peters, R.J.R.W.; van Donkelaar, C.C.; Thies, J.C.; van Rhijn, L.W.; Emans, P.J. Polymers in Cartilage Defect Repair of the Knee: Current Status and Future Prospects. Polymers 2016, 8, 219. [Google Scholar] [CrossRef]
- Jung, Y.; Park, M.S.; Lee, J.W.; Kim, Y.H.; Kim, S.H.; Kim, S.H. Cartilage regeneration with highly-elastic three-dimensional scaffolds prepared from biodegradable poly(L-lactide-co-epsilon-caprolactone). Biomaterials 2008, 29, 4630–4636. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; D’Amora, U.; Russo, T.; Ronca, A.; Gloria, A.; Ambrosio, L. 3D fibre deposition and stereolithography techniques for the design of multifunctional nanocomposite magnetic scaffolds. J. Mater. Sci. Mater. Med. 2015, 26, 250. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Hsieh, M.F.; Fang, C.H.; Jiang, C.P.; Lin, B.J.; Lee, H.M. Osteochondral Regeneration Induced by TGF-β Loaded Photo Cross-Linked Hyaluronic Acid Hydrogel Infiltrated in Fused Deposition-Manufactured Composite Scaffold of Hydroxyapatite and Poly (Ethylene Glycol)-Block-Poly(ε-Caprolactone). Polymers 2017, 9, 182. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, S.H.; You, H.J.; Kim, S.H.; Kim, Y.H.; Min, B.G. Application of an elastic biodegradable poly(l-lactide-co-ε-caprolactone) scaffold for cartilage tissue regeneration. J. Biomater. Sci. Polym. Ed. 2008, 19, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Cheng, W.T.; Kuo, T.F.; Sun, J.S.; Lin, F.H.; Tsai, J.C. Fibrin glue mixed with gelatin/hyaluronic acid/chondroitin-6-sulfate tri-copolymer for articular cartilage tissue engineering: The results of real-time polymerase chain reaction. J. Biomed. Mater. Res. A 2007, 82, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, S.H.; Kim, Y.H.; Kim, S.H. The effect of hybridization of hydrogels and poly(l-lactide-co-epsilon-caprolactone) scaffolds on cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2010, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.H.; Jang, J.; Kim, S.W.; Cho, D.W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Forward Sequence | Reverse Sequence | Product Size (bp) |
|---|---|---|---|
| Aggrecan | TCGAGGACAGCGAGGCC | TCGAGGGTGTAGGCGTGTAGAGA | 94 |
| Proteogly-can | CACCTACCAGGACAAGGT | GCGCAGGCTCTGGATCTC | 78 |
| Type II collagen | CCTGTGCGACGACATAATCTGT | GCAGTGGCGAGGTCAGTAG | 98 |
| Type I collagen | GGGTTTAGACCGTCGTGAGA | TTGCCAGGAGAACCAGCAAGA | 170 |
| GAPDH | GCACCGTCAAGGCTGAGAAC | ATGGTGGTGAAGACGCCAGT | 142 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, K.; Kim, S.H.; Jung, Y. Enhanced Cartilaginous Tissue Formation with a Cell Aggregate-Fibrin-Polymer Scaffold Complex. Polymers 2017, 9, 348. https://doi.org/10.3390/polym9080348
Lee S, Lee K, Kim SH, Jung Y. Enhanced Cartilaginous Tissue Formation with a Cell Aggregate-Fibrin-Polymer Scaffold Complex. Polymers. 2017; 9(8):348. https://doi.org/10.3390/polym9080348
Chicago/Turabian StyleLee, Soojin, Kangwon Lee, Soo Hyun Kim, and Youngmee Jung. 2017. "Enhanced Cartilaginous Tissue Formation with a Cell Aggregate-Fibrin-Polymer Scaffold Complex" Polymers 9, no. 8: 348. https://doi.org/10.3390/polym9080348