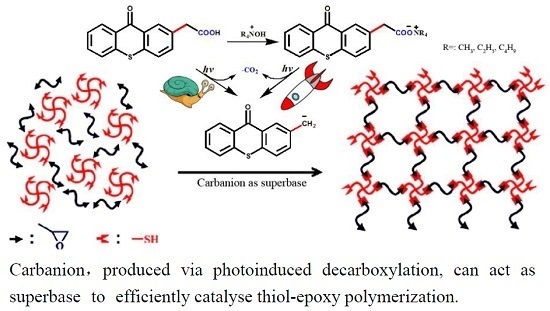
Carbanion as a Superbase for Catalyzing Thiol–Epoxy Photopolymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Photolysis
2.4. Real-Time FT-IR
2.5. Dielectric Analysis
2.6. Thermal Properties Measurements
2.7. Nanoindentation Tests
2.8. Synthetic Procedures
2.8.1. Synthesis of Thioxanthone Acetic Acid (TX)
2.8.2. Synthesis of Compound (9-Oxo-9H-Thioxanthen-2-yl)-Acetatetetraethyl-Ammonium (TX–NEt)
2.8.3. Synthesis of Compound (9-Oxo-9H-Thioxanthen-2-yl)-Acetatetetramethyl-Ammonium (TX–NMe)
2.8.4. Synthesis of Compound (9-Oxo-9H-Thioxanthen-2-yl)-Acetatetetrabutyl-Ammonium (TX–NBu)
3. Results and Discussion
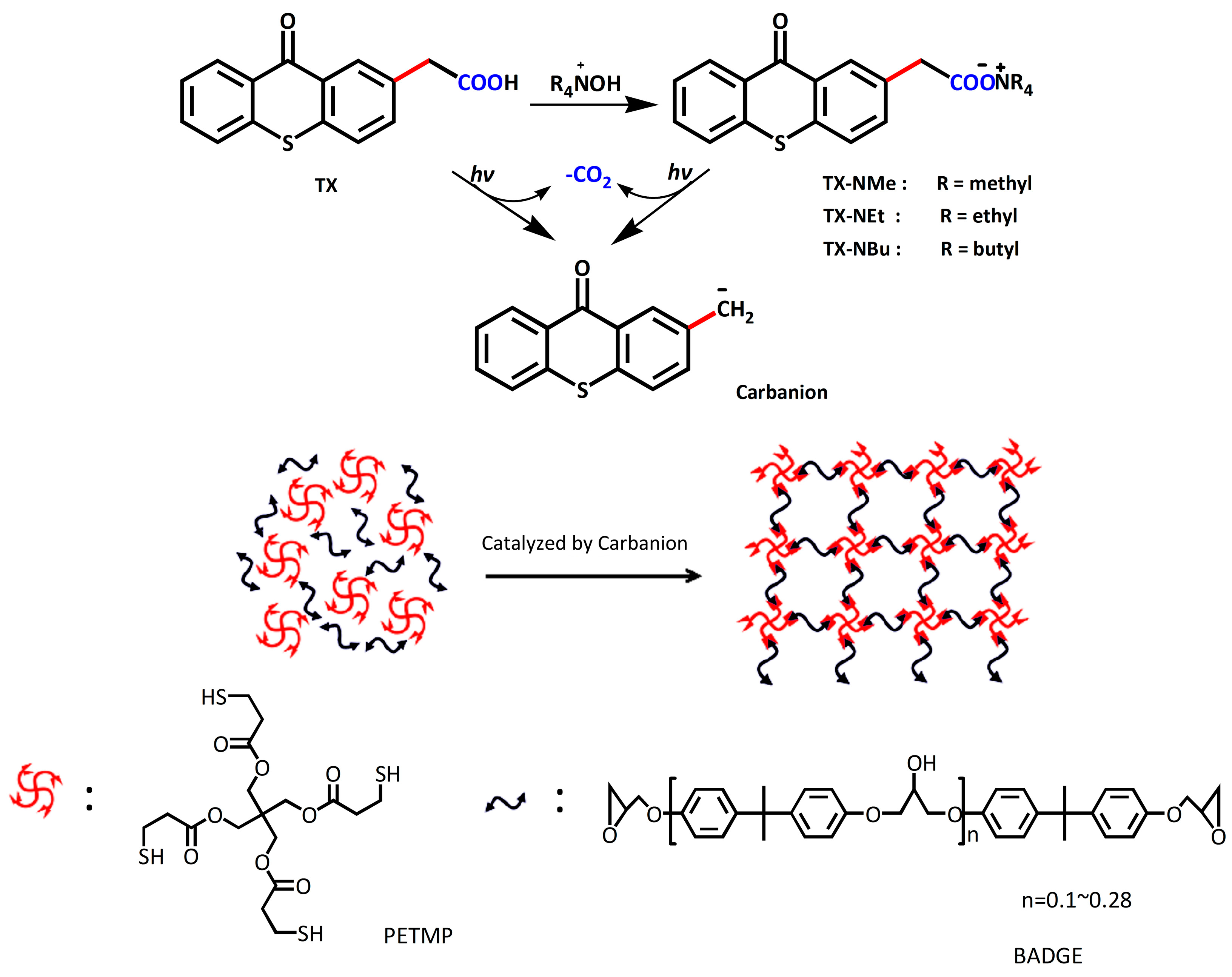
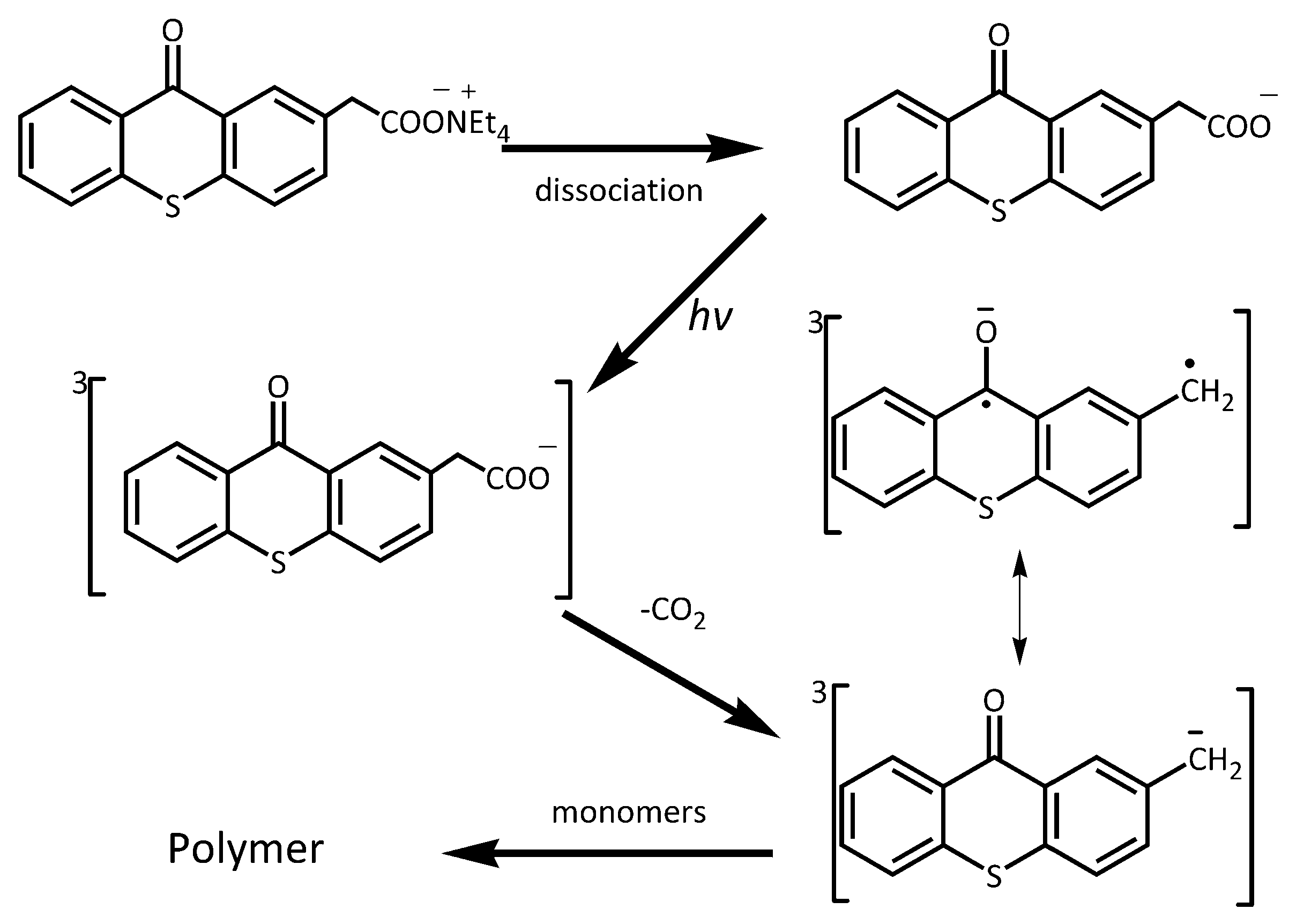
3.1. Synthesis of Photobase Generators
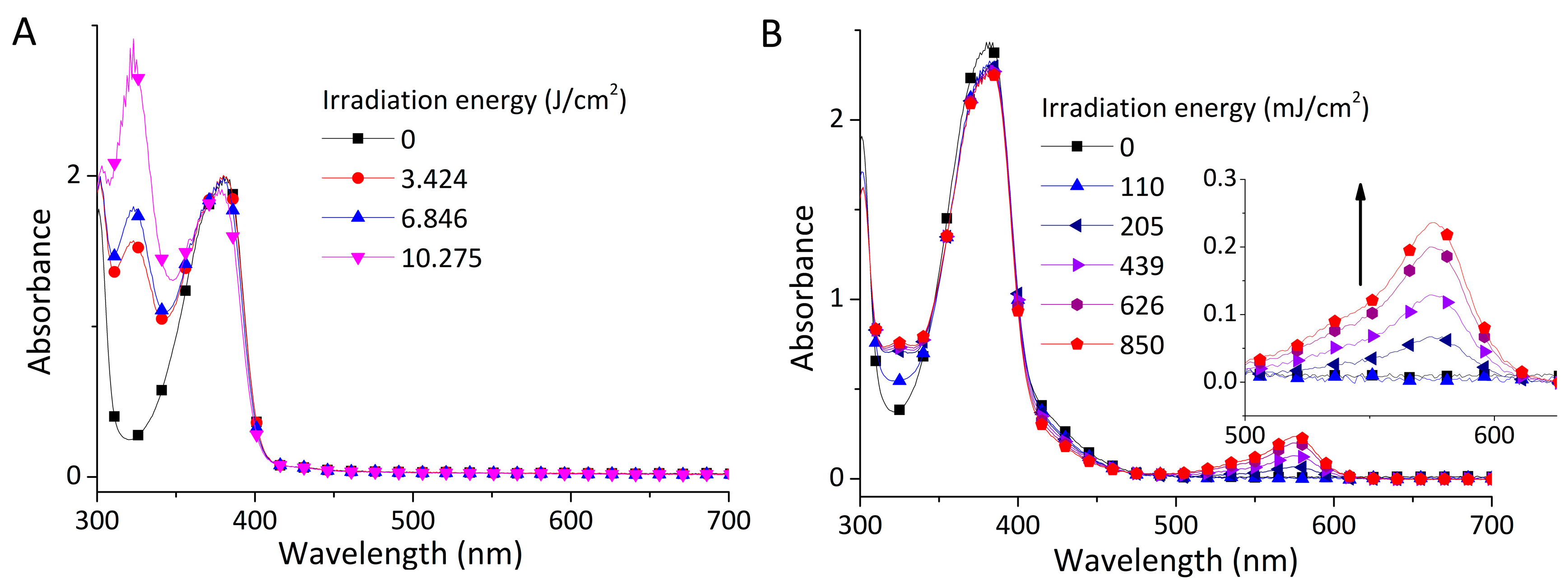
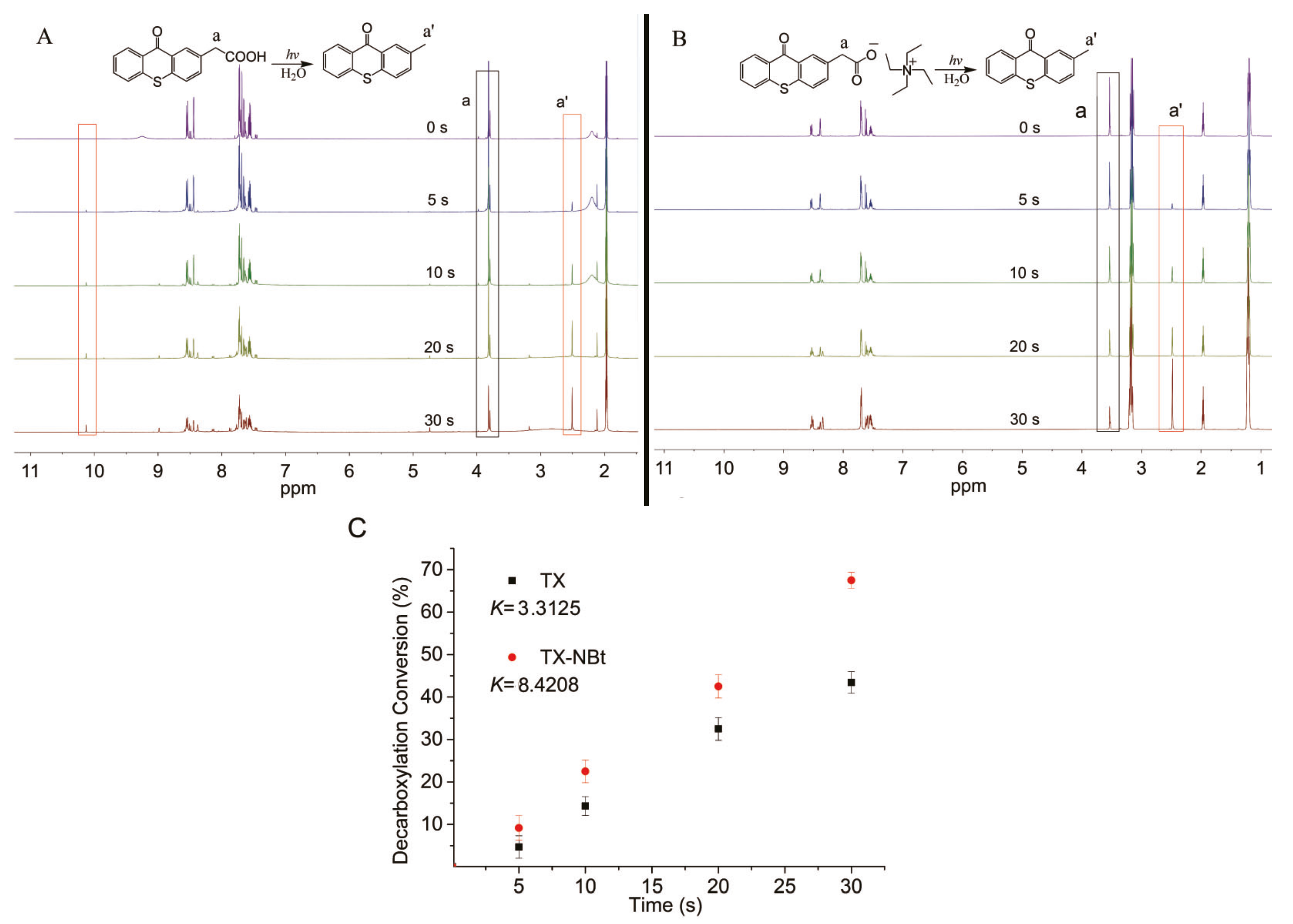
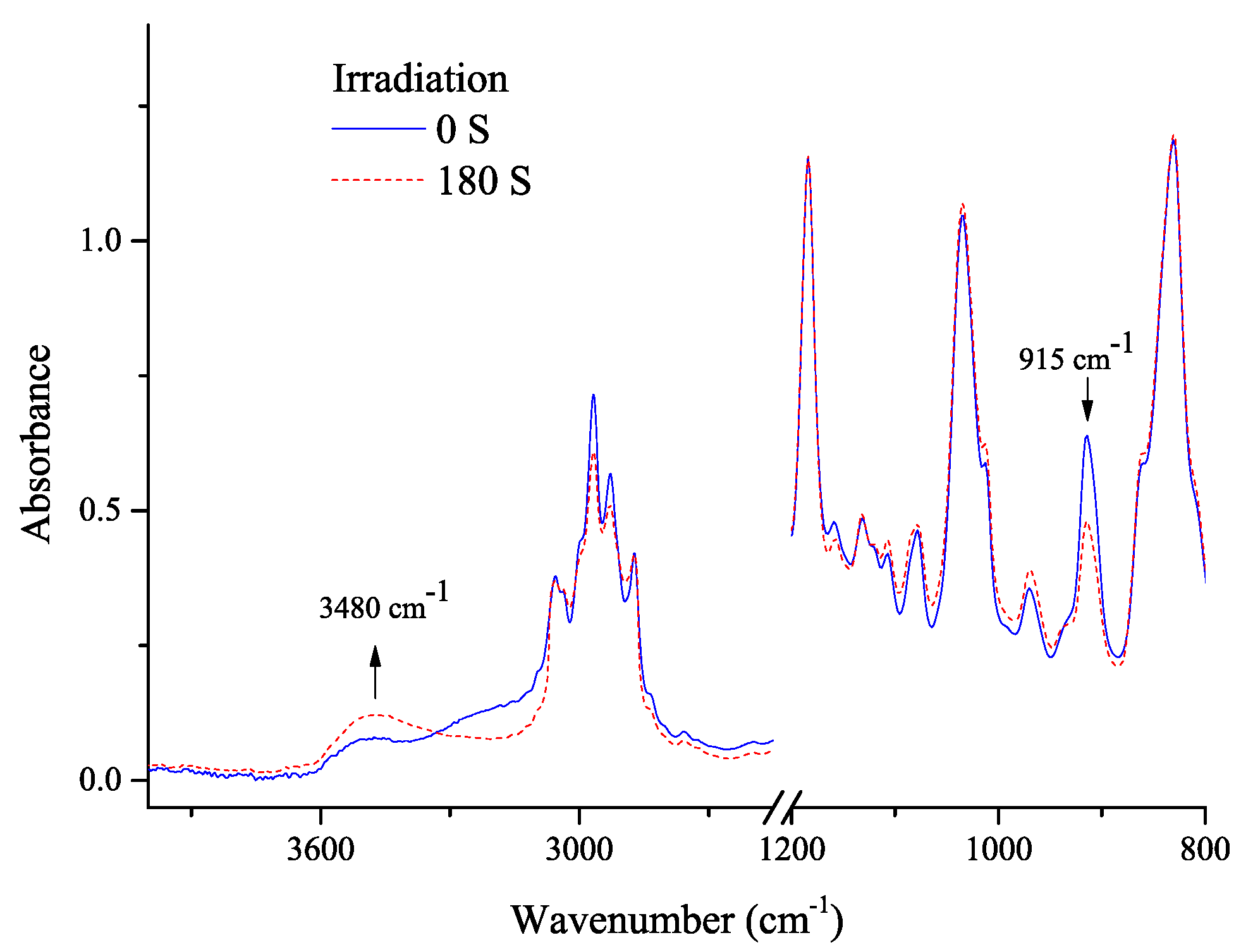
3.2. Photolysis Study
3.3. Carbanion-Catalyzed Ring-Opening Polymerization
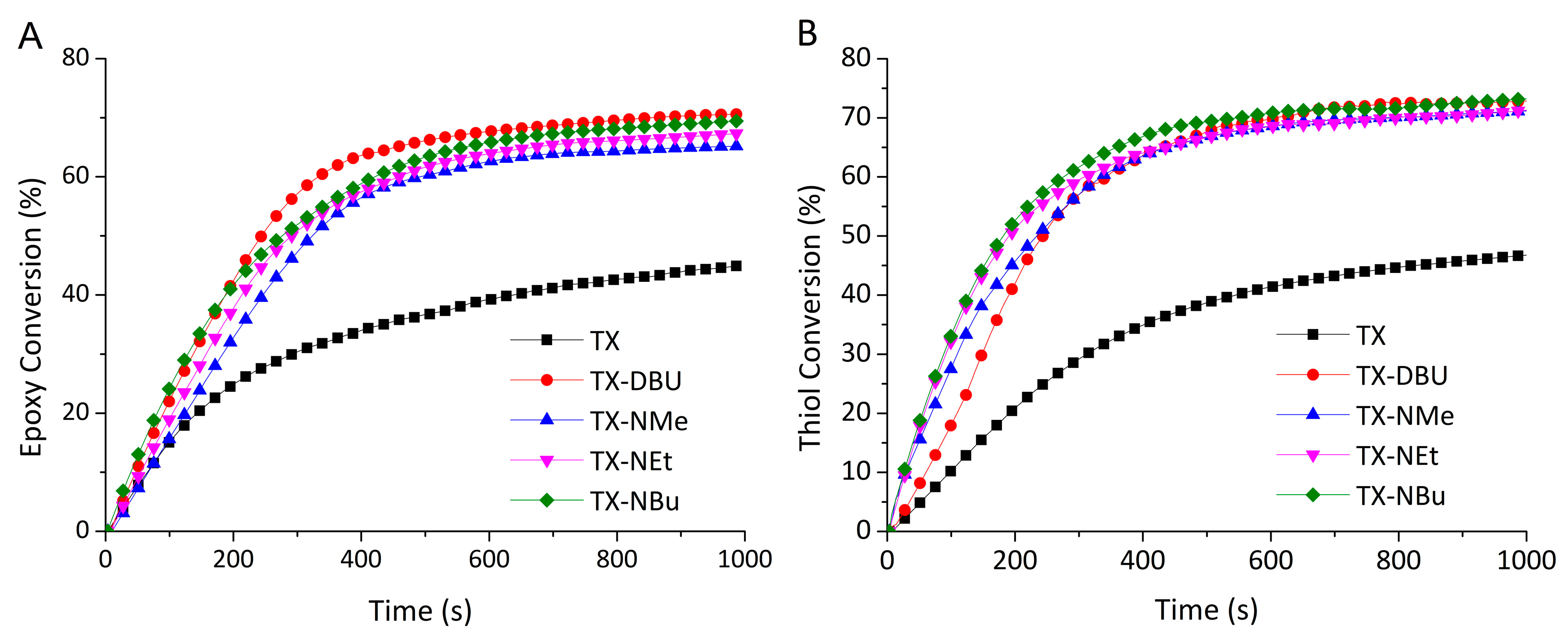
3.4. Carbanion-Catalysed Thiol–Epoxy Photopolymerization
3.5. Dielectric Analysis
3.6. Thermal Properties of Photocured Films
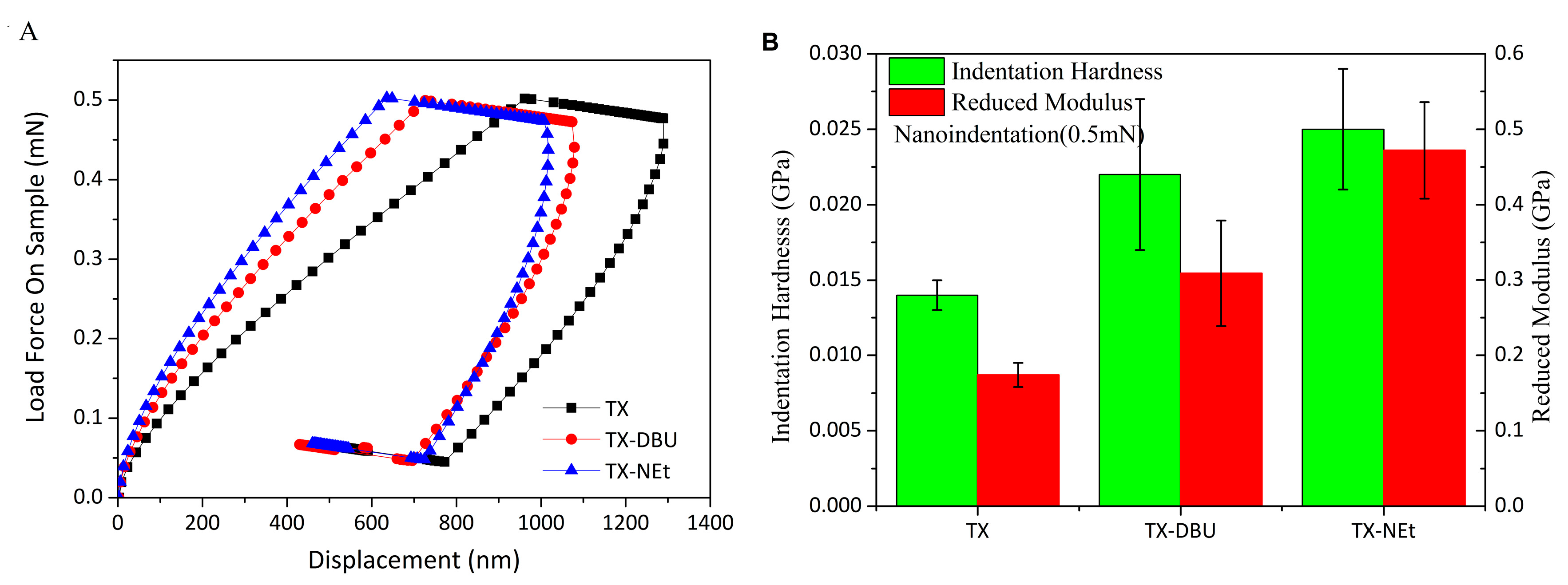
3.7. Mechanical Properties of Photocured Films
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tasdelen, M.A.; Kiskan, B.; Yagci, Y. Externally stimulated click reactions for macromolecular syntheses. Prog. Polym. Sci. 2016, 52, 19–78. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef] [PubMed]
- Stuparu, M.C.; Khan, A. Thiol-epoxy “click” chemistry: Application in preparation and postpolymerization modification of polymers. J. Polym. Sci. Polym. Chem. 2016, 54, 3057–3070. [Google Scholar] [CrossRef]
- Belmonte, A.; Russo, C.; Ambrogi, V.; Fernández-Francos, X.; De la Flor, S. Epoxy-based shape-memory actuators obtained via dual-curing of off-stoichiometric “thiol-epoxy” mixtures. Polymers 2017, 9, 113. [Google Scholar] [CrossRef]
- Hallett-Tapley, G.L.; Wee, T.E.; Hoang, T.; Rananavare, S.B.; Blackwell, J.M.; Scaiano, J.C. Single component photoacid/photobase generators: Potential applications in double patterning photolithography. J. Mater. Chem. C 2013, 1, 2657–2665. [Google Scholar] [CrossRef]
- Chatani, S.; Gong, T.; Earle, B.A.; Podgorski, M.; Bowman, C.N. Visible-Light Initiated Thiol-Michael Addition Photopolymerization Reactions. ACS. Macro Lett. 2014, 3, 315–318. [Google Scholar] [CrossRef]
- Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Evaluation and control of thiol-ene/thiol-epoxy hybrid networks. Polymer 2007, 48, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Arimitsu, K.; Endo, R. Application to photoreactive materials of photochemical generation of superbases with high efficiency based on photodecarboxylation reactions. Chem. Mater. 2013, 25, 4461–4463. [Google Scholar] [CrossRef]
- Guzmán, D. Preparation of click thiol-ene/thiol-epoxy thermosets by controlled photo/thermal dual curing sequence. RSC Adv. 2015, 5, 101623–101633. [Google Scholar] [CrossRef]
- Seubert, C.M.; Nichols, M.E. Epoxy thiol photolatent base clearcoats: Curing and formulation. J. Coat. Technol. Res. 2010, 7, 615–622. [Google Scholar] [CrossRef]
- Sangermano, M.; Vitale, A.; Dietliker, K. Photolatent amines producing a strong base as photocatalyst for the in-situ preparation of organic–inorganic hybrid coatings. Polymer 2014, 55, 1628–1635. [Google Scholar] [CrossRef]
- Katogi, S.; Yusa, M. Photobase generation from amineimide derivatives and their use for curing an epoxide/thiol system. J. Polym. Sci. Polym. Chem. 2002, 40, 4045–4052. [Google Scholar] [CrossRef]
- Salmi, H.; Allonas, X.; Ley, C.; Defoin, A.; Ak, A. Quaternary ammonium salts of phenylglyoxylic acid as photobase generators for thiol-promoted epoxide photopolymerization. Polym. Chem. 2014, 5, 6577–6583. [Google Scholar] [CrossRef]
- Sun, X.; Gao, J.P.; Wang, Z.Y. Bicyclic guanidinium tetraphenylborate: A photobase generator and a photocatalyst for living anionic ring-opening polymerization and cross-linking of polymeric materials containing ester and hydroxy groups. J. Am. Chem. Soc. 2008, 130, 8130–8131. [Google Scholar] [CrossRef] [PubMed]
- Podsiadly, R.; Podemska, K.; Szymczak, A.M. Novel visible photoinitiators systems for free-radical/cationic hybrid photopolymerization. Dyes Pigments 2011, 91, 422–426. [Google Scholar] [CrossRef]
- Jian, Y.; He, Y.; Sun, Y.; Yang, H.; Yang, W.; Nie, J. Thiol-epoxy/thiol-acrylate hybrid materials synthesized by photopolymerization. J. Mater. Chem. C 2013, 1, 4481–4489. [Google Scholar] [CrossRef]
- Shao, J.; Huang, Y.; Fan, Q. Visible light initiating systems for photopolymerization: Status, development and challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Arsu, N.; Aydin, M.; Yagci, Y.; Jockusch, S.; Turro, N.J. Photochemistry and UV Curing: New Trends; Research Signpost: Kerala, India, 2006; pp. 17–29. [Google Scholar]
- Zhang, X.; Xi, W.; Wang, C.; Podgórski, M.; Bowman, C.N. Visible-light-initiated thiol-michael addition polymerizations with coumarin-based photobase generators: Another photoclick reaction strategy. ACS Macro Lett. 2016, 5, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Hu, P.; Zhu, G.; Li, Z.; Liu, R.; Liu, X. Thioxanthone acetic acid ammonium salts: Highly efficient photobase generators based on photodecarboxylation. RSC Adv. 2015, 5, 53342–53348. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, P. Ketoprofen as a photoinitiator for anionic polymerization. Photochem. Photobiol. Sci. 2015, 14, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.A.; Lukeman, M.; Scaiano, J.C. Photolabile protecting groups based on the singlet state photodecarboxylation of xanthone acetic acid. J. Am. Chem. Soc. 2009, 131, 4127–4135. [Google Scholar] [CrossRef] [PubMed]
- Lukeman, M.; Scaiano, J.C. Carbanion-mediated photocages: Rapid and efficient photorelease with aqueous compatibility. J. Am. Chem. Soc. 2005, 127, 7698–7699. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, X.; Fang, W.; Phillips, D.L. Ph- and wavelength-dependent photodecarboxylation of ketoprofen. Org. Lett. 2011, 13, 5472–5475. [Google Scholar] [CrossRef] [PubMed]
- Morton, M. Anionic Polymerization: Principles and Practice; Elsevier: New York, NY, USA, 2012; pp. 86–90. [Google Scholar]
- Ederlé, Y.; Mathis, C. Carbanions on grafted C60 as initiators for anionic polymerization. Macromolecules 1997, 30, 4262–4267. [Google Scholar] [CrossRef]
- Esen, D.S.; Temel, G.; Balta, D.K.; Allonas, X.; Arsu, N. One-component thioxanthone acetic acid derivative photoinitiator for free radical polymerization. Photochem. Photobiol. 2014, 90, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Su, T.; Ma, J.; Liu, M.; Liu, H.; Li, X.; Phillips, D.L. Phototriggered release of a leaving group in ketoprofen derivatives via a benzylic carbanion pathway, but not via a biradical pathway. Chem. Eur. J. 2013, 19, 11241–11250. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yeung, C.S.; Guan, X.; Ma, J.; Li, W.; Ma, C.; Phillips, D.L. Water- and acid-mediated excited-state intramolecular proton transfer and decarboxylation reactions of ketoprofen in water-rich and acidic aqueous solutions. Chem. Eur. J. 2011, 17, 10935–10950. [Google Scholar] [CrossRef] [PubMed]
- Corrales, T.; Catalina, F.; Peinado, C.; Allen, N.S. Free radical macrophotoinitiators: An overview on recent advances. J. Photochem. Photobiol. A 2003, 159, 103–114. [Google Scholar] [CrossRef]
- McLeary, J.B.; McKenzie, J.M.; Tonge, M.P.; Sanderson, R.D.; Klumperman, B. Initialisation in raft-mediated polymerisationpolymerization of methyl acrylateelectronic. Chem. Commun. 2004, 17, 1950–1951. [Google Scholar] [CrossRef] [PubMed]
- McLeary, J.B.; Calitz, F.M.; McKenzie, J.M.; Tonge, M.P.; Sanderson, R.D.; Klumperman, B. A 1H NMR investigation of reversible addition−fragmentation chain transfer polymerization kinetics and mechanisms. Initialization with different initiating and leaving groups. Macromolecules 2005, 38, 3151–3161. [Google Scholar] [CrossRef]
- Brindle, I.D.; Doyle, P.P. Methyl substituted thioxanthones and thioxanthone-10,10-dioxides. Can. J. Chem. 1983, 61, 1869–1871. [Google Scholar] [CrossRef]
- Green, M.; Wong, L.L.; Sella, A. Relationship between intramolecular chemical exchange and NMR-observed rate constants. Organometallics 1992, 7, 2660–2668. [Google Scholar] [CrossRef]
- Reetz, M.T.; Knauf, T.; Minet, U.; Bingel, C. Metal-free carbanion salts as initiators for the anionic polymerization of acrylic and methacrylic acid esters. Angew. Chem. Int. Ed. 1988, 27, 1373–1374. [Google Scholar] [CrossRef]
- Carlborg, C.F.; Vastesson, A.; Liu, Y.; van der Wijngaart, W.; Johansson, M.; Haraldsson, T. Functional off-stoichiometry thiol-ene-epoxy thermosets featuring temporally controlled curing stages via an UV/UV dual cure process. J. Polym. Sci. Polym. Chem. 2014, 52, 2604–2615. [Google Scholar] [CrossRef]
- Terasaki, M.; Kazama, T.; Shiraishi, F.; Makino, M. Identification and estrogenic characterization of impurities in commercial bisphenol a diglycidyl ether (BADGE). Chemosphere 2006, 65, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Scigalski, F.; Paczkowski, J. Tetraalkylammonium salts of amino acids and sulfur-containing amino acids as effective co-initiators of free radical polymerization in the presence of aromatic ketones. Macromol. Chem. Phys. 2008, 209, 1872–1880. [Google Scholar] [CrossRef]
- Aydin, M.; Arsu, N.; Yagci, Y.; Jockusch, S.; Turro, N.J. Mechanistic study of photoinitiated free radical polymerization using thioxanthone thioacetic acid as one-component type II photoinitiator. Macromolecules 2005, 38, 4133–4138. [Google Scholar] [CrossRef]
- Li, Z.; Shen, W.; Liu, X.; Liu, R. Efficient unimolecular photoinitiators for simultaneous hybrid thiol-yne-epoxy photopolymerization under visible led light irradiation. Polym. Chem. 2017, 8, 1579–1588. [Google Scholar] [CrossRef]
- Steinhaus, J.; Hausnerova, B.; Haenel, T.; Großgarten, M.; Möginger, B. Curing kinetics of visible light curing dental resin composites investigated by dielectric analysis (DEA). Dent. Mater. 2014, 30, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. New catalysts for diglycidyl ether of bisphenol a curing based on thiol-epoxy click reaction. Eur. Polym. J. 2014, 59, 377–386. [Google Scholar] [CrossRef]
- Liu, R.; Chen, H.; Li, Z.; Shi, F.; Liu, X. Extremely deep photopolymerization using upconversion particles as internal lamps. Polym. Chem. 2016, 7, 2457–2463. [Google Scholar] [CrossRef]
- Oyen, M.L.; Cook, R.F. A practical guide for analysis of nanoindentation data. J. Mech. Behav. Biomed. 2009, 2, 396–407. [Google Scholar] [CrossRef] [PubMed]


| Type of PBGs | Tg °C | T5% °C | Tmax °C |
|---|---|---|---|
| TX | 6 | 328 | 367 |
| TX–NEt | 15 | 326 | 372 |
| TX–DBU | 18 | 298 | 376 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Hu, P.; Shen, W.; Li, Z.; Liu, R.; Liu, X. Carbanion as a Superbase for Catalyzing Thiol–Epoxy Photopolymerization. Polymers 2017, 9, 400. https://doi.org/10.3390/polym9090400
Dong X, Hu P, Shen W, Li Z, Liu R, Liu X. Carbanion as a Superbase for Catalyzing Thiol–Epoxy Photopolymerization. Polymers. 2017; 9(9):400. https://doi.org/10.3390/polym9090400
Chicago/Turabian StyleDong, Xiaoqing, Peng Hu, Weizhen Shen, Zhiquan Li, Ren Liu, and Xiaoya Liu. 2017. "Carbanion as a Superbase for Catalyzing Thiol–Epoxy Photopolymerization" Polymers 9, no. 9: 400. https://doi.org/10.3390/polym9090400