A Review of Metal and Metal-Oxide Nanoparticle Coating Technologies to Inhibit Agglomeration and Increase Bioactivity for Agricultural Applications
Abstract
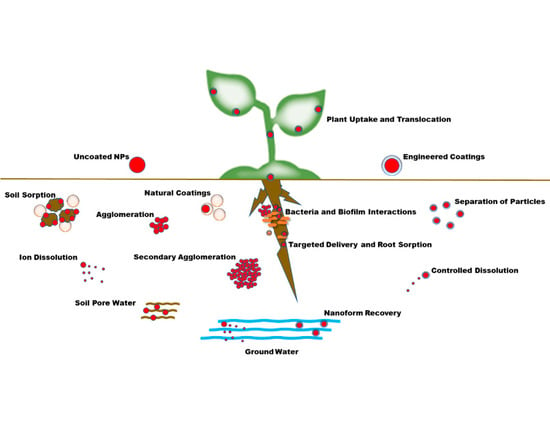
:1. Introduction
1.1. Nanoparticles in Agriculture
1.2. Effects of Soil on Fate and Transport
1.3. Agglomeration in Soils and Complex Media
1.4. Agglomeration during Storage and Transport
2. Coatings
3. Advances in Coating Technology
3.1. Review of the Literature
- Use of spherical core metal or metal-oxide NPs;
- Coatings that fully or partially encapsulate NPs;
- Testing specifically for agglomeration under biologically relevant conditions;
- Agriculturally viable NP-coating systems.
3.2. Natural Organic Coatings
3.3. Polymer Coatings
3.4. Zwitterionic Coatings
3.5. Miscellaneous Coatings (Inorganic Coatings and Small Organic Molecule Coatings)
3.5.1. Inorganic Coatings
3.5.2. Citrate
3.5.3. Other
3.6. Encapsulation
4. Summary and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ali, S.; Rizwan, M.; Zia ur Rehman, M.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Huang, Z.; Rajasekaran, P.; Ozcan, A.; Santra, S. Antimicrobial Magnesium Hydroxide Nanoparticles As an Alternative to Cu Biocide for Crop Protection. J. Agric. Food Chem. 2018, 66, 8679–8686. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. Biotech 2016, 6, 254. [Google Scholar]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Suskova, S.; Mandzhieva, S.; Tsitsuashvili, V.; Chapligin, V.; Fedorenko, A. Effects of Copper Nanoparticles (CuO NPs) on Crop Plants: A Mini Review. BioNanoScience 2018, 8, 36–42. [Google Scholar] [CrossRef]
- Biju, S.; Fuentes, S.; Gupta, D. Silicon improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defense system. Plant Physiol. Biochem. 2017, 119, 250–264. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; Cedeño-Mattei, Y.; Bailón-Ruiz, S.J.; Vazquez-Nuñez, E.; Hernandez-Viezcas, J.A.; Perales-Pérez, O.J.; la Rosa, G.D.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Environmental behavior of coated NMs: Physicochemical aspects and plant interactions. J. Hazard. Mater. 2018, 347, 196–217. [Google Scholar] [CrossRef]
- Agrawal, S.; Rathore, P. Nanotechnology Pros and Cons to Agriculture: A Review. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 43–55. [Google Scholar]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef]
- Phogat, N.; Khan, S.A.; Shankar, S.; Ansary, A.A.; Uddin, I. Fate of Inorganic Nanoparticles in Agriculture. Adv. Mater. Lett. 2016, 7, 3–12. [Google Scholar] [CrossRef]
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T.; Brink, N.V.D.; Nickel, C. Fate and bioavailability of engineered nanoparticles in soils: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764. [Google Scholar] [CrossRef]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm Sci. 2016, 29, 1–13. [Google Scholar]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanoparticle Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Darlington, T.K.; Neigh, A.M.; Spencer, M.T.; Oldenburg, S.J.; Nguyen, O.T. Nanoparticle characteristics affecting environmental fate and transport through soil. Environ. Toxicol. Chem. 2009, 28, 1191–1199. [Google Scholar] [CrossRef]
- Calder, A.J.; Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Johnson, W.; Anderson, A.J. Soil components mitigate the antimicrobial effects of silver nanoparticles towards a beneficial soil bacterium, Pseudomonas chlororaphis O6. Sci. Total Environ. 2012, 429, 215–222. [Google Scholar] [CrossRef]
- Mun, E.A.; Hannell, C.; Rogers, S.E.; Hole, P.; Williams, A.C.; Khutoryanskiy, V.V. On the Role of Specific Interactions in the Diffusion of Nanoparticles in Aqueous Polymer Solutions. Langmuir 2014, 30, 308–317. [Google Scholar] [CrossRef]
- Dimkpa, C.O. Soil properties influence the response of terrestrial plants to metallic nanoparticles exposure. Curr. Opin. Environ. Sci. Health 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Theng, B.K.G.; Yuan, G. Nanoparticles in the soil environment. Elements 2008, 4, 395–399. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Razavi. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Nye, P.H. Changes of pH across the rhizosphere induced by roots. Plant Soil 1981, 61, 7–26. [Google Scholar] [CrossRef]
- Schaller, G. pH changes in the rhizosphere in relation to the pH-buffering of soils. Plant Soil 1987, 97, 439–444. [Google Scholar] [CrossRef]
- Hengl, T.; De Jesus, J.M.; Macmillan, R.A.; Batjes, N.H.; Heuvelink, G.B.M.; Ribeiro, E.; Samuel-Rosa, A.; Kempen, B.; Leenaars, J.G.B.; Walsh, M.G.; et al. SoilGrids1km—Global soil Information based on automated mapping. PLoS ONE 2014, 9, e105992. [Google Scholar] [CrossRef] [Green Version]
- Kirk, G.J.D.; Bajita, J. Root-induced iron oxidation, pH changes and zinc solubilization in the rhizosphere of lowland rice. New Phytol. 1995, 131, 129–137. [Google Scholar] [CrossRef]
- Lorenz, S.; Hamon, R.; McGrath, S.P. Differences between soil solutions obtained from rhizosphere and non-rhizosphere soils by water displacement and soil centrifugation. Eur. J. Soil Sci. 1994, 45, 431–438. [Google Scholar] [CrossRef]
- Sebastia, J.; Labanowski, J.; Lamy, I. Changes in soil organic matter chemical properties after organic amendments. Chemosphere 2007, 68, 1245–1253. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef]
- Dietz, K.J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.A.; Schnoor, J.L.; Wiesner, M. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle Characterization: State of the Art, Challenges, and Emerging Technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrček, I.V.; Pavičić, I.; Crnković, T.; Jurašin, D.D.; Babič, M.; Jendelova, P.; Lovrić, M.; Ferhatović, L.; Ćurlin, M.; Gajović, S. Does surface coating of metallic nanoparticles modulate their interference with in vitro assays? RSC Adv. 2015, 5, 70787–70807. [Google Scholar] [CrossRef]
- Inci, G.; Arnold, A.; Kronenburg, A.; Weeber, R. Modeling Nanoparticle Agglomeration using Local Interactions. Aerosol. Sci. Technol. 2014, 48, 842–852. [Google Scholar] [CrossRef]
- Raichman, Y.; Kazakevich, M.; Rabkin, E.; Tsur, Y. Inter-Nanoparticle Bonds in Agglomerates Studied by Nanoindentation. Adv. Mater. 2006, 18, 2028–2030. [Google Scholar] [CrossRef]
- Eggersdorfer, M.L.; Pratsinis, S.E. Agglomerates and aggregates of nanoparticles made in the gas phase. Adv. Powder Technol. 2014, 25, 71–90. [Google Scholar] [CrossRef]
- Kim, S.C.; Wang, J.; Emery, M.S.; Shin, W.G.; Mulholland, G.W.; Pui, D.Y.H. Structural property effect of nanoparticle agglomerates on particle penetration through fibrous filter. Aerosol Sci. Technol. 2009, 43, 344–355. [Google Scholar] [CrossRef]
- Wang, C.; Bobba, A.D.; Attinti, R.; Shen, C.; Lazouskaya, V.; Wang, L.P.; Jin, Y. Retention and transport of silica nanoparticles in saturated porous media: Effect of concentration and particle size. Environ. Sci. Technol. 2012, 46, 7151–7158. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Cheng, B.; Ngan, A.H.W. The sintering and densification behaviour of many copper nanoparticles: A molecular dynamics study. Comput. Mater. Sci. 2013, 74, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nono, M.D.C.D.A. Compaction Behavior Study of Powder Composed by Nanoparticle Agglomerates and Aggregates. Mater. Sci. Forum 2006, 530, 461–466. [Google Scholar] [CrossRef]
- Kocjan, A.; Logar, M.; Shen, Z. The agglomeration, coalescence and sliding of nanoparticles, leading to the rapid sintering of zirconia nanoceramics. Sci. Rep. 2017, 7, 2541. [Google Scholar] [CrossRef] [PubMed]
- Wakuda, D.; Kim, K.; Suganuma, K. Room temperature sintering of Ag nanoparticles by drying solvent. Scr. Mater. 2008, 59, 649–652. [Google Scholar] [CrossRef]
- Nanda, K.K.; Maisels, A.; Kruis, F.E.; Fissan, H.; Stappert, S. Higher surface energy of free nanoparticles. Phys. Rev. Lett. 2003, 91, 106102. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Zhang, W.; Mujumdar, A.S.; Huang, L. Progress in drying technology for nanomaterials. Dry Technol. 2005, 23, 7–32. [Google Scholar] [CrossRef]
- Viguié, J.-R.; Sukmanowski, J.; Nölting, B.; Royer, F.-X. Study of agglomeration of alumina nanoparticles by atomic force microscopy (AFM) and photon correlation spectroscopy (PCS). Colloids Surf. A 2007, 302, 269–275. [Google Scholar] [CrossRef]
- Blute, I.; Pugh, R.J.; Van De Pas, J.; Callaghan, I. Silica nanoparticle sols. Part 3: Monitoring the state of agglomeration at the air/water interface using the Langmuir–Blodgett technique. J. Colloid Interface Sci. 2009, 336, 584–591. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.-K.; Lee, J.-K.; Jeong, Y.-M.; Cheong, S.; Ahn, Y.-C.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Peng, C.; Shen, C.; Zheng, S.; Yang, W.; Hu, H.; Liu, J.; Shi, J. Transformation of CuO nanoparticles in the aquatic environment: Influence of pH, electrolytes and natural organic matter. Nanomaterials 2017, 7, 326. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef]
- Yu, L. Universal Polymer Coatings with Tailorable Bioinert and Biospecific Function. Ph.D. Thesis, Freie Universität, Berlin, Germany, 2018. [Google Scholar]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hsu, C.; Zhu, L.; Tseng, S.; Hsu, J.-P. Influence of metal oxide nanoparticles concentration on their zeta potential. J. Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interface Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef]
- Lankoff, A.; Sandberg, W.J.; Węgierek-Ciuk, A.; Lisowska, H.; Refsnes, M.; Sartowska, B.; Schwarze, P.E.; Męczyńska-Wielgosz, S.; Wojewódzka, M.; Kruszewski, M. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol. Lett. 2012, 208, 197–213. [Google Scholar] [CrossRef]
- Alias, S.; Ismail, A.; Mohamad, A.A. Effect of pH on ZnO nanoparticle properties synthesized by sol–gel centrifugation. J. Alloys Compd. 2010, 499, 231–237. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Baumann, J.; Köser, J.; Arndt, D.; Filser, J. The coating makes the difference: Acute effects of iron oxide nanoparticles on Daphnia magna. Sci. Total Environ. 2014, 484, 176–184. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Varela-Ramirez, A.; Castillo-Michel, H.; Li, C.; Zhang, J.; Aguilera, R.J.; Keller, A.A.; Gardea-Torresdey, J.L. Effect of surface coating and organic matter on the uptake of CeO2 NPs by corn plants grown in soil: Insight into the uptake mechanism. J. Hazard. Mater. 2012, 225, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zook, J.M.; MacCuspie, R.; Locascio, L.E.; Halter, M.D.; Elliott, J.T. Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology 2011, 5, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Lin, Y.; Chen, R.; Ding, L.; Jiang, W. Effects of humic acid and bovine serum albumin on the agglomeration and sedimentation of oxide nanoparticles. J. Zhejiang Univ. Sci. A 2014, 15, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, V.; Gusain, D.; Sharma, Y.C. Critical Review on the Toxicity of Some Widely Used Engineered Nanoparticles. Ind. Eng. Chem. Res. 2015, 54, 6209–6233. [Google Scholar] [CrossRef]
- Deng, Y.; White, J.C.; Xing, B. Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. Sci. A 2014, 15, 552–572. [Google Scholar] [CrossRef] [Green Version]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.-J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeleye, A.S.; Conway, J.R.; Perez, T.; Rutten, P.; Keller, A.A. Influence of Extracellular Polymeric Substances on the Long-Term Fate, Dissolution, and Speciation of Copper-Based Nanoparticles. Environ. Sci. Technol. 2014, 48, 12561–12568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baalousha, M.; Nur, Y.; Römer, I.; Tejamaya, M.; Lead, J.R. Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles. Sci. Total Environ. 2013, 454, 119–131. [Google Scholar] [CrossRef]
- Dutta, T.; Bagchi, D.; Bera, A.; Das, S.; Adhikari, T.; Pal, S.K. Surface Engineered ZnO-Humic/Citrate Interfaces: Photoinduced Charge Carrier Dynamics and Potential Application for Smart and Sustained Delivery of Zn Micronutrient. ACS Sustain. Chem. Eng. 2019, 7, 10920–10930. [Google Scholar] [CrossRef]
- Estephan, Z.G.; Jaber, J.A.; Schlenoff, J.B. Zwitterion-Stabilized Silica Nanoparticles: Toward Nonstick Nano. Langmuir 2010, 26, 16884–16889. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chu, S.-H.; Li, C.-H.; Lee, T.R. Surface modification with zwitterionic cysteine betaine for nanoshell-assisted near-infrared plasmonic hyperthermia. Colloids Surf. B 2016, 145, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.; Liu, X.; Xu, J.; Ji, J.; Shen, J. Zwitterionic phosphorylcholine-protected water-soluble Ag nanoparticles. Sci. China Ser. B-Chem. 2009, 52, 64–68. [Google Scholar] [CrossRef]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Impact of exopolysaccharides on the stability of silver nanoparticles in water. Water Res. 2011, 45, 5184–5190. [Google Scholar] [CrossRef]
- Liu, X.; Huang, H.; Jin, Q.; Ji, J. Mixed Charged Zwitterionic Self-Assembled Monolayers as a Facile Way to Stabilize Large Gold Nanoparticles. Langmuir 2011, 27, 5242–5251. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Gao, B.; Sun, Y.; Shi, X.; Xu, H.; Wu, J. Effects of Humic Acid and Solution Chemistry on the Retention and Transport of Cerium Dioxide Nanoparticles in Saturated Porous Media. Water Air Soil Pollut. 2014, 225, 2167. [Google Scholar] [CrossRef]
- Ranka, M.; Brown, P.; Hatton, T.A. Responsive Stabilization of Nanoparticles for Extreme Salinity and High-Temperature Reservoir Applications. ACS Appl. Mater. Interfaces 2015, 7, 19651–19658. [Google Scholar] [CrossRef] [PubMed]
- Rouhana, L.L.; Jaber, J.A.; Schlenoff, J.B. Aggregation-Resistant Water-Soluble Gold Nanoparticles. Langmuir 2007, 23, 12799–12801. [Google Scholar] [CrossRef]
- Tebbe, M.; Kuttner, C.; Männel, M.J.; Fery, A.; Chanana, M. Colloidally stable and surfactant-free protein-coated gold nanorods in biological media. ACS Appl. Mater. Interfaces 2015, 7, 5984–5991. [Google Scholar] [CrossRef] [PubMed]
- Whitley, A.R.; Levard, C.; Oostveen, E.; Bertsch, P.; Matocha, C.J.; Von Der Kammer, F.; Unrine, J.M. Behavior of Ag nanoparticles in soil: Effects of particle surface coating, aging and sewage sludge amendment. Environ. Pollut. 2013, 182, 141–149. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Wang, S.; White, A.D.; Jiang, S. Functionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serum. Biomaterials 2009, 30, 5617–5621. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Trindade, T.; Duarte, A.C.; Pereira, E.; Koopmans, G.F.; Römkens, P.F.A.M. A framework to measure the availability of engineered nanoparticles in soils: Trends in soil tests and analytical tools. TrAC Trends Anal. Chem. 2016, 75, 129–140. [Google Scholar] [CrossRef]
- Wagner, S.; Gondikas, A.; Neubauer, E.; Hofmann, T.; von der Kammer, F. Spot the Difference: Engineered and Natural Nanoparticles in the Environment-Release, Behavior, and Fate. Angew. Chem. Int. Ed. 2014, 53, 12398–12419. [Google Scholar] [CrossRef]
- Oriekhova, O.; Stoll, S. Stability of uncoated and fulvic acids coated manufactured CeO2 nanoparticles in various conditions: From ultrapure to natural Lake Geneva waters. Sci. Total Environ. 2016, 562, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gunsolus, I.L.; Mousavi, M.P.S.; Hussein, K.; Bühlmann, P.; Haynes, C.L. Effects of humic and fulvic acids on silver nanoparticle stability, dissolution, and toxicity. Environ. Sci. Technol. 2015, 49, 8078–8086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackowiak, C.L.; Grossl, P.R.; Bugbee, B.G. Beneficial effects of humic acid on micronutrient availability to wheat. Soil Sci. Soc. Am. J. 2001, 65, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Z.; Zhang, W.; Xia, M.; Dai, G.; Zeng, G.; Zou, B.; Zhang, P. Facile synthesis of humic acid-coated iron oxide nanoparticles and their applications in wastewater treatment. Funct. Mater. Lett. 2011, 4, 373–376. [Google Scholar] [CrossRef]
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef]
- Hawke, D.; Powell, K.; Gregor, J. Determination of the aluminium complexing capacity of fulvic acids and natural waters, with examples from five New Zealand rivers. Mar. Freshw. Res. 1996, 47, 11–17. [Google Scholar] [CrossRef]
- Erhayem, M.; Sohn, M. Stability studies for titanium dioxide nanoparticles upon adsorption of Suwannee River humic and fulvic acids and natural organic matter. Sci. Total Environ. 2014, 468, 249–257. [Google Scholar] [CrossRef]
- Li, Z. Mechanistic Insight into the Effect of Polymer and NOM Coatings on Adhesion and Interactions between Nanoparticles and Bacteria. Ph.D. Thesis, Carnegie Melon University, Pittsburgh, PA, USA, 2011. [Google Scholar]
- Jain, R.; Jordan, N.; Weiss, S.; Foerstendorf, H.; Heim, K.; Kacker, R.; Hübner, R.; Kramer, H.; van Hullebusch, E.D.; Farges, F.; et al. Extracellular Polymeric Substances Govern the Surface Charge of Biogenic Elemental Selenium Nanoparticles. Environ. Sci. Technol. 2015, 49, 1713–1720. [Google Scholar] [CrossRef]
- Fischer, H. The Role of Biofilms in the Uptake and Transformation of Dissolved Organic Matter. In Aquatic Ecosystems; Elsevier: Amsterdam, The Netherlands, 2003; pp. 285–313. [Google Scholar]
- Tripathi, S.; Champagne, D.; Tufenkji, N. Transport Behavior of Selected Nanoparticles with different Surface Coatings in Granular Porous Media coated with Pseudomonas aeruginosa Biofilm. Environ. Sci. Technol. 2012, 46, 6942–6949. [Google Scholar] [CrossRef]
- Xiao, Y.; Wiesner, M.R. Transport and Retention of Selected Engineered Nanoparticles by Porous Media in the Presence of a Biofilm. Environ. Sci. Technol. 2013, 47, 2246–2253. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Magnacca, G.; Allera, A.; Montoneri, E.; Celi, L.; Benito, D.E.; Gagliardi, L.G.; Gonzalez, M.C.; Mártire, D.O.; Carlos, L. Novel Magnetite Nanoparticles Coated with Waste-Sourced Biobased Substances as Sustainable and Renewable Adsorbing Materials. ACS Sustain. Chem. Eng. 2014, 2, 1518–1524. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, I.; Cwiertny, D.M.; Walker, S.L. Combined Factors Influencing the Aggregation and Deposition of nano-TiO2 in the Presence of Humic Acid and Bacteria. Environ. Sci. Technol. 2012, 46, 6968–6976. [Google Scholar] [CrossRef] [PubMed]
- White, G.F. Bacterial biodegradation of ethoxylated surfactants. Pestic. Sci. 1993, 37, 159–166. [Google Scholar] [CrossRef]
- Watson, G.; Jones, N. The biodegradation of polyethylene glycols by sewage bacteria. Water Res. 1977, 11, 95–100. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.-M.; Liu, P.-X.; Liang, X.-J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [Green Version]
- Haines, J.R.; Alexander, M. Microbial Degradation of Polyethylene Glycols. Appl. Microbiol. 1975, 29, 621–625. [Google Scholar] [CrossRef] [Green Version]
- Bagwe, R.P.; Hilliard, L.R.; Tan, W. Surface Modification of Silica Nanoparticles to Reduce Aggregation and Nonspecific Binding. Langmuir 2006, 22, 4357–4362. [Google Scholar] [CrossRef] [Green Version]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Brandenberger, C.; Mühlfeld, C.; Ali, Z.; Lenz, A.-G.; Schmid, O.; Parak, W.J.; Gehr, P.; Rothen-Rutishauser, B. Quantitative evaluation of cellular uptake and trafficking of plain and polyethylene glycol-coated gold nanoparticles. Small 2010, 6, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, S.; Wiesner, M.R. Influence of natural organic matter on transport and retention of polymer coated silver nanoparticles in porous media. J. Hazard. Mater. 2014, 264, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Hase, H.; Saito, K. Further studies on the adsorption of plant phenols by synthetic polymers. Acta Soc. Bot. Pol. 1989, 58, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Mulens-Arias, V.; Nicolás-Boluda, A.; Gehanno, A.; Balfourier, A.; Carn, F.; Gazeau, F. Polyethyleneimine-assisted one-pot synthesis of quasi-fractal plasmonic gold nanocomposites as a photothermal theranostic agent. Nanoscale 2019, 11, 3344–3359. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, K.; De Schamphelaere, K.A.C.; Ali, Z.; Zhang, F.; Elsaesser, A.; Rivera-Gil, P.; Parak, W.J.; Smagghe, G.; Howard, C.V.; Janssen, C.R. Ecotoxicity and uptake of polymer coated gold nanoparticles. Nanotoxicology 2013, 7, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef]
- Hill, M.R.; MacKrell, E.J.; Forsthoefel, C.P.; Jensen, S.P.; Chen, M.; Moore, G.A.; He, Z.L.; Sumerlin, B.S. Biodegradable and pH-Responsive Nanoparticles Designed for Site-Specific Delivery in Agriculture. Biomacromolecules 2015, 16, 1276–1282. [Google Scholar] [CrossRef]
- Biehl, P.; von der Lühe, M.; Dutz, S.; Schacher, F. Synthesis, Characterization, and Applications of Magnetic Nanoparticles Featuring Polyzwitterionic Coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Yang, Q.; Mi, B. Novel antifouling surface with improved hemocompatibility by immobilization of polyzwitterions onto silicon via click chemistry. Appl. Surf. Sci. 2016, 363, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Binder, W.H. Click-chemistry for nanoparticle-modification. J. Mater. Chem. 2011, 21, 16717–16734. [Google Scholar] [CrossRef]
- Estephan, Z.G.; Schlenoff, P.S.; Schlenoff, J.B. Zwitteration As an Alternative to PEGylation. Langmuir 2011, 27, 6794–6800. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, J.; Li, L.; Jiang, S. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: Insights into nonfouling properties of zwitterionic materials. J. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef] [PubMed]
- Seetho, K.; Zhang, S.; Pollack, K.A.; Zou, J.; Raymond, J.E.; Martinez, E.; Wooley, K.L. Facile synthesis of a phosphorylcholine-based zwitterionic amphiphilic copolymer for anti-biofouling coatings. ACS Macro Lett. 2015, 4, 505–510. [Google Scholar] [CrossRef]
- Virtanen, J.; Arotçaréna, M.; Heise, B.; Ishaya, S.; Laschewsky, A.; Tenhu, H. Dissolution and Aggregation of a Poly(NIPA-block-sulfobetaine) Copolymer in Water and Saline Aqueous Solutions. Langmuir 2002, 18, 5360–5365. [Google Scholar] [CrossRef]
- Sun, J.-T.; Yu, Z.-Q.; Hong, C.-Y.; Pan, C.-Y. Biocompatible Zwitterionic Sulfobetaine Copolymer-Coated Mesoporous Silica Nanoparticles for Temperature-Responsive Drug Release. Macromol. Rapid Commun. 2012, 33, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Laschewsky, A. Structures and synthesis of zwitterionic polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Cao, B.; Tang, Q.; Cheng, G. Recent advances of zwitterionic carboxybetaine materials and their derivatives. J. Biomater. Sci. Polym. Ed. 2014, 25, 1502–1513. [Google Scholar] [CrossRef]
- Noy, J.-M.; Cao, C.; Stenzel, M. Length of the Stabilizing Zwitterionic Poly(2-methacryloyloxyethyl phosphorycholine) Block Influences the Activity of the Conjugated Arsenic Drug in Drug-Directed Polymerization-Induced Self-Assembly Particles. ACS Macro Lett. 2019, 8, 57–63. [Google Scholar] [CrossRef]
- Vasantha, V.A.; Junhui, C.; Ying, T.B.; Parthiban, A. Salt-Responsive Polysulfabetaines from Acrylate and Acrylamide Precursors: Robust Stabilization of Metal Nanoparticles in Hyposalinity and Hypersalinity. Langmuir 2015, 31, 11124–11134. [Google Scholar] [CrossRef]
- Olteanu, N.L.; Lazăr, C.A.; Petcu, A.R.; Meghea, A.; Rogozea, E.A.; Mihaly, M. “One-pot” synthesis of fluorescent Au@SiO2 and SiO2@Au nanoparticles. Arab. J. Chem. 2016, 9, 854–864. [Google Scholar] [CrossRef] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Hartlen, K.D.; Athanasopoulos, A.P.; Kitaev, V. Facile preparation of highly monodisperse small silica spheres (15 to> 200 nm) suitable for colloidal templating and formation of ordered arrays. Langmuir 2008, 24, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Sakamoto, Y.; Terasaki, O.; Kubota, Y.; Okubo, T.; Tatsumi, T. Periodic arrangement of silica nanospheres assisted by amino acids. J. Am. Chem. Soc. 2006, 128, 13664–13665. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Hassabo, A.G.; Mohamed, A.; Shaheen, T.I. Surface modification of SiO2 coated ZnO nanoparticles for multifunctional cotton fabrics. J. Colloid Interface Sci. 2017, 498, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Rusakova, I.; Hoffman, D.M.; Jacobson, A.J.; Lee, T.R. Monodisperse SnO2-coated gold nanoparticles are markedly more stable than analogous SiO2-coated gold nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 2479–2484. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Prabu, P.; Rajendran, V.; Kannan, N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanopart. Res. 2012, 14, 1294. [Google Scholar] [CrossRef]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Wirick, S.; Bertsch, P.M. Bioavailability of Gold Nanomaterials to Plants: Importance of Particle Size and Surface Coating. Environ. Sci. Technol. 2012, 46, 8467–8474. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Y.; Li, K.; Huang, Y.; Chen, Y. Influence of dissolved oxygen on aggregation kinetics of citrate-coated silver nanoparticles. Environ. Pollut. 2011, 159, 3757–3762. [Google Scholar] [CrossRef]
- Pokhrel, L.R.; Dubey, B.; Scheuerman, P.R. Impacts of Select Organic Ligands on the Colloidal Stability, Dissolution Dynamics, and Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2013, 47, 12877–12885. [Google Scholar] [CrossRef]
- Zhang, M.; He, F.; Zhao, D.; Hao, X. Degradation of soil-sorbed trichloroethylene by stabilized zero valent iron nanoparticles: Effects of sorption, surfactants, and natural organic matter. Water Res. 2011, 45, 2401–2414. [Google Scholar] [CrossRef]
- Veiseh, O.; Kievit, F.M.; Mok, H.; Ayesh, J.; Clark, C.; Fang, C.; Leung, M.; Arami, H.; Park, J.O.; Zhang, M. Cell transcytosing poly-arginine coated magnetic nanovector for safe and effective siRNA delivery. Biomaterials 2011, 32, 5717–5725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.D.; Wang, G.; Uludağ, H.; Unsworth, L.D. Poly-l-lysine-coated albumin nanoparticles: Stability, mechanism for increasing in vitro enzymatic resilience, and siRNA release characteristics. Acta Biomater. 2010, 6, 4277–4284. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Debnath, K.; Jana, N.R.; Jana, N.R. Trehalose-functionalized gold nanoparticle for inhibiting intracellular protein aggregation. Langmuir 2017, 33, 13996–14003. [Google Scholar] [CrossRef]
- Debnath, K.; Pradhan, N.; Singh, B.K.; Jana, N.R.; Jana, N.R. Poly (trehalose) nanoparticles prevent amyloid aggregation and suppress polyglutamine aggregation in a Huntington’s Disease model mouse. ACS Appl. Mater. Interfaces 2017, 9, 24126–24139. [Google Scholar] [CrossRef] [PubMed]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Malcolm, A.C.; Parnis, J.M.; Vreugdenhil, A.J. Size control and characterization of Au nanoparticle agglomeration during encapsulation in sol–gel matrices. J. Non-Cryst. Solids 2011, 357, 1203–1208. [Google Scholar] [CrossRef]
- Sokic, S.; Christenson, M.C.; Larson, J.C.; Appel, A.A.; Brey, E.M.; Papavasiliou, G. Evaluation of MMP substrate concentration and specificity for neovascularization of hydrogel scaffolds. Biomater. Sci. 2014, 2, 1343–1354. [Google Scholar] [CrossRef]
- Castelló, J.; Gallardo, M.; Busquets, M.A.; Estelrich, J. Chitosan (or alginate)-coated iron oxide nanoparticles: A comparative study. Colloids Surf. A 2015, 468, 151–158. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Application of copper-chitosan nanoparticles stimulate growth and induce resistance in finger millet (Eleusine coracana Gaertn.) plants against blast disease. J. Agric. Food Chem. 2018, 66, 1784–1790. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.; Kumari, S.; Sharma, S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef] [PubMed]



| NP and Source Details | NP Size (Technique) | Surface Charge (mV) | Testing Conditions Where Stability was Observed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Coating (Classification) | Core NP(s) | Bare Size (nm) | Coated Size (nm) | Medium | pH | Bare NP Zeta Potential | Coated NP Zeta Potential | pH | Temperature | Ionic Stability | Biological Components | Organic Matter/Soils/Polymers | Time |
| Adeleye 2014 [66] | Soil Matter (NOM) | Cu, CuO, Kocite | 40, 50, 50,000 (SEM) 2590, 280, 1532 (DLS) | -, 308, - (DLS) | 0.5 mM PBS | 7 | −34 | −25 (EPS) −45 (SRN) | 4,7,11 | - | 1, 10, 100 mM NaCl | 5 mg-C/L (EPS)5 mg/L (SRN) | - | 90 days |
| Baalousha 2013 [67] | FA (NOM) | citrate-Ag | 29.9 (DLS) 17 (TEM) 20.8 (Flow-Field Flow Fractionation) | - | DI Water | 7 | EPM: −3.88 × 10−8 m2/s | EPM: Reported as "Lower" in the presence of FA | ~7 | - | NaCl, NaNO3, Na2SO4, CaCl2, Ca(NO3)2, CaSO4, MgCl2, MgSO4, and NaCl/CaCl2 | - | Very Soft Water, Soft Water, Sea Water | - |
| Dutta 2019 [68] | HA (NOM) | ZnO | 30 (Sigma-Aldrich) | 25 (SEM) | - | 4, 7, 12 | - | - | - | - | 10 days (10 ppm HA) | |||
| Citrate (Misc) | ZnO | 30 (Sigma-Aldrich) | 16 (SEM) | - | 4, 7, 12 | - | - | - | - | 10 days (10 ppm citrate—performed worse than HA) | ||||
| Estephan 2010 [69] | SBS (Zwitterion) | Silica | 16.8–17.5 (SEM) | - | - | 7.4 | Up to 37 C | Up to 3M | 50% FBS | - | 15 days | |||
| Huang 2016 [70] | Cysteine Betaine (Zwitterion) | Au-Ag | 40–100 (SEM/DLS) | 102 (DLS) | - | 7.4 | Up to 55 C | Up to 3M | Gram positive/gram negative bacteria in 1% FBS | - | 24 h | |||
| Jin 2009 [71] | HS-PC (Zwitterion) | Ag | 7.8 (TEM) | 7.8 (TEM) | - | 7.4 | Up to 37 C | Up to 2M | Various | - | 24 h | |||
| Khan 2011 [72] | EPS (NOM) | Ag | ~50 (DLS) | ~70 (DLS) | DI Water | 7 | 10.23 | −29.32 (10 mg/L EPS) to ~ −42 (250 mg/L EPS) | ~3–12 | - | Up to 1 M NaCl | - | - | >72 h |
| Liu 2011 [73] | HS-C10-SN4 (Zwitterion) | citrate-Au | 16, 50, 100 (DLS/TEM) | ~ 17.5, 55, 105 (DLS) * | - | 1–13 | 37 C | Up to 2M NaCl, PBS | DMEM + 10% FBS, Lysozyme, BSA, Plasma | - | 6 month | |||
| HS-PEG2000 (Polymer) | citrate-Au | 16, 50, 100 (DLS/TEM) | ~ 45, 80, 135 (DLS) * | - | 2–12 | 37 C | 0.1 M NaCl, PBS | DMEM + 10% FBS | - | 4 month | ||||
| Citrate (Misc) | Au | 16, 50, 100 (DLS/TEM) | ~16, 50, 100 (DLS/TEM) | - | 7.4 | 37 C | - | - | - | 72 h | ||||
| Lv 2014 [74] | HA (NOM) | CeO2 | 538.2 (DLS) | 282.8 (5 mg/L HA, DLS) 249.1 (10 mg/L HA, DLS) | DI Water | 7 | −7.96 | −33.58 (5 mg/L HA) −35.72 (10 mg/L HA) | 7–10 | - | Up to 0.1 M NaCl | - | Sand | - |
| Ranka 2015 [75] | Random Copolymer MA/SBMA (Zwitterion) | Silica | 22 (Ludox-40) | ~180 (in water, DLS) ~350 (in brine, DLS) | - | - | Up to 90 C | Water, Seawater, Arab D Brine | Up to 120k mg/dm3 (3 types of electrolytes) | - | >30 days | |||
| Block Copolymer MA/SBMA (Zwitterion) | Silica | 22 (Ludox-40) | ~55–65 (DLS) | - | - | Up to 90 C | Water, Seawater, Arab D Brine | Up to 120k mg/dm3 (3 types of electrolytes) | - | >30 days | ||||
| Rouhana 2007 [76] | Disulfide Zwitterion (Zwitterion) | citrate-Au | 2.8 (QELS) 3.2 (TEM) | 3.2 (QELS) | - | - | - | - | BSA, Lysozyme | PSS, PAA, PDADMAC, PAH, PVBTMAC | - | |||
| Citrate (Misc) | Au | N/A | 2.8 (QELS) 3.4 (TEM) | - | - | - | - | BSA | PSS, PAA | - | ||||
| Tebbe 2015 [77] | Albumin/Proteins (NOM) | Au | ~80 nm (TEM) +Different aspect ratios | - | PBS, DMEM, NCS | 7.5 | - | −35 (pH 12) +20 (pH 1) | 2-12 | - | - | Up to 20 mg/mL biological media (PBS, DMEM) | - | - |
| Whitley 2013 [78] | PVP (Polymer) | Ag | 51.4 (DLS) 53 (TEM) | 51.4 (DLS) 53 (TEM) | DI Water | 6 | −24.7 | −101 * | - | - | - | - | Soil, Sewer Sludge (1% and 3% w/w) | - |
| Citrate (Misc) | Ag | 51.4 (DLS) 53 (TEM) | 84.4 (DLS) 84 (TEM) | DI Water | 6 | −24.7 | −24.7 * | - | - | - | - | Soil, Sewer Sludge (1% and 3% w/w) | - | |
| Yang W. 2008 [79] | pCBAA (Zwitterion) | Au | 18.5 (TEM) | 58.4 (DLS) | - | 7.4 | - | 20% NaCl | Up to 100% BSA, 1 mg/ml Lysozyme | - | - | |||
| PEG5000 (Polymer) | Au | 18.5 (TEM) | 53.4 (DLS) | - | 7.4 | - | - | - | - | - | ||||
| OEGMA (Polymer) | Au | 18.5 (TEM) | 74.3 (DLS) | - | 7.4 | - | - | - | - | - | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartwright, A.; Jackson, K.; Morgan, C.; Anderson, A.; Britt, D.W. A Review of Metal and Metal-Oxide Nanoparticle Coating Technologies to Inhibit Agglomeration and Increase Bioactivity for Agricultural Applications. Agronomy 2020, 10, 1018. https://doi.org/10.3390/agronomy10071018
Cartwright A, Jackson K, Morgan C, Anderson A, Britt DW. A Review of Metal and Metal-Oxide Nanoparticle Coating Technologies to Inhibit Agglomeration and Increase Bioactivity for Agricultural Applications. Agronomy. 2020; 10(7):1018. https://doi.org/10.3390/agronomy10071018
Chicago/Turabian StyleCartwright, Anthony, Kyle Jackson, Christina Morgan, Anne Anderson, and David W. Britt. 2020. "A Review of Metal and Metal-Oxide Nanoparticle Coating Technologies to Inhibit Agglomeration and Increase Bioactivity for Agricultural Applications" Agronomy 10, no. 7: 1018. https://doi.org/10.3390/agronomy10071018