Determination of Adequate Substrate Water Content for Mass Production of a High Value-Added Medicinal Plant, Crepidiastrum denticulatum (Houtt.) Pak & Kawano
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Conditions
2.2. Treatments of Substrate Water Content
2.3. Plant Growth Parameters
2.4. Photosynthetic Parameters
2.5. Sap Flow
2.6. Total Phenolic Content and Antioxidant Capacity
2.7. Hydroxycinnamic Acids
2.8. Statistical Analysis
3. Results
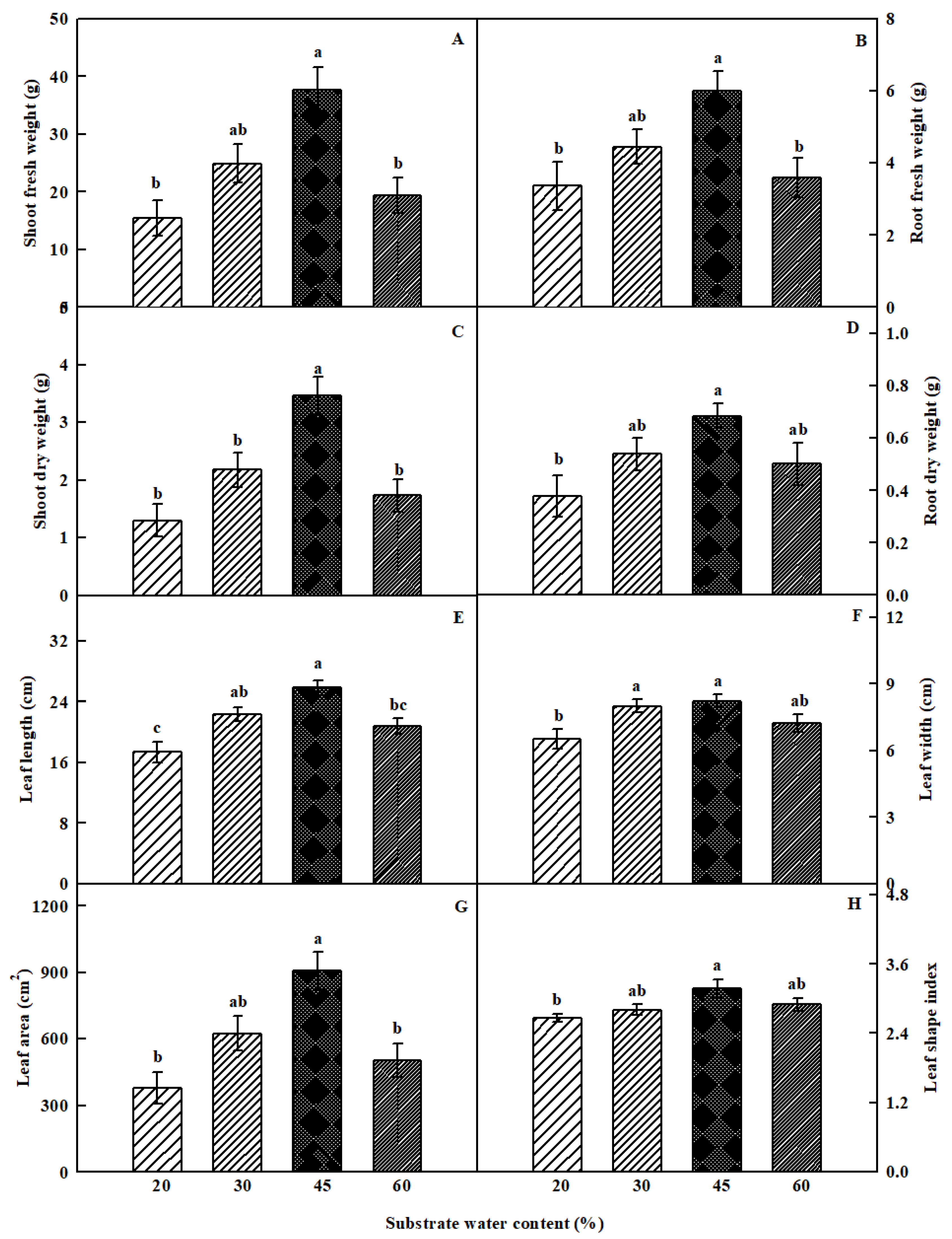
3.1. Plant Growth Parameters
3.2. Photosynthetic Parameters
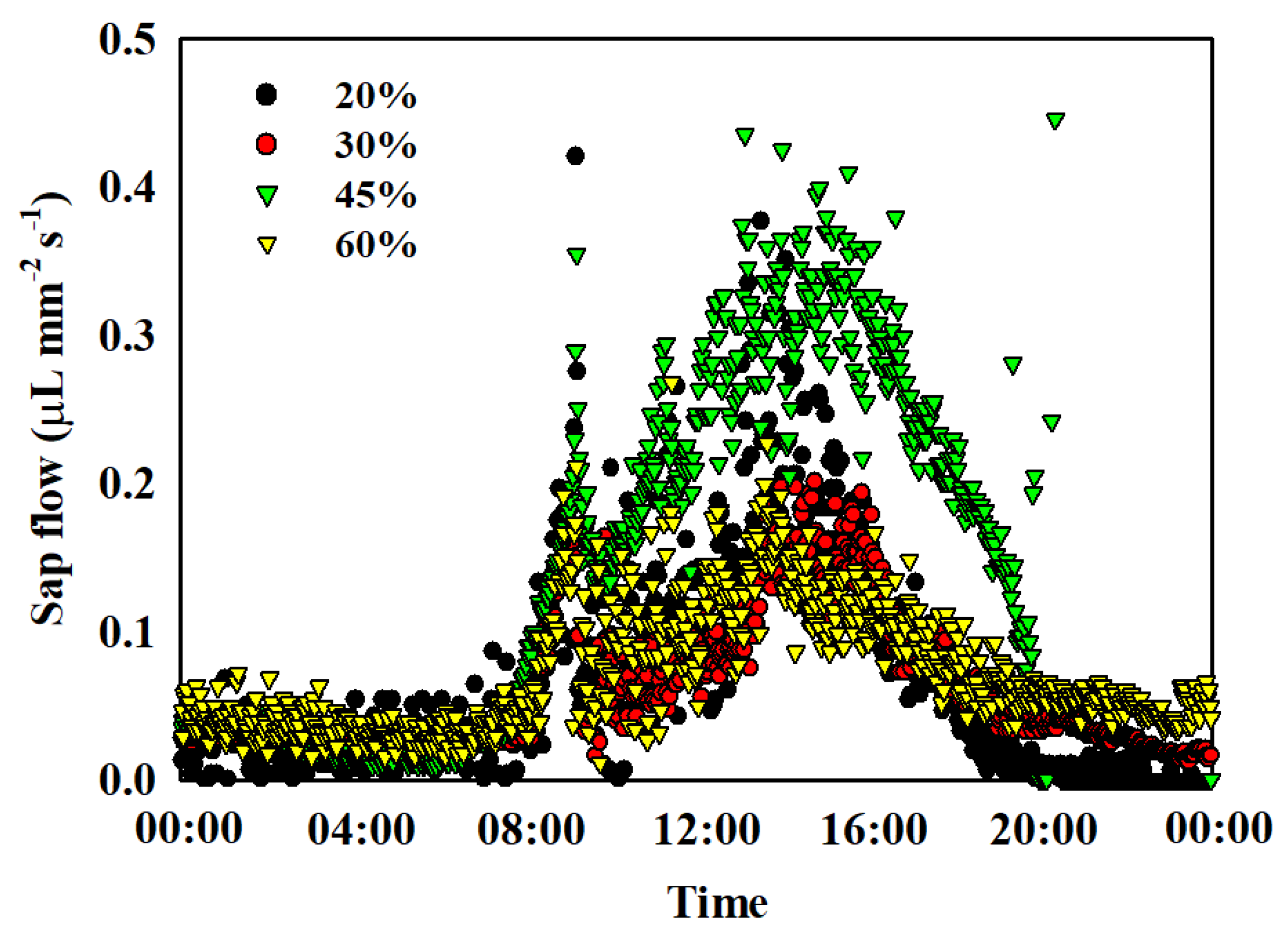
3.3. Sap Flow
3.4. Total Phenolic Content and Antioxidant Capacity
3.5. Hydroxycinnamic Acids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Akıncı, Ş.; Lösel, D.M. Plant water-stress response mechanisms. In Water Stress; Rahman, I.M.M., Hasegawa, H., Eds.; InTechOpen: Rijeka, Croatia, 2012; pp. 15–42. [Google Scholar]
- Liao, C.; Lin, C. Physiological adaptation of crop plants to flooding stress. In Proceedings of the National Science Council, Republic of China, Part B, Life Sciences, Taipei, Taiwan, 1 July 2001; Volume 25, pp. 148–157. [Google Scholar]
- Ahsan, N.; Lee, D.; Lee, S.; Kang, K.Y.; Bahk, J.D.; Choi, M.S.; Lee, I.; Renaut, J.; Lee, B. A comparative proteomic analysis of tomato leaves in response to waterlogging stress. Physiol. Plant. 2007, 131, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Phytochemical and physiological changes in the leaves of St. John’s wort plants under a water stress condition. Environ. Exp. Bot. 2007, 59, 109–116. [Google Scholar] [CrossRef]
- Lee, H.J.; Cha, K.H.; Kim, C.Y.; Nho, C.W.; Pan, C. Bioavailability of hydroxycinnamic acids from Crepidiastrum denticulatum using simulated digestion and Caco-2 intestinal cells. J. Agric. Food Chem. 2014, 62, 5290–5295. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kang, K.; Yun, J.H.; Kim, M.A.; Nho, C.W. Crepidiastrum denticulatum extract protects the liver against chronic alcohol-induced damage and fat accumulation in rats. J. Med. Food 2014, 17, 432–438. [Google Scholar] [CrossRef]
- Ahn, H.R.; Lee, H.J.; Kim, K.; Kim, C.Y.; Nho, C.W.; Jang, H.; Pan, C.; Lee, C.Y.; Jung, S.H. Hydroxycinnamic acids in Crepidiastrum denticulatum protect oxidative stress-induced retinal damage. J. Agric. Food Chem. 2014, 62, 1310–1323. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, G.; Randy, A.; Kim, H.S.; Nho, C.W. Chicoric acid attenuate a nonalcoholic steatohepatitis by inhibiting key regulators of lipid metabolism, fibrosis, oxidation, and inflammation in mice with methionine and choline deficiency. Mol. Nutr. Food Res. 2017, 61, 1600632. [Google Scholar] [CrossRef]
- Rural Development Administration (RDA) Agriculture Science Library. Available online: http://lib.rda.go.kr/search/searchDetailART.do?ctrl=000001165804 (accessed on 15 July 2010).
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Regulated water deficits improve phytochemical concentration in lettuce. J. Am. Soc. Hort. Sci. 2010, 135, 223–229. [Google Scholar] [CrossRef]
- Lee, J.Y.; Oh, M.M. Mild water deficit increases the contents of bioactive compounds in dropwort. Hortic. Environ. Biotechnol. 2017, 58, 458–466. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Ruiz, J.M.; Ferreres, F.; Moreno, D.A. Phenolic metabolism in grafted versus nongrafted cherry tomatoes under the influence of water stress. J. Agric. Food Chem. 2011, 59, 8839–8846. [Google Scholar] [CrossRef]
- Afshar, R.K.; Chaichi, M.R.; Jovini, M.A.; Jahanzad, E.; Hashemi, M. Accumulation of silymarin in milk thistle seeds under drought stress. Planta 2015, 242, 539–543. [Google Scholar]
- Park, S.Y.; Oh, S.B.; Kim, S.M.; Cho, Y.Y.; Oh, M.M. Evaluating the effects of a newly developed nutrient solution on growth, antioxidants, and chicoric acid contents in Crepidiastrum denticulatum. Hortic. Environ. Biotechnol. 2016, 57, 478–486. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Mullet, J.E.; Whitsitt, M.S. Plant cellular responses to water deficit. In Drought Tolerance in Higher Plants: Genetical, Physiological and Molecular Biological Analysis; Belhassen, E., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 41–46. [Google Scholar]
- Nonami, H.; Boyer, J.S. Wall extensibility and cell hydraulic conductivity decrease in enlarging stem tissues at low water potentials. Plant Physiol. 1990, 93, 1610–1619. [Google Scholar] [CrossRef]
- Watanabe, S.; Sato, M.; Sawada, Y.; Tanaka, M.; Matsui, A.; Kanno, Y.; Hirai, M.Y.; Seki, M.; Sakamoto, A.; Seo, M. Arabidopsis molybdenum cofactor sulfurase ABA3 contributes to anthocyanin accumulation and oxidative stress tolerance in ABA-dependent and independent ways. Sci. Rep. 2018, 8, 16592. [Google Scholar] [CrossRef]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Kim, J.; Malladi, A.; Van Iersel, M.W. Physiological and molecular responses to drought in Petunia: The importance of stress severity. J. Exp. Bot. 2012, 63, 6335–6345. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Akhtar, I.; Nazir, N. Effect of waterlogging and drought stress in plants. Int. J. Water Resour. Environ. Sci. 2013, 2, 34–40. [Google Scholar]
- Rodríguez-Gamir, J.; Ancillo, G.; González-Mas, M.C.; Primo-Millo, E.; Iglesias, D.J.; Forner-Giner, M.A. Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol. Biochem. 2011, 49, 636–645. [Google Scholar] [CrossRef]
- Currey, C.J.; Flax, N.J.; Litvin, A.G.; Metz, V.C. Substrate volumetric water content controls growth and development of containerized culinary herbs. Agronomy 2019, 9, 667. [Google Scholar] [CrossRef]
- Rood, S.B.; Nielsen, J.L.; Shenton, L.; Gill, K.M.; Letts, M.G. Effects of flooding on leaf development, transpiration, and photosynthesis in narrowleaf cottonwood, a willow-like poplar. Photosynth. Res. 2010, 104, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mutava, R.N.; Prince, S.J.K.; Syed, N.H.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2015, 86, 109–120. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Bettaieb, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Khan, M.; Ulrichs, C.; Mewis, I. Effect of water stress and aphid herbivory on flavonoids in broccoli (Brassica oleracea var. italica Plenck). J. Appl. Bot. Food Qual. 2011, 84, 178–182. [Google Scholar]
- Son, J.E.; Oh, M.M.; Lu, Y.J.; Kim, K.S.; Giacomelli, G.A. Nutrient-flow wick culture system for potted plant production: System characteristics and plant growth. Sci. Hortic. 2006, 107, 392–398. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Kim, J.; Oh, M.-M. Determination of Adequate Substrate Water Content for Mass Production of a High Value-Added Medicinal Plant, Crepidiastrum denticulatum (Houtt.) Pak & Kawano. Agronomy 2020, 10, 388. https://doi.org/10.3390/agronomy10030388
Park S-Y, Kim J, Oh M-M. Determination of Adequate Substrate Water Content for Mass Production of a High Value-Added Medicinal Plant, Crepidiastrum denticulatum (Houtt.) Pak & Kawano. Agronomy. 2020; 10(3):388. https://doi.org/10.3390/agronomy10030388
Chicago/Turabian StylePark, Song-Yi, Jongyun Kim, and Myung-Min Oh. 2020. "Determination of Adequate Substrate Water Content for Mass Production of a High Value-Added Medicinal Plant, Crepidiastrum denticulatum (Houtt.) Pak & Kawano" Agronomy 10, no. 3: 388. https://doi.org/10.3390/agronomy10030388
APA StylePark, S.-Y., Kim, J., & Oh, M.-M. (2020). Determination of Adequate Substrate Water Content for Mass Production of a High Value-Added Medicinal Plant, Crepidiastrum denticulatum (Houtt.) Pak & Kawano. Agronomy, 10(3), 388. https://doi.org/10.3390/agronomy10030388




