Genotypic Variation in Nitrogen Use-Efficiency Traits of 28 Tobacco Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tobacco Genotypes and Growth Conditions
2.2. Plant Analysis and N Use-Efficiency Indexes
3. Results
3.1. Visual Symptoms of N Deficiency, Dry Mass (DM) Production, N Contents, and N Accumulation in Tobacco Genotypes
3.2. N Use-Efficiency Traits
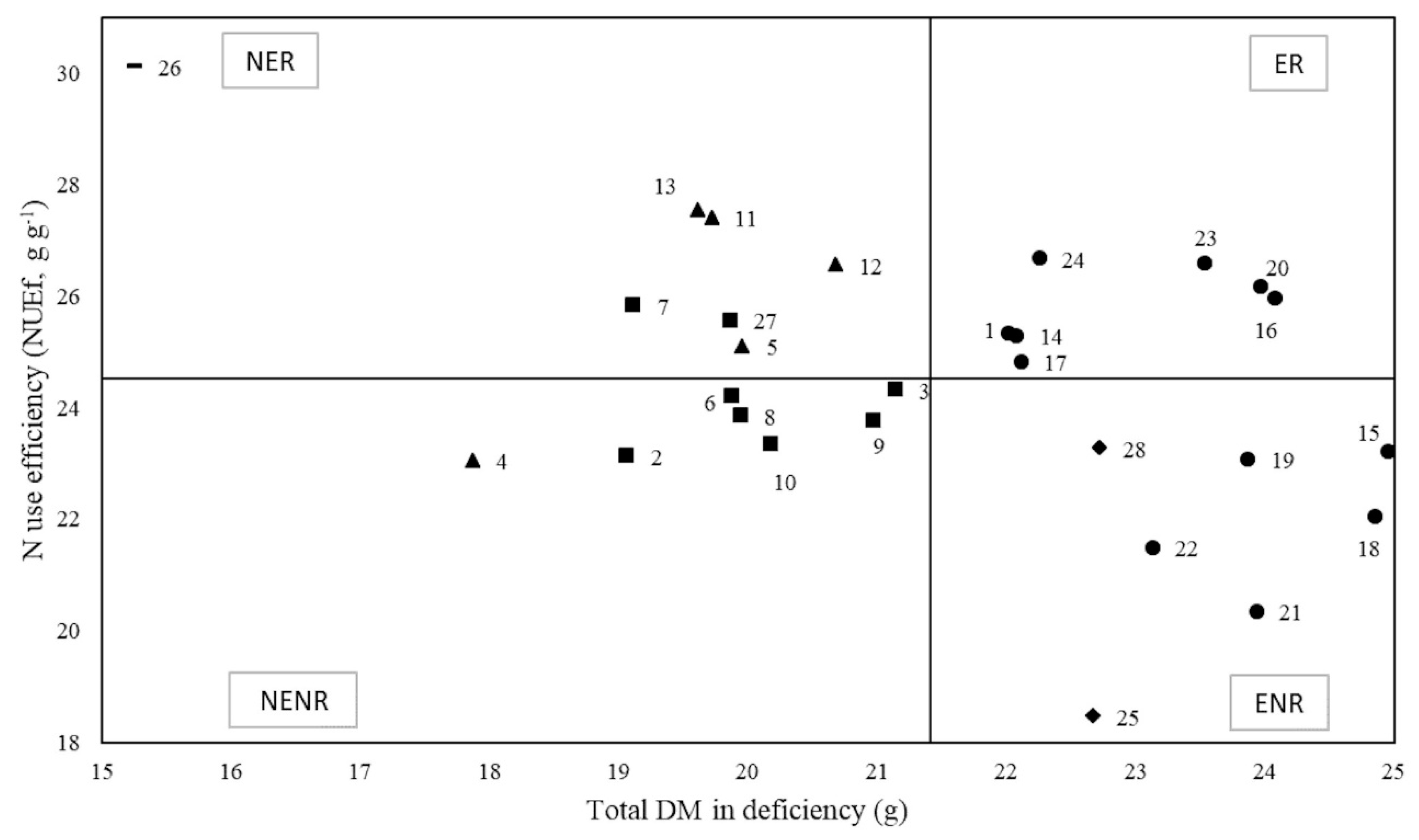
3.3. Grouping Tobacco Genotypes According to their Efficiency and Responsiveness to N Supply
4. Discussion
4.1. Variation of N Utilization Efficiency (NUE) Traits among Genotypes and N Supplies
4.2. Classification of Genotypes: Efficiency and Responsiveness to N Supply of Contrasting Tobacco Genotypes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kant, S.; Bi, Y.-M.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajari, E.; Snyman, S.J.; Watt, M.P. Nitrogen use efficiency of sugarcane (Saccharum spp.) varieties under in vitro conditions with varied N supply. Plant Cell. Tissue Organ Cult. 2015, 122, 21–29. [Google Scholar] [CrossRef]
- Hajari, E.; Snyman, S.J.; Watt, M.P. Inorganic nitrogen uptake kinetics of sugarcane (Saccharum spp.) varieties under in vitro conditions with varying N supply. Plant Cell. Tissue Organ Cult. 2014, 117, 361–371. [Google Scholar] [CrossRef]
- Schum, A.; Meise, P.; Jansen, G.; Seddig, S.; Ordon, F. Evaluation of nitrogen efficiency associated traits of starch potato cultivars under in vitro conditions. Plant Cell. Tissue Organ Cult. 2017, 130, 651–665. [Google Scholar] [CrossRef]
- Schum, A.; Jansen, G. In vitro method for early evaluation of nitrogen use efficiency associated traits in potato. J. Appl. Bot. Food Qual. 2014, 87, 256–264. [Google Scholar]
- Ray, D.; Sheshshayee, M.S.; Mukhopadhyay, K.; Bindumadhava, H.; Prasad, T.G.; Udaya Kumar, M. High nitrogen use efficiency in rice genotypes is associated with higher net photosynthetic rate at lower Rubisco content. Biol. Plant. 2003, 46, 251–256. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Brueck, H.; Senbayram, M. Low nitrogen supply decreases water-use efficiency of oriental tobacco. J. Plant Nutr. Soil Sci. 2009, 172, 216–223. [Google Scholar] [CrossRef]
- Pearson, B.; Bush, L.; Pfeufer, E.; Snell, W.; Townsend, L.; Purschwitz, M.; Swetnam, L. 2017-2018 Burley and Dark Tobacco Production Guide; University of Kentucky Cooperative Extension Service: Lexington, KY, USA, 2016. [Google Scholar]
- Li, Y.; Shi, H.; Yang, H.; Zhou, J.; Wang, J.; Bai, R.; Xu, D. Difference between Burley Tobacco and Flue-Cured Tobacco in Nitrate Accumulation and Chemical Regulation of Nitrate and TSNA Contents. J. Chem. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Sisson, V.A.; Rufty, T.W.; Williamson, R.E. Nitrogen-Use Efficiency among Flue-Cured Tobacco Genotypes. Crop Sci. 1991, 31, 1615. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Rivero, R.M.; Cervilla, L.M.; Castellano, R.; Romero, L. Grafting to improve nitrogen-use efficiency traits in tobacco plants. J. Sci. Food Agric. 2006, 86, 1014–1021. [Google Scholar] [CrossRef]
- Fan, T.F.; He, M.J.; Li, C.J.; Shi, D.X.; Yang, C.; Chen, Y.Y.; Ke, J.; Chen, Y.X.; Zhang, L.; Li, D.Q.; et al. Physiological dissection revealed that both uptake and assimilation are the major components regulating different growth responses of two tobacco cultivars to nitrogen nutrition. Plant Biol. 2018, 20, 39–49. [Google Scholar] [CrossRef]
- Teng, W.; Li, W.; Li, C. Comparison of N uptake and internal use efficiency in two tobacco varieties. Crop J. 2015, 3, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Law, A.D.; Fisher, C.; Jack, A.; Moe, L.A. Tobacco, Microbes, and Carcinogens: Correlation Between Tobacco Cure Conditions, Tobacco-Specific Nitrosamine Content, and Cured Leaf Microbial Community. Microb. Ecol. 2016, 72, 120–129. [Google Scholar] [CrossRef]
- Cai, B.; Ji, H.; Fannin, F.F.; Bush, L.P. Contribution of Nicotine and Nornicotine toward the Production of N ′-Nitrosonornicotine in Air-Cured Tobacco (Nicotiana tabacum). J. Nat. Prod. 2016, 79, 754–759. [Google Scholar] [CrossRef]
- Edwards, K.D.; Fernandez-Pozo, N.; Drake-Stowe, K.; Humphry, M.; Evans, A.D.; Bombarely, A.; Allen, F.; Hurst, R.; White, B.; Kernodle, S.P.; et al. A reference genome for Nicotiana tabacum enables map-based cloning of homeologous loci implicated in nitrogen utilization efficiency. BMC Genom. 2017, 18, 448. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–39. [Google Scholar]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análises de Solo, Plantas e Outros Materiais, 2nd ed.; UFRGS: Porto Alegre, Brazil, 1995. [Google Scholar]
- Amirhandeh, M.A.S.; Norouzi, M.; Nosratabad, A.R.F. Effects of nitrogen fertilization on nitrogen use efficiency of Coker (flue-cured) tobacco inoculated with Azotobacter chroococcum. Adv. Environ. Biol. 2013, 7, 968–977. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef] [Green Version]
- Pilbeam, D.J. The Utilization of Nitrogen by Plants: A Whole Plant Perspective. In Annual Plant Reviews Online; John Wiley & Sons, Ltd: Chichester, UK, 2018; Volume 42, pp. 305–351. ISBN 9781119312994. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R. Some aspects of the equilibrium between overground and underground plant parts. Jaarb. Inst. Biol. Scheikd. Onderz. Landbouwgewassen Wageningen 1963, 31–40. [Google Scholar]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Vann, M.C.; Inman, M.D. Managing Nutrients. In 2018 Flue-Cured Tobacco Guide; Fisher, L., Ed.; NC State Extension: Raleigh, NC, USA, 2018; pp. 57–76. [Google Scholar]
- Pearce, R.C.; Bailey, W.A.; Bush, L.P.; Green, J.D.; Jack, A.M.; Miller, R.D.; Snell, W.M.; Townsend, L.H.; Purschwitz, M.A.; Swetnam, L.D.; et al. 2015–2016 Burley and Dark Tobacco Production Guide. Agric. Nat. Resour. Publ. 2016, 162. [Google Scholar]
- Sifola, M.I.; Postiglione, L. The effect of nitrogen fertilization on nitrogen use efficiency of irrigated and non-irrigated tobacco (Nicotiana tabacum L.). Plant Soil 2003, 252, 313–323. [Google Scholar] [CrossRef]
- Ju, X.-T.; Chao, F.-C.; Li, C.-J.; Jiang, R.-F.; Christie, P.; Zhang, F.-S. Yield and Nicotine Content of Flue-Cured Tobacco as Affected by Soil Nitrogen Mineralization. Pedosphere 2008, 18, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Amirhandeh, M.S.; Nosratabad, A.F.; Norouzi, M.; Harutyunyan, S. Response of Coker (Flue-cured) tobacco (Nicotiana tabacum) to inoculation with Azotobacter chroococcum at various levels of nitrogen fertilization. Aust. J. Crop Sci. 2012, 6, 861–868. [Google Scholar]
- Lu, J.; Zhang, L.; Lewis, R.S.; Bovet, L.; Goepfert, S.; Jack, A.M.; Crutchfield, J.D.; Ji, H.; Dewey, R.E. Expression of a constitutively active nitrate reductase variant in tobacco reduces tobacco-specific nitrosamine accumulation in cured leaves and cigarette smoke. Plant Biotechnol. J. 2016, 14, 1500–1510. [Google Scholar] [CrossRef] [Green Version]
| Genotypes | DM 1 (g plant−1) | Root/Shoot Ratio 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | ||||||
| 2 mM | 10 mM | 2 mM | 10 mM | 2 mM | 10 mM | 2 mM | 10 mM | |
| BAG 06 | 5.23 B | 8.05 E | 4.20 A | 14.20 B | 12.58 A | 24.31 A | 0.31 E | 0.21 D |
| BAT 2101 | 6.52 A | 10.28 C | 1.70 B | 9.05 E | 10.85 B | 21.39 B | 0.52 A | 0.34 A |
| BAT 2301 | 5.99 A | 8.51 D | 3.77 A | 13.00 C | 11.39 B | 21.75 B | 0.39 C | 0.25 C |
| BAT 3004 | 5.34 B | 8.76 D | 2.56 B | 9.60 E | 9.98 B | 20.55 B | 0.42 C | 0.29 B |
| BAT 3201 | 6.55 A | 9.58 C | 2.88 B | 10.90 D | 10.53 B | 23.08 B | 0.49 B | 0.28 B |
| CSC 221 | 6.08 A | 9.93 C | 3.12 B | 11.43 D | 10.67 B | 20.32 B | 0.44 C | 0.31 A |
| CSC 2305 | 6.07 A | 9.29 C | 2.82 B | 13.60 B | 10.23 B | 22.83 B | 0.47 B | 0.25 C |
| CSC 2307 | 6.38 A | 9.83 C | 2.40 B | 11.54 D | 11.16 B | 22.02 B | 0.47 B | 0.29 B |
| CSC 259 | 6.90 A | 10.61 B | 2.93 B | 12.10 C | 11.13 B | 21.24 B | 0.49 B | 0.32 A |
| CSC 2602 | 6.95 A | 9.79 C | 2.61 B | 10.49 D | 10.62 B | 22.55 B | 0.53 A | 0.30 B |
| CSC 302 | 6.33 A | 11.42 A | 2.87 B | 12.48 C | 10.51 B | 22.56 B | 0.48 B | 0.32 A |
| CSC 3702 | 6.82 A | 11.88 A | 2.17 B | 11.13 D | 11.69 B | 23.09 B | 0.49 B | 0.35 A |
| CSC 3703 | 6.14 A | 10.92 B | 2.25 B | 9.45 E | 11.22 B | 25.53 A | 0.46 B | 0.31 A |
| CSC 416 | 5.17 B | 7.71 E | 3.76 A | 12.18 C | 13.15 A | 25.12 A | 0.30 E | 0.20 D |
| CSC 4303 | 5.98 A | 8.94 D | 4.86 A | 12.38 C | 14.10 A | 26.12 A | 0.31 E | 0.23 C |
| CSC 4304 | 5.64 B | 8.76 D | 3.92 A | 13.73 B | 14.50 A | 26.99 A | 0.31 E | 0.22 D |
| CSC 439 | 5.01 B | 8.62 D | 3.96 A | 13.38 B | 13.15 A | 24.52 A | 0.29 E | 0.23 C |
| CSC 444 | 5.91 A | 8.71 D | 3.82 A | 12.09 C | 15.13 A | 24.97 A | 0.31 E | 0.23 C |
| CSC 447 | 5.48 B | 7.99 E | 3.89 A | 13.23 C | 14.49 A | 25.27 A | 0.30 E | 0.21 D |
| CSC 4501 | 5.43 B | 9.43 C | 4.02 A | 14.76 A | 14.52 A | 24.89 A | 0.29 E | 0.24 C |
| CSC 4703 | 6.12 A | 9.30 C | 2.92 B | 11.89 C | 14.90 A | 21.89 B | 0.34 D | 0.27 B |
| CSC 4704 | 5.51 B | 8.21 D | 3.05 B | 10.45 D | 14.56 A | 24.84 A | 0.31 E | 0.24 C |
| CSC 4707 | 5.36 B | 7.10 E | 4.71 A | 15.64 A | 13.47 A | 27.66 A | 0.29 E | 0.16 E |
| CSC 497 | 4.84 B | 8.43 D | 4.23 A | 13.96 B | 13.19 A | 25.52 A | 0.28 E | 0.22 D |
| CSC 500 | 5.37 B | 7.56 E | 3.17 B | 10.50 D | 14.13 A | 20.40 B | 0.31 E | 0.24 C |
| Dark O.S. | 4.09 B | 9.43 C | 2.83 B | 12.13 C | 8.33 B | 21.51 B | 0.36 D | 0.28 B |
| HB 4488P | 5.85 A | 8.33 D | 3.29 B | 12.71 C | 10.72 B | 24.33 A | 0.42 C | 0.22 C |
| New cultivar | 5.77 A | 9.01 D | 2.62 B | 9.91 E | 14.32 A | 26.11 A | 0.34 D | 0.25 C |
| Average | 5.82 | 9.16 | 3.26 | 12.07 | 12.33 | 23.62 | 0.38 | 0.26 |
| Genotypes | N content (mg g−1) plant−1 | |||||
|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | ||||
| 2 mM | 10 mM | 2 mM | 10 mM | 2 mM | 10 mM | |
| BAG 06 | 17.37 A | 30.31 B | 15.28 C | 23.77 B | 16.28 B | 30.68 B |
| BAT 2101 | 16.66 A | 29.65 C | 20.94 A | 28.00 A | 16.75 B | 33.03 A |
| BAT 2301 | 18.17 A | 27.14 D | 14.87 C | 24.50 B | 16.33 B | 32.85 A |
| BAT 3004 | 18.10 A | 28.67 C | 19.03 B | 27.04 A | 18.83 B | 35.81 A |
| BAT 3201 | 19.23 A | 26.80 D | 16.93 C | 23.65 B | 17.67 B | 34.36 A |
| CSC 221 | 18.85 A | 28.10 C | 14.99 C | 25.51 A | 18.74 B | 34.19 A |
| CSC 2305 | 19.03 A | 29.70 C | 15.65 C | 24.14 B | 19.57 B | 34.47 A |
| CSC 2307 | 19.23 A | 32.42 B | 16.77 C | 26.11 A | 17.82 B | 33.04 A |
| CSC 259 | 18.60 A | 29.22 C | 18.40 B | 26.92 A | 16.62 B | 33.06 A |
| CSC 2602 | 18.24 A | 29.01 C | 17.35 B | 27.06 A | 17.41 B | 33.85 A |
| CSC 302 | 20.74 A | 28.97 C | 18.74 B | 23.07 B | 17.72 B | 32.16 A |
| CSC 3702 | 20.23 A | 26.99 D | 20.38 A | 25.71 A | 14.94 C | 30.75 B |
| CSC 3703 | 19.84 A | 28.77 C | 18.37 B | 26.39 A | 17.05 B | 29.36 B |
| CSC 416 | 20.36 A | 32.15 B | 16.03 C | 24.12 B | 14.74 C | 29.00 B |
| CSC 4303 | 19.79 A | 31.64 B | 14.63 C | 24.12 B | 13.50 C | 29.92 B |
| CSC 4304 | 19.38 A | 31.54 B | 16.08 C | 23.02 B | 13.18 C | 27.96 B |
| CSC 439 | 19.23 A | 31.01 B | 17.12 B | 24.06 B | 15.18 C | 31.21 B |
| CSC 444 | 18.54 A | 30.42 B | 14.81 C | 22.61 B | 13.41 C | 31.32 B |
| CSC 447 | 19.43 A | 31.77 B | 14.91 C | 23.87 B | 13.88 C | 31.18 B |
| CSC 4501 | 19.36 A | 28.62 C | 15.40 C | 23.54 B | 13.69 C | 28.49 B |
| CSC 4703 | 20.03 A | 31.71 B | 18.29 B | 26.63 A | 13.06 C | 31.98 A |
| CSC 4704 | 18.24 A | 31.03 B | 16.16 C | 28.64 A | 13.79 C | 30.99 B |
| CSC 4707 | 19.54 A | 34.54 A | 15.53 C | 22.75 B | 13.42 C | 27.82 B |
| CSC 497 | 18.84 A | 28.96 C | 13.59 C | 22.70 B | 16.39 B | 30.51 B |
| CSC 500 | 18.19 A | 29.27 C | 14.99 C | 25.85 A | 14.48 C | 34.95 A |
| Dark O.S. | 19.03 A | 27.40 D | 17.56 B | 23.98 B | 24.45 A | 32.60 A |
| HB 4488P | 18.25 A | 29.15 C | 16.03 C | 24.09 B | 17.86 B | 32.86 A |
| New cultivar | 19.81 A | 26.89 D | 15.67 C | 25.01 B | 14.32 C | 31.75 A |
| Average | 19.01 | 29.71 | 16.59 | 24.89 | 16.11 | 31.79 |
| Genotypes | N accumulation (mg) plant−1 | |||||
|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | ||||
| 2 mM | 10 Mm | 2 mM | 10 mM | 2 mM | 10 Mm | |
| BAG 06 | 91.02 B | 244.36 D | 64.22 A | 337.34 A | 202.71 A | 745.81 B |
| BAT 2101 | 108.54 B | 305.10 A | 35.55 A | 253.09 D | 181.81 A | 704.53 C |
| BAT 2301 | 108.40 B | 230.80 D | 55.67 A | 317.75 B | 185.57 A | 710.01 C |
| BAT 3004 | 96.48 B | 251.36 D | 48.58 A | 259.20 D | 187.65 A | 734.77 C |
| BAT 3201 | 125.90 A | 257.22 C | 48.84 A | 256.15 D | 186.08 A | 787.80 A |
| CSC 221 | 114.50 A | 280.79 B | 46.79 A | 290.56 C | 198.90 A | 689.64 C |
| CSC 2305 | 115.15 A | 275.03 C | 43.75 A | 327.36 B | 198.65 A | 785.22 A |
| CSC 2307 | 122.23 A | 318.42 A | 40.23 A | 300.60 B | 198.41 A | 724.76 C |
| CSC 259 | 128.67 A | 309.69 A | 53.85 A | 324.82 B | 184.77 A | 700.05 C |
| CSC 2602 | 126.76 A | 283.75 B | 45.22 A | 282.42 C | 184.44 A | 760.98 B |
| CSC 302 | 131.27 A | 330.20 A | 53.85 A | 288.30 C | 182.83 A | 725.17 C |
| CSC 3702 | 138.00 A | 320.49 A | 44.09 A | 285.25 C | 174.71 A | 708.08 C |
| CSC 3703 | 121.72 A | 313.82 A | 40.81 A | 246.82 D | 190.20 A | 746.71 B |
| CSC 416 | 105.18 B | 247.94 D | 60.11 A | 291.35 C | 193.12 A | 726.13 C |
| CSC 4303 | 117.45 A | 281.69 B | 71.23 A | 296.06 C | 189.63 A | 769.56 B |
| CSC 4304 | 109.27 B | 275.71 C | 63.20 A | 316.20 B | 190.96 A | 750.72 B |
| CSC 439 | 96.38 B | 267.06 C | 68.12 A | 321.74 B | 199.15 A | 758.15 B |
| CSC 444 | 109.29 B | 264.21 C | 56.52 A | 272.95 C | 202.64 A | 779.41 A |
| CSC 447 | 106.33 B | 253.09 D | 58.07 A | 315.59 B | 201.10 A | 776.71 A |
| CSC 4501 | 104.96 B | 271.13 C | 61.90 A | 347.38 A | 198.28 A | 706.71 C |
| CSC 4703 | 122.34 A | 294.78 B | 53.25 A | 315.92 B | 194.22 A | 699.40 C |
| CSC 4704 | 100.47 B | 254.88 C | 49.39 A | 286.49 C | 200.89 A | 756.97 B |
| CSC 4707 | 104.71 B | 244.91 D | 73.22 A | 355.90 A | 180.71 A | 767.62 B |
| CSC 497 | 90.63 B | 244.73 D | 57.61 A | 315.74 B | 212.85 A | 762.15 B |
| CSC 500 | 97.55 B | 221.76 D | 47.60 A | 271.01 C | 204.69 A | 711.63 C |
| Dark O.S. | 76.00 B | 257.85 C | 48.70 A | 290.90 C | 199.37 A | 698.61 C |
| HB 4488P | 106.69 B | 242.99 D | 52.90 A | 306.16 B | 191.37 A | 799.77 A |
| New cultivar | 114.25 A | 242.07 D | 39.99 A | 247.36 D | 203.89 A | 827.05 A |
| Average | 110.36 | 270.92 | 52.98 | 297.16 | 193.56 | 743.36 |
| Genotypes | NUE 1 (g DM g−1 N) | PNUE (g2 DM mg−1 N) | NHI (%) | NUpE 2 (mg N g−1 roots) | RENU (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2 mM | 10 mM | 2 mM | 10 mM | 2 mM | 10 mM | 2 mM | 10 mM | ||
| BAG 06 | 61.51 B | 35.08 A | 1.36 Ab | 1.63 Aa | 56.64 Ba | 56.16 Ba | 69.04 A | 165.42 B | 47.23 C |
| BAT 2101 | 58.51 C | 32.24 A | 1.12 Ba | 1.31 Ba | 55.78 Ba | 55.78 Ba | 50.02 B | 122.80 E | 46.92 C |
| BAT 2301 | 60.47 B | 34.33 A | 1.28 Ba | 1.49 Ba | 53.07 Ca | 56.39 Ba | 58.67 B | 148.44 C | 48.97 C |
| BAT 3004 | 53.75 C | 31.24 A | 0.96 Ba | 1.21 Ba | 56.39 Ba | 59.02 Aa | 62.56 B | 142.37 D | 45.97 C |
| BAT 3201 | 55.33 C | 33.44 A | 1.11 Bb | 1.46 Ba | 51.58 Cb | 60.61 Aa | 55.10 B | 136.23 D | 46.06 C |
| CSC 221 | 55.17 C | 33.00 A | 1.10 Bb | 1.38 Ba | 55.22 Ba | 54.87 Ba | 59.36 B | 130.16 D | 47.90 C |
| CSC 2305 | 53.40 C | 32.92 A | 1.03 Bb | 1.51 Ba | 55.50 Ba | 56.60 Ba | 59.15 B | 149.83 C | 41.80 D |
| CSC 2307 | 55.28 C | 32.25 A | 1.11 Bb | 1.40 Ba | 55.00 Ba | 53.97 Ba | 56.94 B | 136.86 D | 46.02 C |
| CSC 259 | 57.08 C | 32.89 A | 1.20 Ba | 1.45 Ba | 50.32 Ca | 52.50 Ba | 53.31 B | 126.83 E | 47.77 C |
| CSC 2602 | 56.59 C | 32.23 A | 1.14 Ba | 1.38 Ba | 51.76 Cb | 57.32 Aa | 51.31 B | 135.77 D | 47.25 C |
| CSC 302 | 53.63 C | 34.60 A | 1.06 Bb | 1.61 Aa | 49.74 Cb | 53.98 Ba | 58.15 B | 117.95 E | 42.41 D |
| CSC 3702 | 58.00 C | 35.07 A | 1.20 Bb | 1.62 Aa | 48.98 Cb | 53.88 Ba | 52.38 B | 110.65 E | 44.97 C |
| CSC 3703 | 55.54 C | 35.10 A | 1.09 Bb | 1.61 Aa | 53.98 Ba | 57.08 Aa | 57.50 B | 120.10 E | 42.78 D |
| CSC 416 | 61.53 B | 35.56 A | 1.36 Aa | 1.60 Aa | 53.94 Ba | 57.40 Aa | 69.36 A | 165.44 B | 49.08 C |
| CSC 4303 | 65.90 A | 35.09 A | 1.65 Aa | 1.68 Aa | 50.16 Cb | 57.16 Aa | 63.66 A | 153.09 C | 53.02 B |
| CSC 4304 | 66.23 A | 36.84 A | 1.59 Aa | 1.83 Aa | 52.55 Ca | 55.93 Ba | 64.68 A | 153.62 C | 48.73 C |
| CSC 439 | 60.87 B | 34.51 A | 1.35 Aa | 1.61 Aa | 54.85 Ba | 56.27 Ba | 72.59 A | 156.69 B | 47.64 C |
| CSC 444 | 67.50 A | 34.74 A | 1.68 Aa | 1.59 Aa | 55.01 Bb | 59.19 Aa | 62.59 B | 151.93 C | 54.43 B |
| CSC 447 | 65.30 A | 34.52 A | 1.56 Aa | 1.62 Aa | 55.02 Ba | 57.70 Aa | 66.78 A | 168.58 B | 51.63 B |
| CSC 4501 | 65.62 A | 37.02 A | 1.57 Aa | 1.82 Aa | 54.28 Ba | 53.38 Ba | 67.41 A | 141.32 D | 48.85 C |
| CSC 4703 | 64.67 A | 32.87 A | 1.55 Aa | 1.42 Ba | 52.51 Ca | 53.38 Ba | 60.49 B | 141.50 D | 55.54 A |
| CSC 4704 | 65.97 A | 33.42 A | 1.52 Aa | 1.47 Ba | 57.27 Ba | 58.30 Aa | 63.67 A | 158.31 B | 53.94 B |
| CSC 4707 | 65.58 A | 36.85 A | 1.54 Ab | 1.86 Aa | 50.35 Cb | 56.12 Ba | 66.93 A | 194.02 A | 46.68 C |
| CSC 497 | 61.52 B | 36.14 A | 1.38 Ab | 1.74 Aa | 59.00 Aa | 57.61 Aa | 75.18 A | 158.70 B | 46.51 C |
| CSC 500 | 64.84 A | 31.90 A | 1.47 Aa | 1.23 Ba | 58.49 Aa | 59.11 Aa | 65.27 A | 160.28 B | 59.17 A |
| Dark O.S. | 46.94 D | 34.55 A | 0.74 Bb | 1.49 Ba | 61.66 Aa | 56.02 Bb | 82.53 A | 133.76 D | 35.16 E |
| HB 4488P | 56.60 C | 33.66 A | 1.12 Bb | 1.53 Ba | 54.54 Bb | 59.27 Aa | 60.01 B | 162.16 B | 43.77 D |
| New cultivar | 63.41 B | 34.19 A | 1.45 Aa | 1.54 Ba | 56.96 Bb | 62.78 Aa | 62.07 B | 146.31 C | 50.37 B |
| Average | 59.88 | 34.15 | 1.30 | 1.54 | 54.31 | 56.71 | 62.38 | 146.04 | 47.88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, A.B.; Guelfi, D.R.; Faquin, V.; Coelho, F.S.; de C. Souza, C.S.; Faquin, G.P.; Souza, K.R.D.; Chagas, W.F.T. Genotypic Variation in Nitrogen Use-Efficiency Traits of 28 Tobacco Genotypes. Agronomy 2020, 10, 572. https://doi.org/10.3390/agronomy10040572
Andrade AB, Guelfi DR, Faquin V, Coelho FS, de C. Souza CS, Faquin GP, Souza KRD, Chagas WFT. Genotypic Variation in Nitrogen Use-Efficiency Traits of 28 Tobacco Genotypes. Agronomy. 2020; 10(4):572. https://doi.org/10.3390/agronomy10040572
Chicago/Turabian StyleAndrade, André B., Douglas R. Guelfi, Valdemar Faquin, Fabrício S. Coelho, Carolina S. de C. Souza, Giulianno P. Faquin, Kamila R. D. Souza, and Wantuir F. T. Chagas. 2020. "Genotypic Variation in Nitrogen Use-Efficiency Traits of 28 Tobacco Genotypes" Agronomy 10, no. 4: 572. https://doi.org/10.3390/agronomy10040572





