Mixed Rigid and Flexible Component Design for High-Performance Polyimide Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
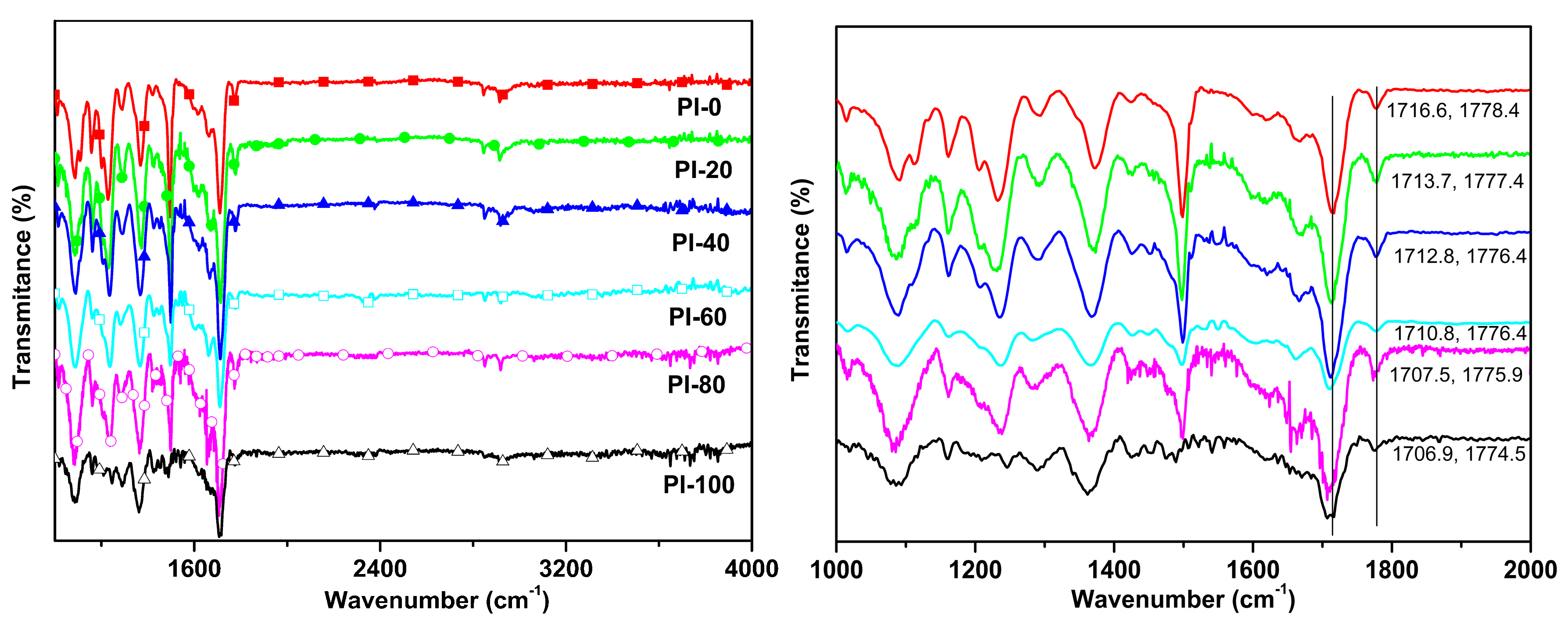
2.2.1. Infrared Spectroscopic Analysis
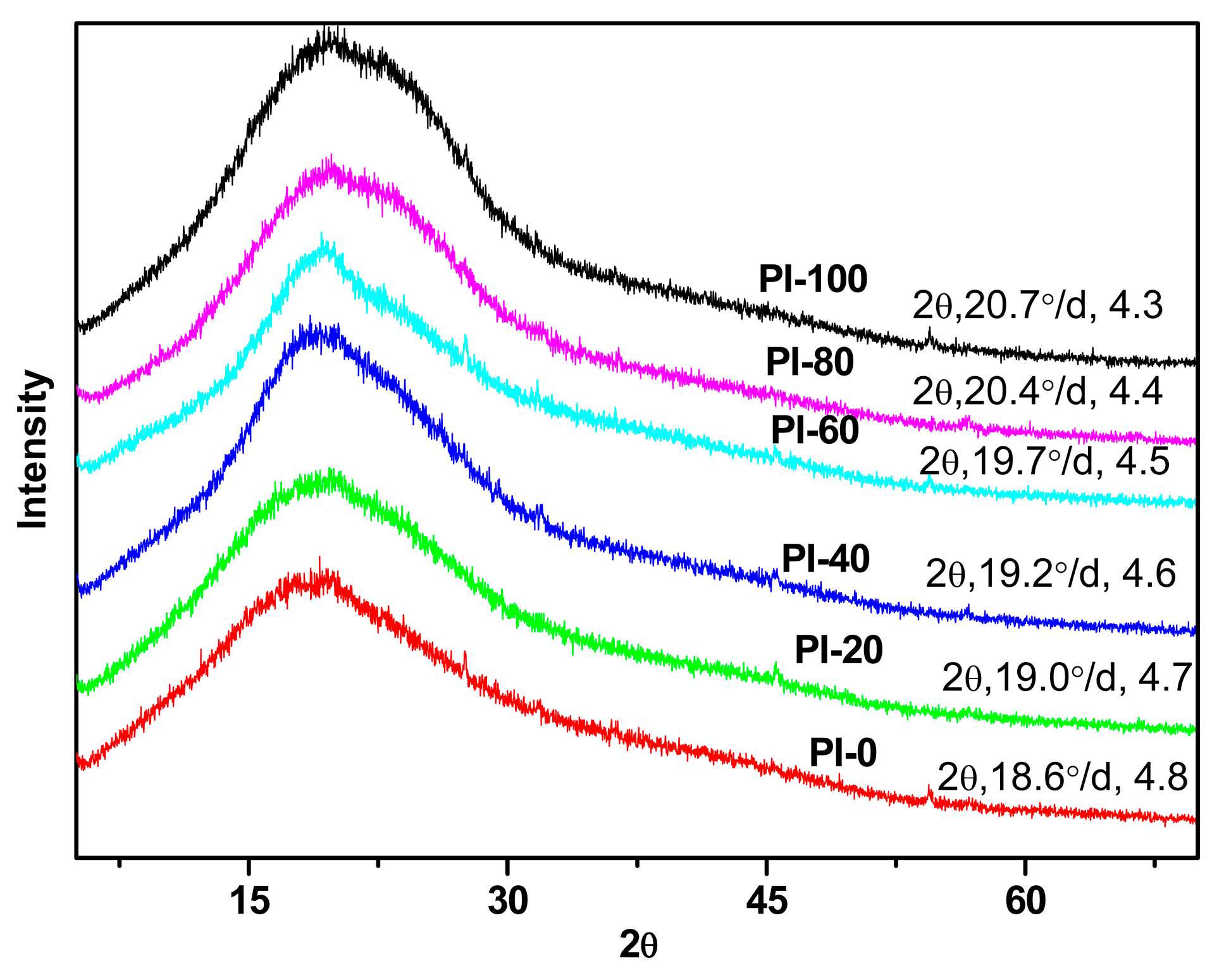
2.2.2. Morphology and Structural Analysis
2.2.3. Thermal Analysis
2.2.4. Mechanical Analysis
2.2.5. Adhesion Properties
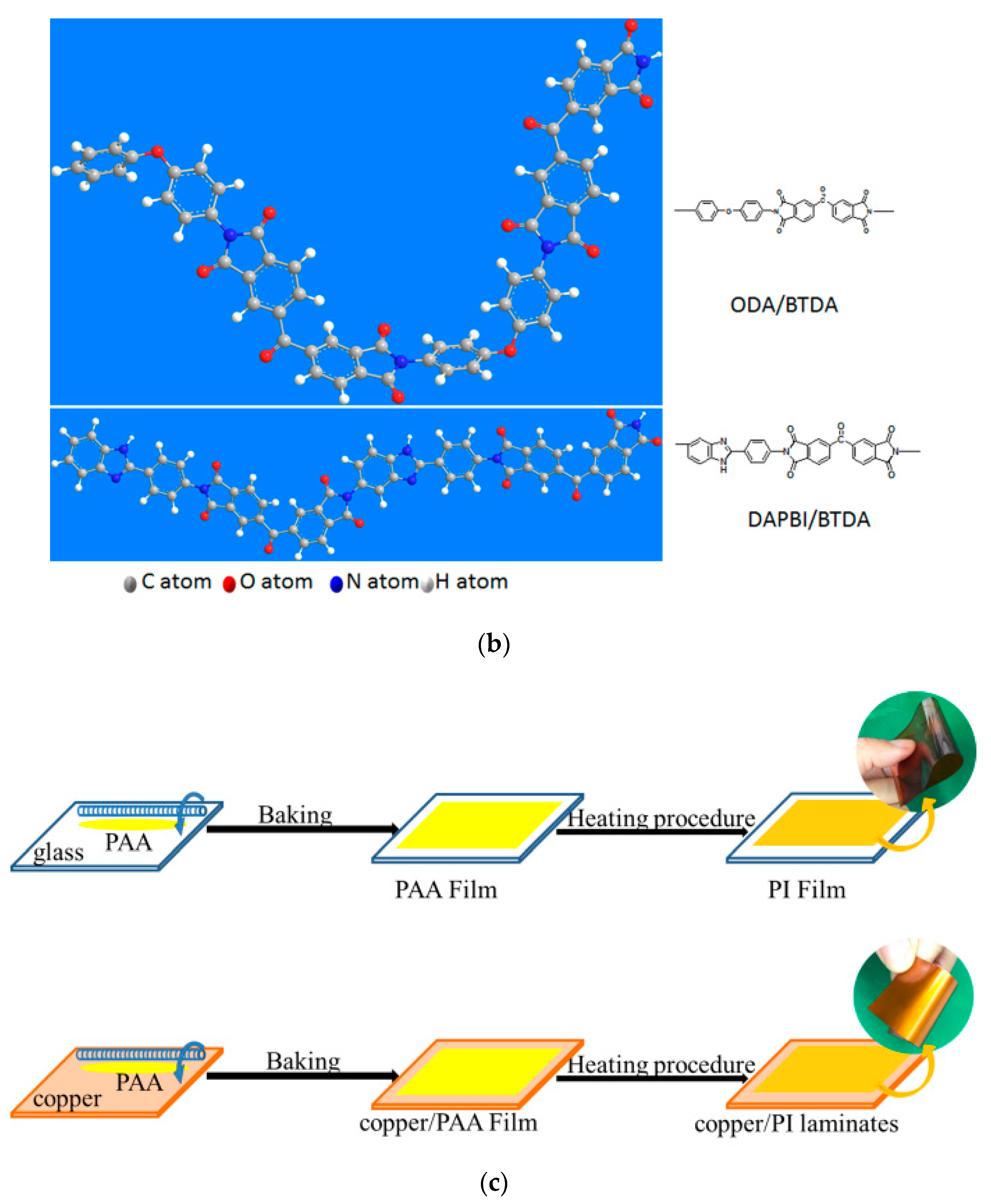
2.2.6. Synthesis of PAA Precursors
2.2.7. Thermal Imidization of PAA Films
3. Results
3.1. Synthesis of PAAs and Fabrication of PI Films
3.2. Viscosities of PAAs and FTIR Analysis of the PI Films
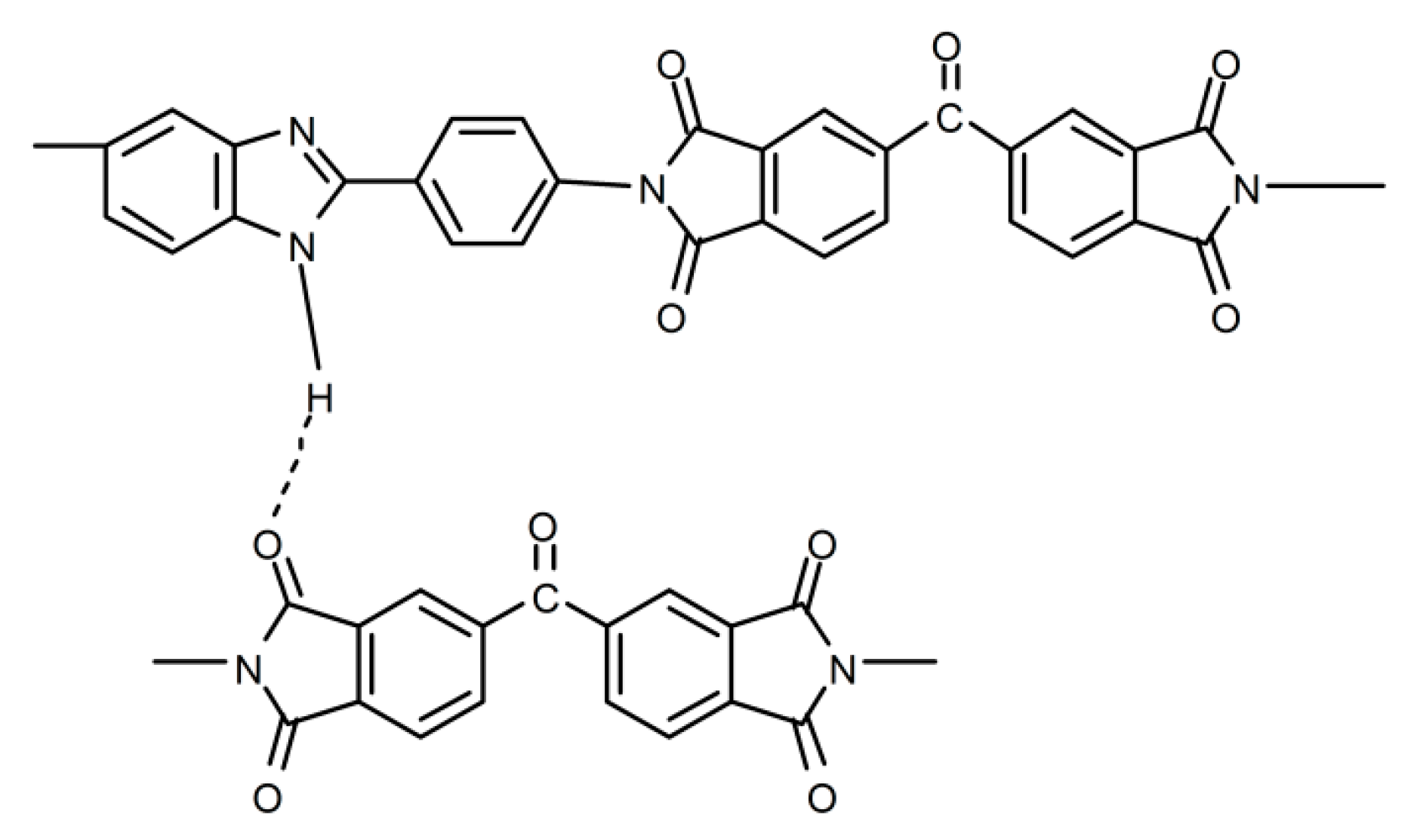
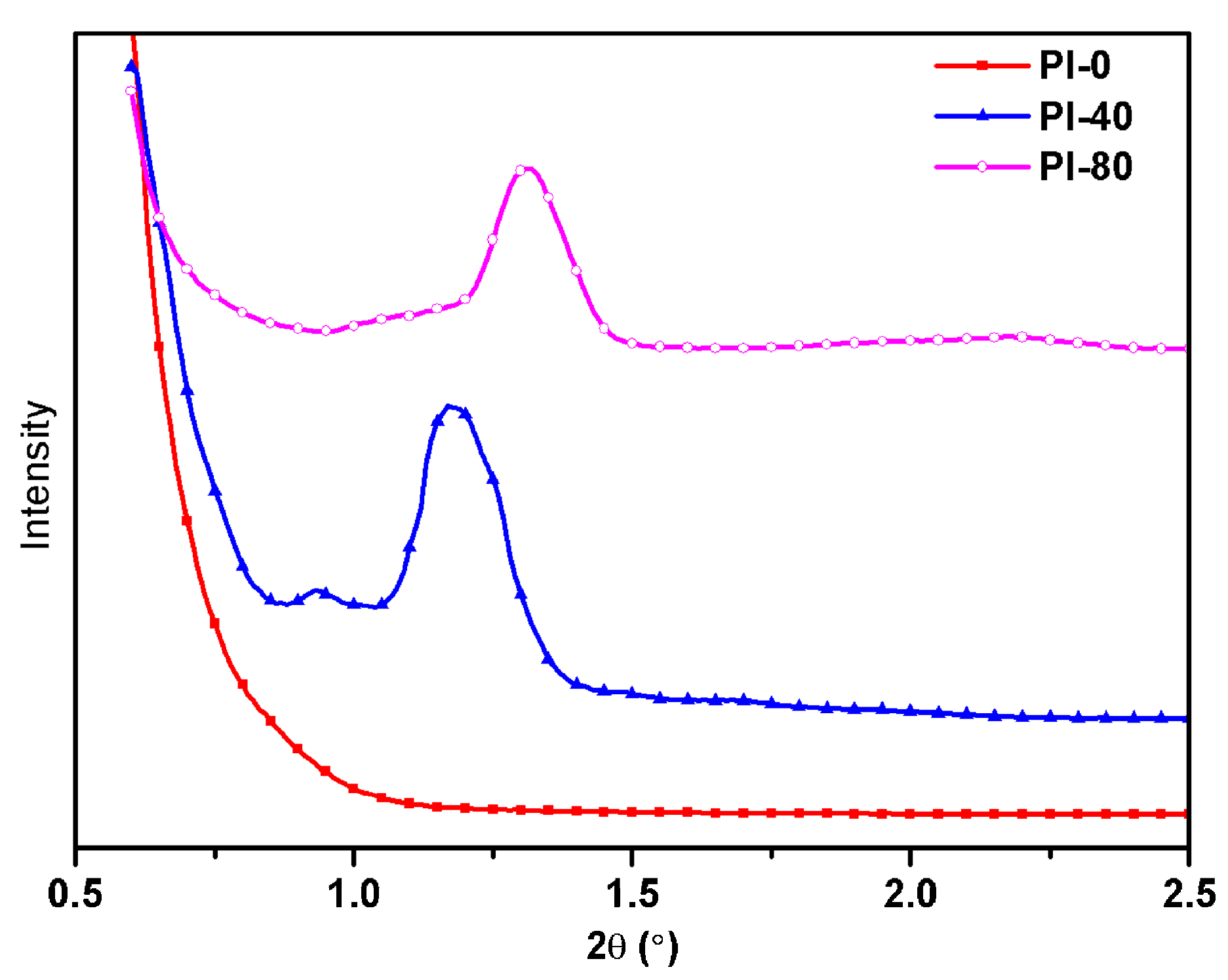
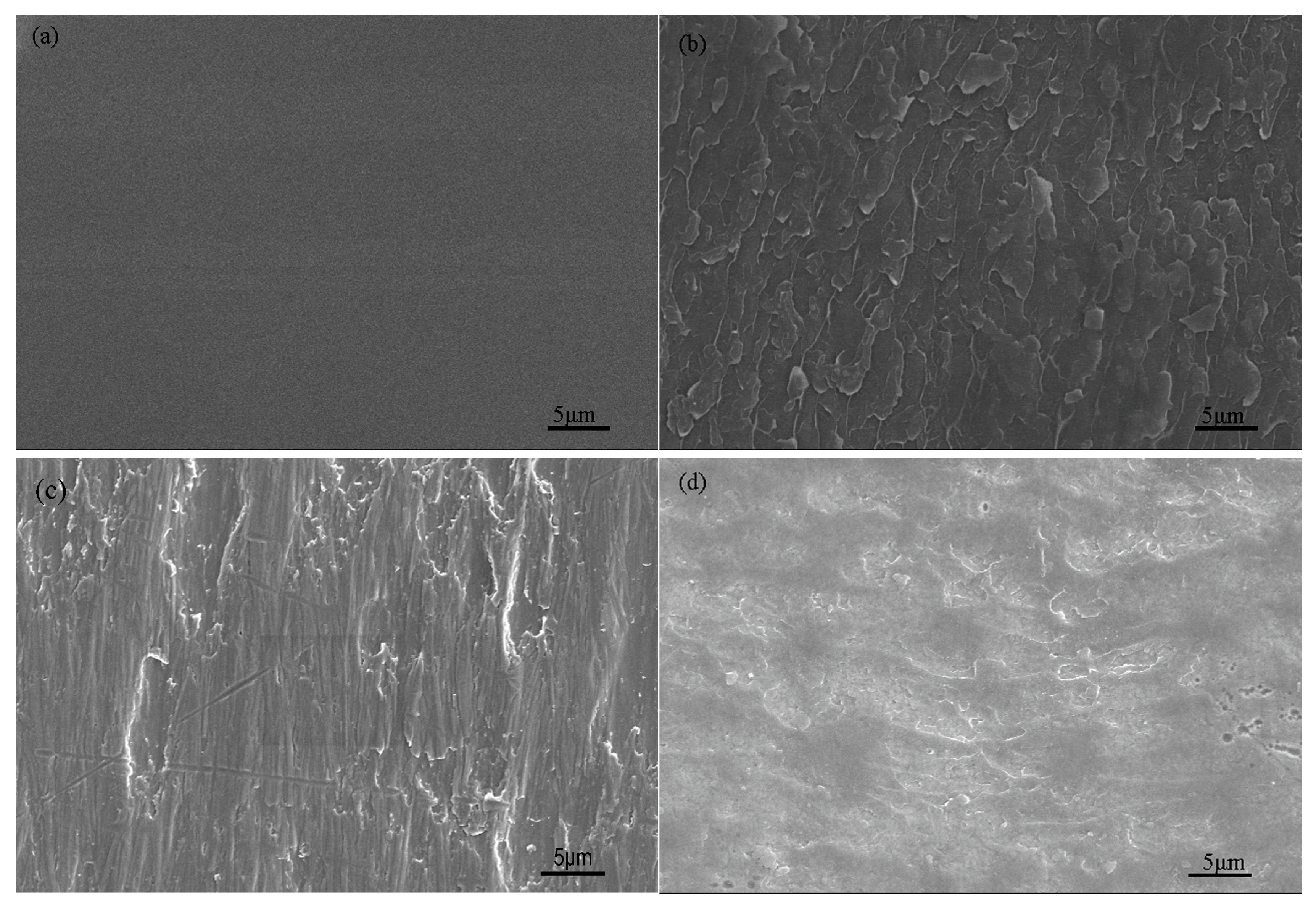
3.3. Morphology and Structures
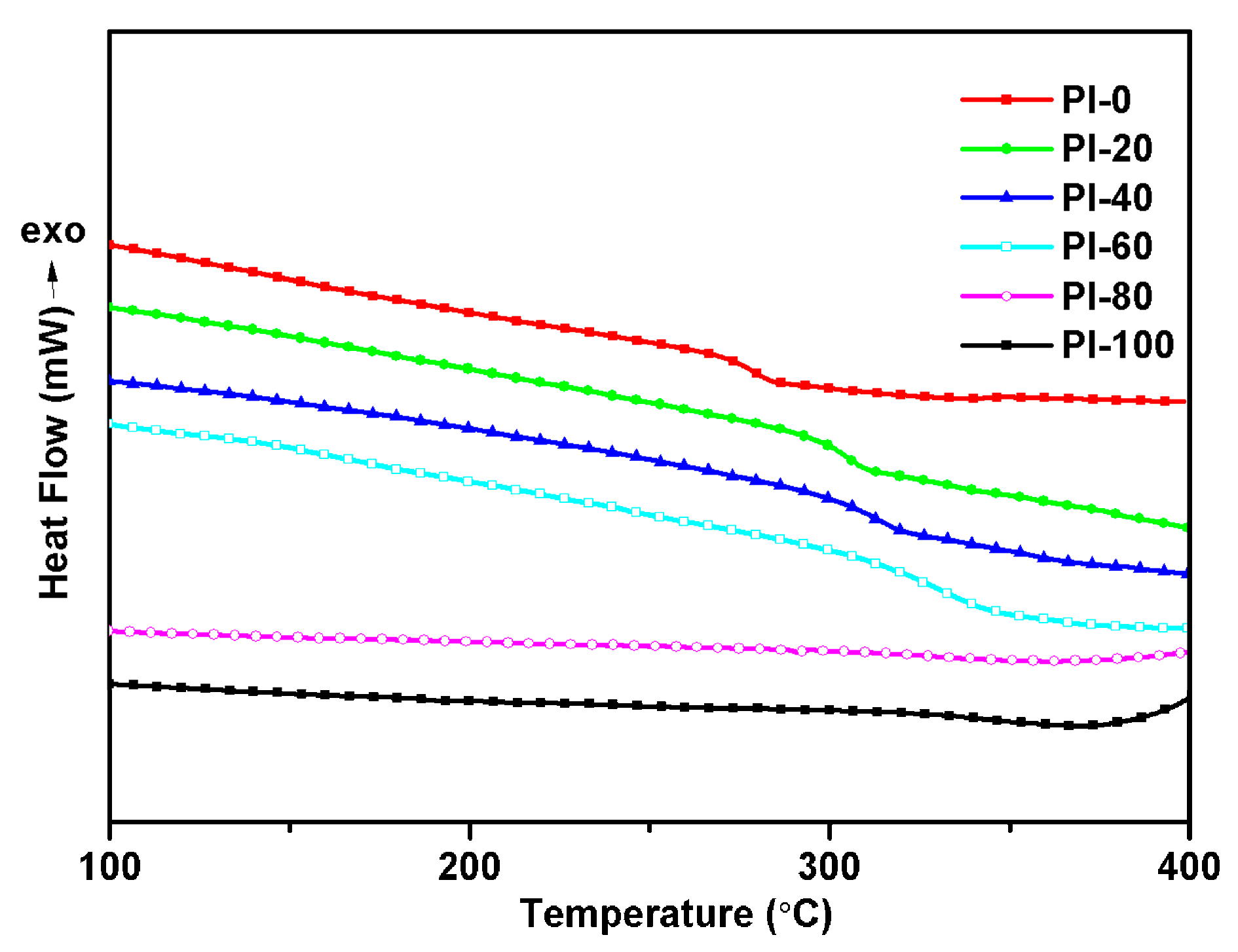
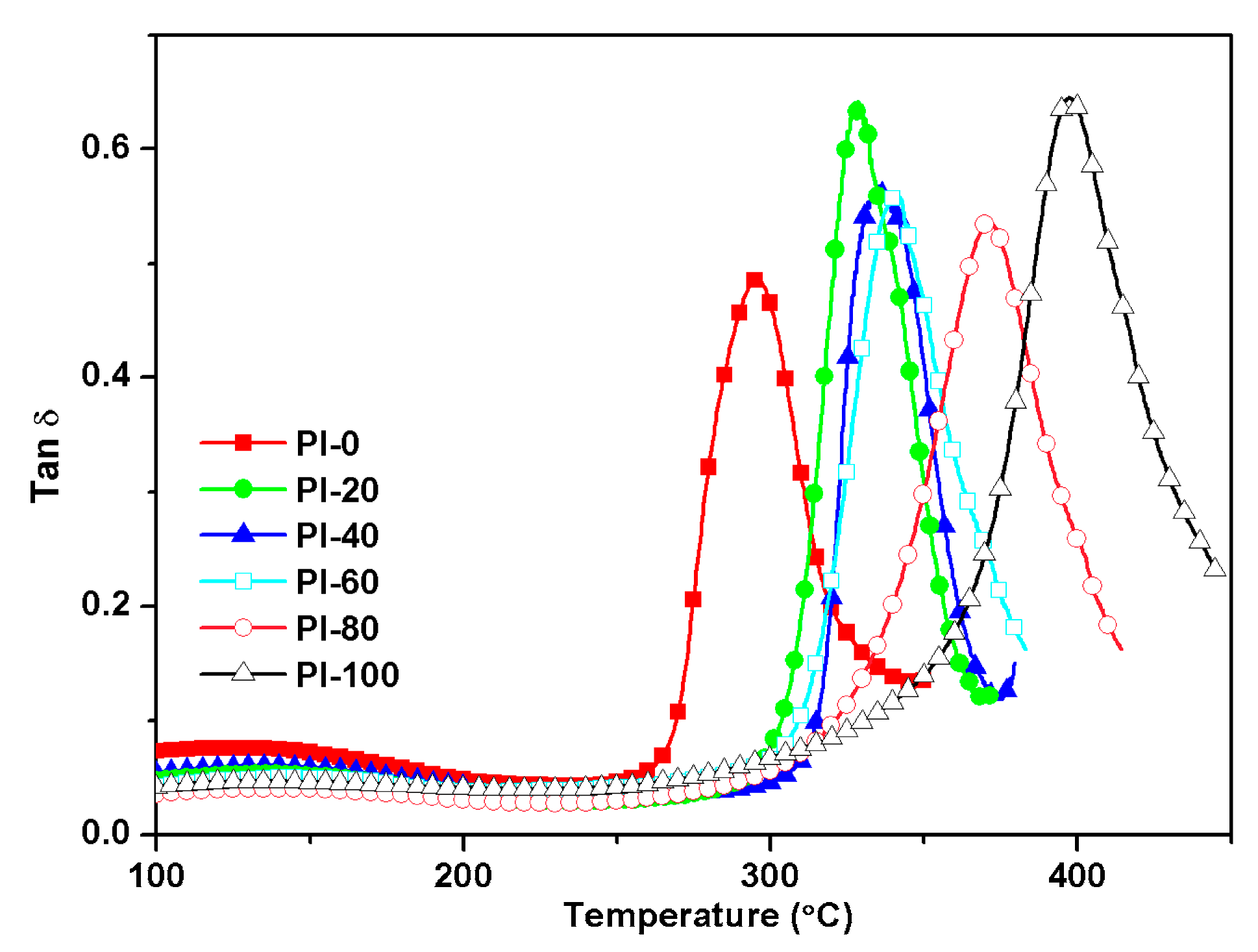
3.4. Thermal Properties
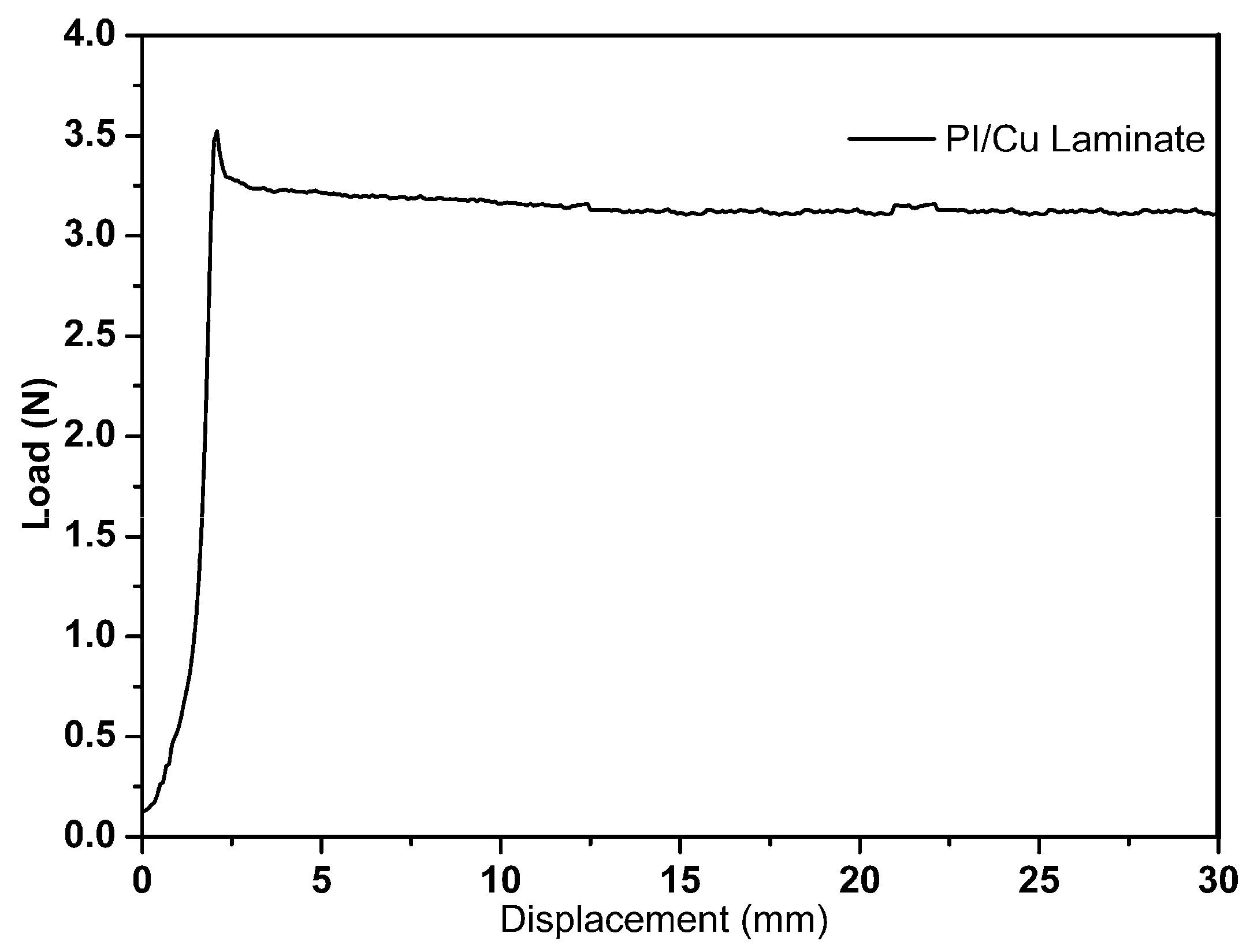
3.5. Adhesion Properties of PI-80/Copper Laminate
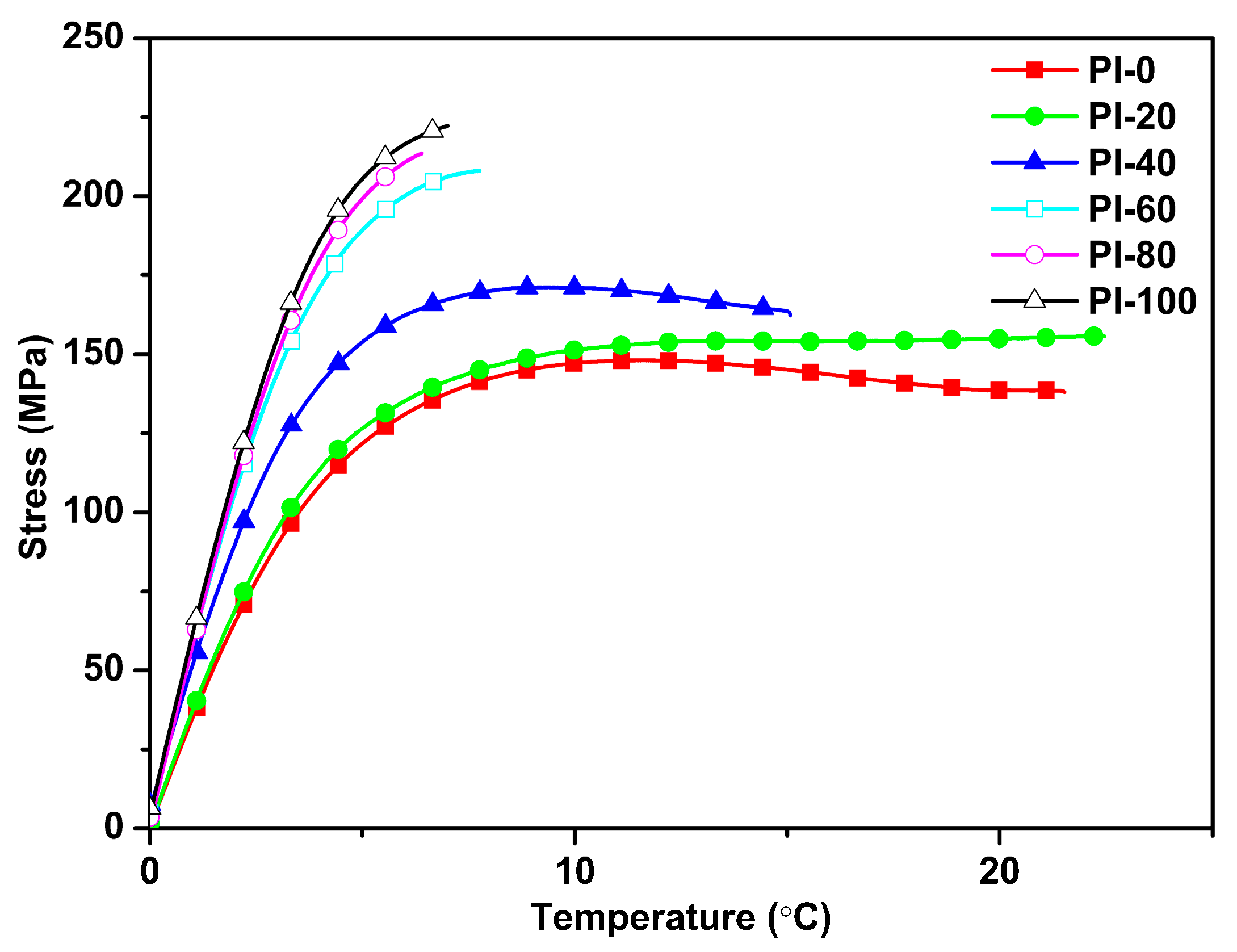
3.6. Mechanical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Jia, M.C.; Li, Y.J.; He, C.Q.; Huang, X.Y. Soluble perfluorocyclobutyl aryl ether-based polyimide for high-performance dielectric material. Appl. Mater. Interfaces 2016, 8, 26352–26358. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.W.; Xu, W.H.; Chen, L.L.; Ding, Y.C.; Chen, S.L.; Wang, X.Y.; Hou, H.Q. Polyimide complexes with high dielectric performance: Toward polymer film capacitor applications. J. Mater. Chem. C 2016, 4, 6452–6456. [Google Scholar] [CrossRef]
- Xu, L.L.; Jiang, S.D.; Li, B.; Hou, W.; Li, G.X.; Memon, M.A.; Huang, Y.; Geng, J.X. Graphene oxide: A versatile agent for polyimide foams with improved foaming capability and enhanced flexibility. Chem. Mater. 2015, 27, 4358–4367. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhou, W.C.; Li, R.; Qing, Y.C.; Luo, F.; Zhu, D.M. Electroless plating preparation and electromagnetic properties of co-coated carbonyl iron particles/polyimide composite. J. Magn. Magn. Mater. 2016, 401, 251–258. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriouleventis, C.; Mohite, D.P.; Larimore, Z.J.; Mang, J.T.; Churu, G.; Lu, H.B. Polyimide aerogels by ring-opening metathesis polymerization (ROMP). Chem. Mater. 2011, 23, 2250–2261. [Google Scholar] [CrossRef]
- Li, X.D.; Zhong, Z.X.; Han, S.H.; Lee, S.H.; Lee, M.H. Facile modifications of polyimide via chloromethylation: II. Synthesis and characterization of thermocurable transparent polyimide having methylene acrylate side groups. Polym. Int. 2005, 54, 406–411. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Bruening, M.L. Ultrathin, gas-selective polyimide membranes prepared from multilayer films. Chem. Mater. 2003, 15, 281–287. [Google Scholar] [CrossRef]
- Lim, J.; Yeo, H.; Goh, M.; Ku, B.C.; Kim, S.G.; Lee, H.S.; Park, B.; You, N.H. Grafting of polyimide onto chemically-functionalized graphene nanosheets for mechanically-strong barrier membranes. Chem. Mater. 2015, 27, 2040–2047. [Google Scholar] [CrossRef]
- Zhuang, Y.B.; Seong, J.G.; Lee, W.H.; Do, Y.S.; Lee, M.J.; Wang, G.; Guiver, M.D.; Lee, Y.M. Mechanically tough, thermally rearranged (TR) random/block poly(benzoxazole-co-imide) gas separation membranes. Macromolecules 2015, 48, 5286–5299. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Chang, H.Y.; Li, P.; Liu, R.H.; Xue, Q.Z. Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sensor. Actuators B Chem. 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Triambulo, R.E.; Cheong, H.G.; Lee, G.H.; Yi, I.S.; Park, J.W. A transparent conductive oxide electrode with highly enhanced flexibility achieved by controlled crystallinity by incorporating Ag nanoparticles on substrates. J. Alloys Compd. 2015, 620, 340–349. [Google Scholar] [CrossRef]
- Lim, H.; Cho, W.J.; Ha, C.S.; Ando, S.; Kim, Y.K.; Park, C.H.; Lee, K. Flexible organic electroluminescent devices based on fluorine-containing colorless polyimide substrates. Adv. Mater. 2002, 14, 1275–1279. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, T.W.; Stryakhilev, D.; Lee, J.S.; An, S.G.; Pyo, Y.S.; Lee, D.S.; Mo, Y.G.; Jin, D.U.; Chung, H.K. Flexible full color organic light-emitting diode display on polyimide plastic substrate driven by amorphous indium gallium zinc oxide thin-film transistors. Appl. Phys. Lett. 2009, 95, 013503. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Zhang, Y.; Liu, S.W.; Chi, Z.G.; Chen, X.D.; Xu, J.R. Flexible and highly fluorescent aromatic polyimide: Design, synthesis, properties, and mechanism. J. Mater. Chem. C. 2016, 4, 10509–10517. [Google Scholar] [CrossRef]
- Wang, H.M.; Hsiao, S.H. Ambipolar, multi-electrochromic polypyromellitimides and polynaphthalimides containing di(tert-butyl)-substituted bis(triarylamine) units. J. Mater. Chem. C. 2014, 2, 1553–1564. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, S.X.; Wang, Q.Y.; Liu, S.W.; Qiao, Z.P.; Chi, Z.G.; Xu, J.R.; Economy, J. Thermally conductive, insulated polyimide nanocomposites by AlO(OH)-coated MWCNTs. J. Mater. Chem. 2011, 21, 14563–14568. [Google Scholar] [CrossRef]
- Lewis, J. Material challenge for flexible organic devices. Mater. Today 2006, 9, 38–45. [Google Scholar] [CrossRef]
- Choi, M.C.; Kim, Y.; Ha, C.S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.F.; Zhang, J.J.; Ding, G.L.; Xu, G.J.; Liu, Z.H.; Cui, G.L. A superior thermostable and nonflammable composite membrane towards high power battery separator. Nano Energy 2014, 10, 277–287. [Google Scholar] [CrossRef]
- Chen, J.L.; Liu, C.T. Technology advances in flexible displays and substrates. Access IEEE. 2013, 1, 150–158. [Google Scholar] [CrossRef]
- Chen, H.L.; Ho, S.H.; Wang, T.H.; Chen, K.M.; Pan, J.P.; Liang, S.M.; Hung, A. Curl-free high-adhesion polyimide/copper laminate. J. Appl. Polym. Sci. 1994, 51, 1647–1652. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choa, Y.H. Adhesion enhancement of ink-jet printed conductive copper patterns on a flexible substrate. J. Mater. Chem. 2012, 22, 12517–12522. [Google Scholar] [CrossRef]
- Long, K.; Kattamis, A.; Cheng, I.C.; Gao, Y.X.; Gleskova, H.; Wagner, S.; Sturm, J.C. High-temperature (250 °C) amorphous silicon TFT’s on clear plastic substrates. SID Dig. 2005, 36, 313–315. [Google Scholar] [CrossRef]
- Ding, M.X. Polyimides: Chemistry, Relationship between Structure and Properties and Materials; Science Press: Beijing, China, 2006; pp. 515–517. ISBN 7030165322. [Google Scholar]
- Wang, L.N.; Yu, X.H.; Wang, D.M.; Zhao, X.G.; Yang, D.; Urrehman, S.; Chen, C.H.; Zhou, H.W.; Dang, G.D. High modulus and high strength ultra-thin polyimide films with hot-stretch induced molecular orientation. Mater. Chem. Phys. 2013, 139, 968–974. [Google Scholar] [CrossRef]
- Nishino, T.; Miki, N.; Mitsuoka, Y.; Nakamae, K.; Saito, T.; Kikuchi, T. Elastic modulus of the crystalline regions of polyimide derived from poly(amic acid)–biphtalic dianhydride and p-phenylene diamine. J. Polym. Sci. Polym. Phys. 1999, 37, 3294–3301. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, T.J.; Liu, S.F.; Jeng, J.L.; Guan, C.E. Thermal and mechanical properties of stretched recyclable polyimide film. J. Appl. Polym. Sci. 2011, 122, 210–219. [Google Scholar] [CrossRef]
- Ma, X.Y.; Ma, X.F.; Qiu, X.P.; Liu, F.F.; Jin, R.; Kang, C.Q.; Guo, H.Q.; GAO, L.X. Preparation and properties of imidazole-containing polyimide/silica hybrid films. Chem. Res. Chin. Univ. 2014, 30, 1047–1050. [Google Scholar] [CrossRef]
- Jin, H.S.; Chang, J.H. Colorless polyimide nanocomposite films: Thermomechanical properties, morphology, and optical transparency. J. Appl. Polym. Sci. 2008, 1, 109–117. [Google Scholar] [CrossRef]
- Huang, J.C.; Xiao, Y.; Mya, K.Y.; Liu, X.M.; He, C.B.; Dai, J.; Siow, Y.P. Thermomechanical properties of polyimide-epoxy nanocomposites from cubic silsesquioxane epoxides. J. Mater. Chem. 2004, 14, 2858–2863. [Google Scholar] [CrossRef]
- Geng, Z.; Ba, J.Y.; Zhang, S.L.; Luan, J.S.; Jiang, X.; Huo, P.F.; Wang, G.B. Ultra low dielectric constant hybrid films via side chain grafting reaction of poly(ether ether ketone) and phosphotungstic acid. J. Mater. Chem. 2012, 22, 23534–23540. [Google Scholar] [CrossRef]
- Jin, H.S.; Chang, J.H. Synthesis and characterization of colorless polyimide nanocomposite films containing pendant trifluoromethyl groups. Macromol. Res. 2008, 16, 503–509. [Google Scholar] [CrossRef]
- An, L.; Pan, Y.Z.; Shen, X.W.; Lu, H.B.; Yang, Y.L. Rod-like attapulgite/polyimide nanocomposites with simultaneously improved strength, toughness, thermal stability and related mechanisms. J. Mater. Chem. 2008, 18, 4928–4941. [Google Scholar] [CrossRef]
- Chang, H.C.; Sohn, B.H.; Chang, J.H. Colorless and transparent polyimide nanocomposites: Comparison of the properties of homo- and co-polymers. J. Ind. Eng. Chem. 2013, 19, 1593–1599. [Google Scholar]
- Xenopoulos, C.; Mascia, L.; Shaw, S.J. Polyimide-silica hybrids derived from an isoimide oligomer precursor. J. Mater. Chem. 2002, 12, 213–218. [Google Scholar] [CrossRef]
- Song, G.L.; Wang, S.; Wang, D.M.; Zhou, H.W.; Chen, C.H.; Zhao, X.G.; Dang, G.D. Rigidity enhancement of polyimides containing benzimidazole moieties. J. Appl. Polym. Sci. 2013, 130, 1653–1658. [Google Scholar] [CrossRef]
- Chen, J.C.; Wu, J.A.; Lee, C.Y.; Tsai, M.C.; Chen, K.H. Novel polyimides containing benzimidazole for temperature proton exchange membrane fuel. J. Mater. Chem. 2015, 483, 144–154. [Google Scholar] [CrossRef]
- Song, G.L.; Wang, D.M.; Zhao, X.G.; Dang, G.D.; Zhou, H.W.; Chen, C.H. Synthesis and properties of polyimides-containing benzoxazole units in the main chain. High Perform. Polym. 2013, 25, 354–360. [Google Scholar] [CrossRef]
- Hasegawa, M. Semi-aromatic polyimides with low dielectric constant and low CTE. High Perform. Polym. 2001, 13, S93–S106. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.W.; Dang, G.D.; Chen, C.H. Synthesis and characterization of thermally stable, high-modulus polyimides containing benzimidazole moieties. J. Polym. Sci. Polym. Chem. 2009, 47, 2024–2031. [Google Scholar] [CrossRef]
- Yin, C.Q.; Dong, J.; Zhang, Z.X.; Zhang, Q.H.; Lin, J.Y. Structure and properties of polyimide fibers containing benzimidazole and amide units. J. Polym. Sci. Polym. Phys. 2015, 53, 183–191. [Google Scholar] [CrossRef]
- Song, G.L.; Wang, D.M.; Dang, G.D.; Zhou, H.W.; Chen, C.H.; Zhao, X.G. Thermal expansion behavior of polyimide films containing benzoxazole units in the main chain. High Perform. Polym. 2014, 26, 413–419. [Google Scholar] [CrossRef]
- Xue, G.; Shi, G.Q.; Ding, J.F.; Chang, W.M.; Chen, R.S. Complex-induced coupling effect: Adhesion of some polymers to copper metal promoted by benzimidazole. J. Adhes. Sci. Technol. 1990, 4, 723–732. [Google Scholar] [CrossRef]
- Chen, K.M.; Ho, S.M.; Wang, T.H.; King, J.S.; Chang, W.C.; Cheng, R.P.; Hung, A. Studies on the adhesion of polyimide coatings on copper foil. J. Appl. Polym. Sci. 1992, 45, 947–956. [Google Scholar] [CrossRef]
- Ahn, T.K.; Kim, A.M.; Choe, S. Hydrogen-bonding strength in the blends of polybenzimidazole with btda- and dsda-based polyimides. Macromol. 1997, 30, 3369–3374. [Google Scholar] [CrossRef]
- Song, G.L.; Zhang, Y.; Wang, D.M.; Chen, C.H.; Zhou, H.W.; Zhao, X.G.; Dang, G.D. Intermolecular interactions of polyimides containing benzimidazole and benzoxazole moieties. Polymer 2013, 54, 2335–2340. [Google Scholar] [CrossRef]

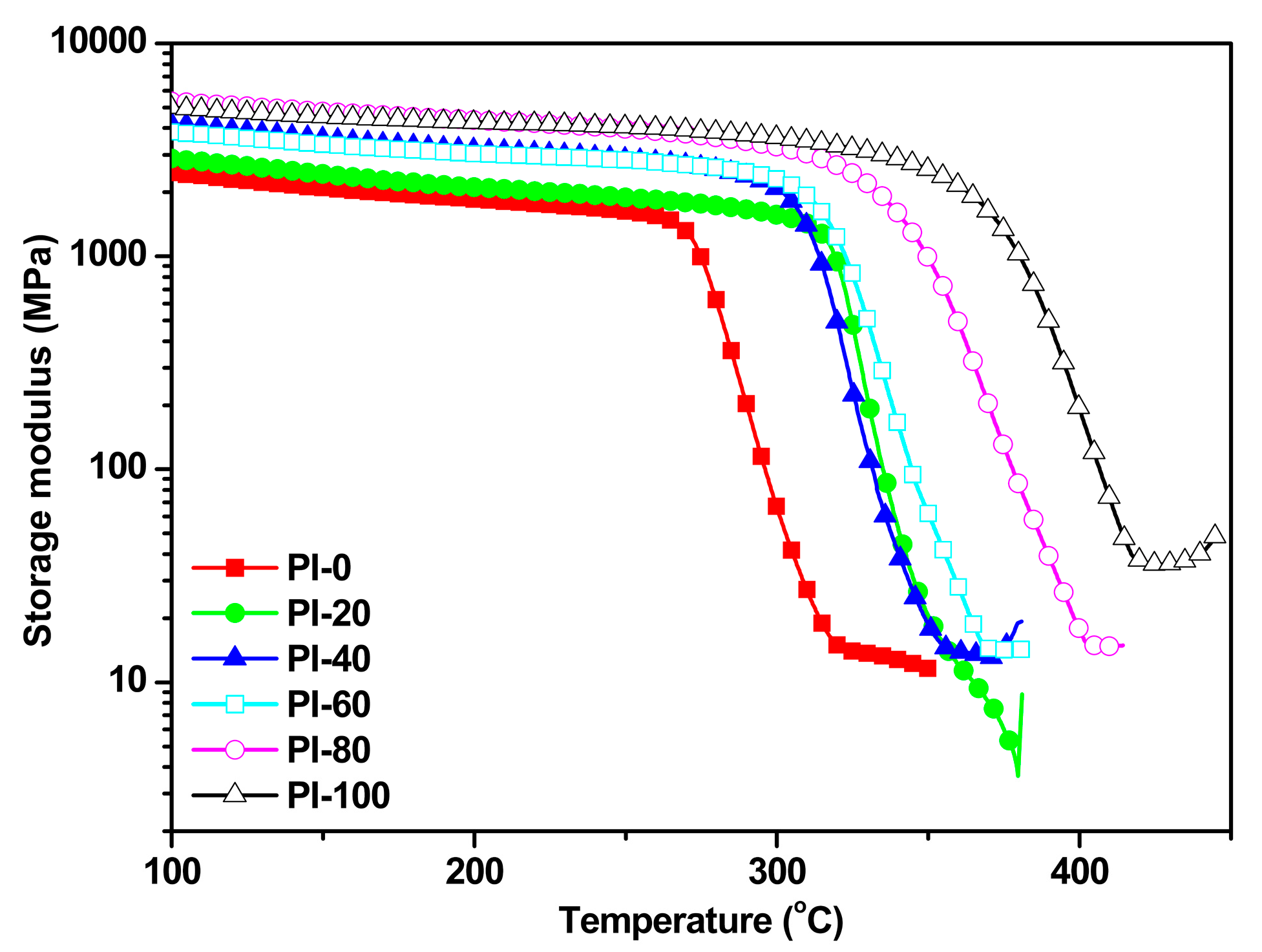
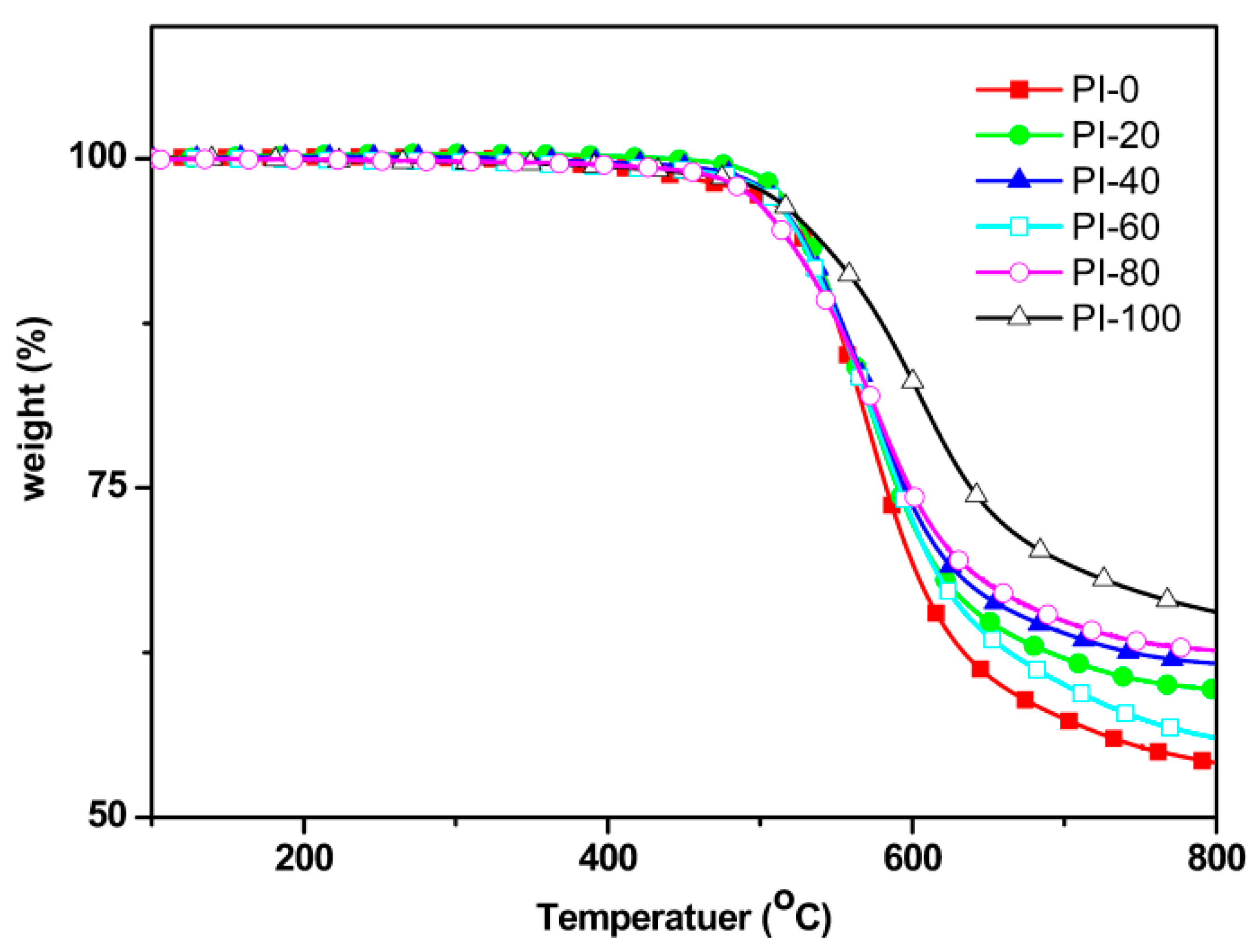
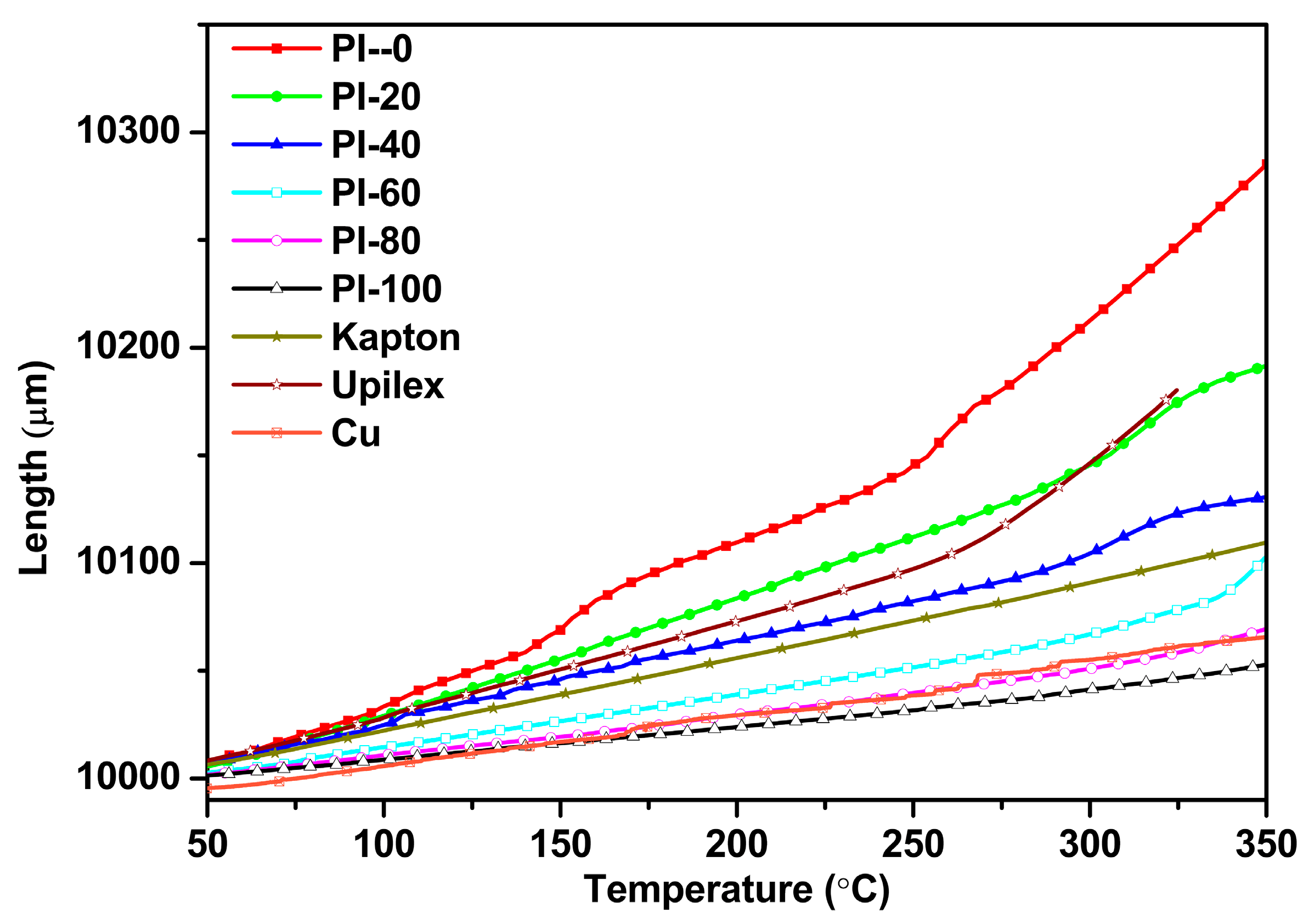
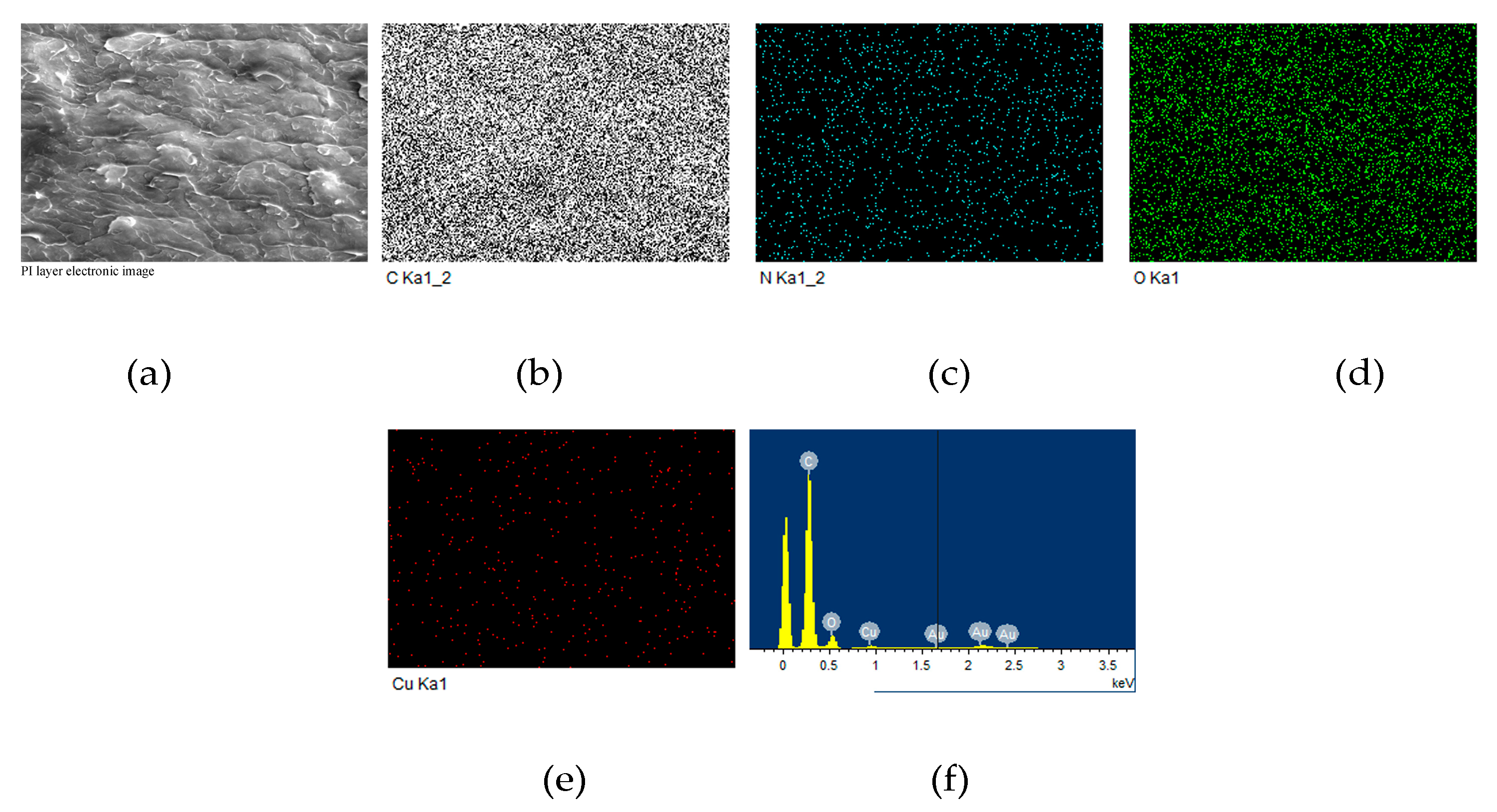
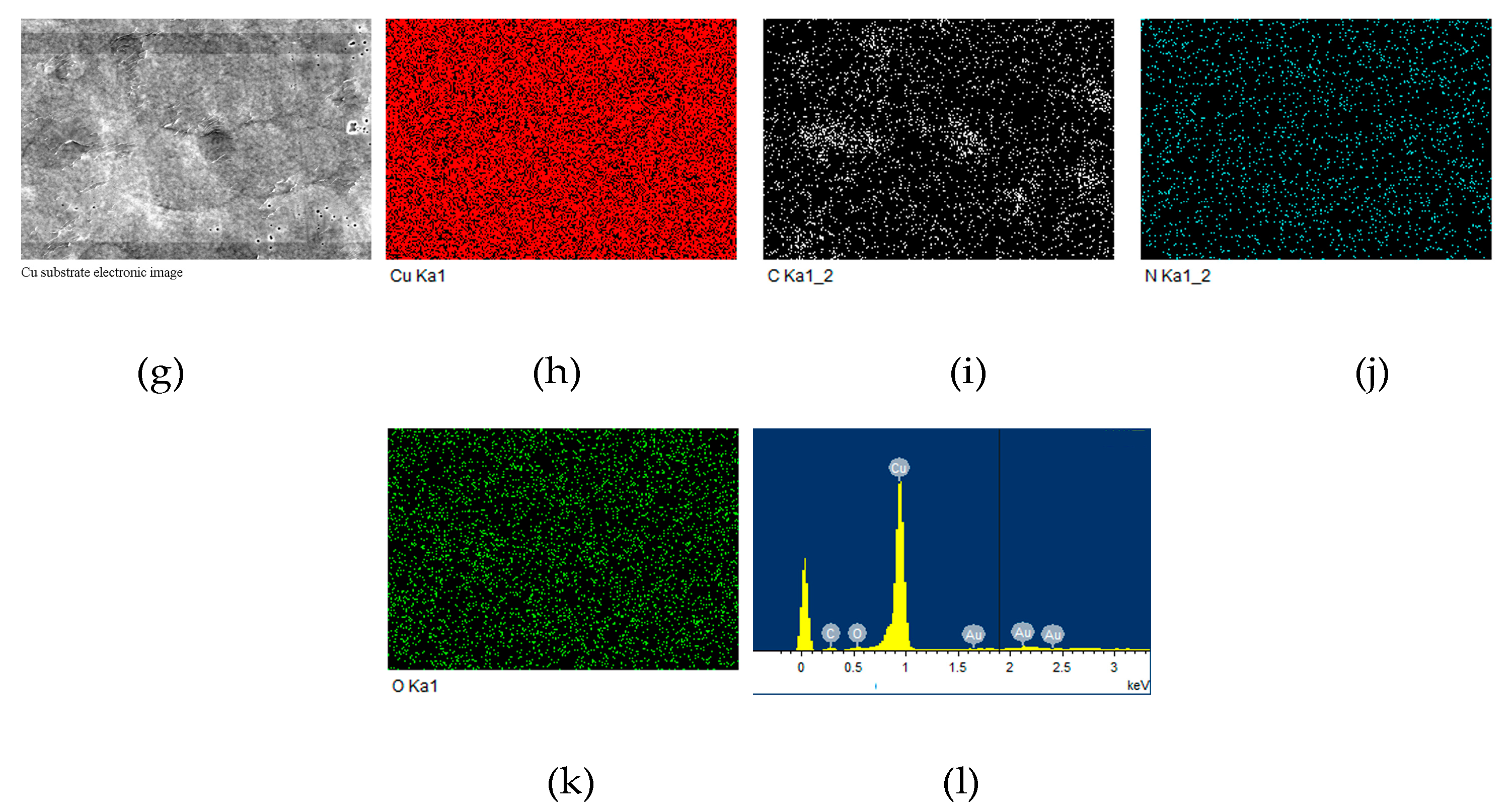
| Samples | ηinh 1 (dL/g) | Tg 2 (°C) | Td5% 3 (°C) | R800 4 (%) | Water absorption (%) | |
|---|---|---|---|---|---|---|
| DSC | DMA | |||||
| PI-0 | 1.85 | 278 | 296 | 523 | 54.2 | 2.0 |
| PI-20 | 2.06 | 307 | 328 | 527 | 59.7 | 2.3 |
| PI-40 | 2.20 | 314 | 334 | 522 | 61.7 | 2.5 |
| PI-60 | 2.25 | 343 | 340 | 521 | 56.1 | 2.9 |
| PI-80 | 2.30 | NF 5 | 371 | 523 | 62.7 | 2.5 |
| PI-100 | 2.15 | NF | 397 | 529 | 65.6 | 3.0 |
| Samples | CTE (50–250 °C) ppm/K | CTE (50–300 °C) ppm/K | CTE (100–200 °C) ppm/K |
|---|---|---|---|
| PI-0 | 67.9 | 82.0 | 76.2 |
| PI-20 | 53.3 | 55.8 | 54.5 |
| PI-40 | 37.8 | 38.6 | 38.9 |
| PI-60 | 24.4 | 25.6 | 24.4 |
| PI-80 | 19.0 | 19.6 | 18.8 |
| PI-100 | 15.2 | 15.9 | 15.4 |
| Cu | 19.0 | 19.4 | 18.6 |
| Kapton | 33.5 | 34.2 | 33.0 |
| Upilex | 44.9 | 55.3 | 43.4 |
| Samples | Elastic (GPa) | Max stress (MPa) | Break strain (%) |
|---|---|---|---|
| PI-0 | 3.4 ± 0.05 | 146.0 ± 1.9 | 16.6 ± 4.9 |
| PI-20 | 3.8 ± 0.14 | 157.8 ± 3.4 | 21.5 ± 8.7 |
| PI-40 | 4.9 ± 0.16 | 173.3 ± 4.8 | 9.4 ± 3.8 |
| PI-60 | 5.8 ± 0.07 | 205.6 ± 8.8 | 7.3 ± 1.0 |
| PI-80 | 5.7 ± 0.48 | 209.0 ± 5.9 | 6.2 ± 0.4 |
| PI-100 | 6.2 ± 0.37 | 220.8 ± 10.0 | 6.8 ± 1.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Liang, W.; Cao, J.; Wu, D. Mixed Rigid and Flexible Component Design for High-Performance Polyimide Films. Polymers 2017, 9, 451. https://doi.org/10.3390/polym9090451
Yu X, Liang W, Cao J, Wu D. Mixed Rigid and Flexible Component Design for High-Performance Polyimide Films. Polymers. 2017; 9(9):451. https://doi.org/10.3390/polym9090451
Chicago/Turabian StyleYu, Xiaohui, Weihua Liang, Jianhua Cao, and Dayong Wu. 2017. "Mixed Rigid and Flexible Component Design for High-Performance Polyimide Films" Polymers 9, no. 9: 451. https://doi.org/10.3390/polym9090451





