Evaluation of the Changes in Thermal, Qualitative, and Antioxidant Properties of Terebinth (Pistacia atlantica) Fruit under Different Drying Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Treatments
2.2.1. Hot Air
2.2.2. Microwave
2.2.3. Infrared
2.2.4. Hot Air–Microwave
2.2.5. Hot Air–Infrared
2.3. Moisture Ratio
2.4. Calculation of Effective Moisture Diffusivity
2.5. Specific Energy Consumption
2.6. Energy Efficiency
2.7. Color
2.8. Shrinkage
2.9. Rehydration Ratio (RR)
2.10. Measurement of AntiOX Properties by Diphenyl Picrylhydrazine (DPPH) Method
2.11. Total Phenol Content (TPC)
2.12. Flavonoid Content (TFC)
2.13. pH
2.14. Statistical Analysis
3. Results and Discussion
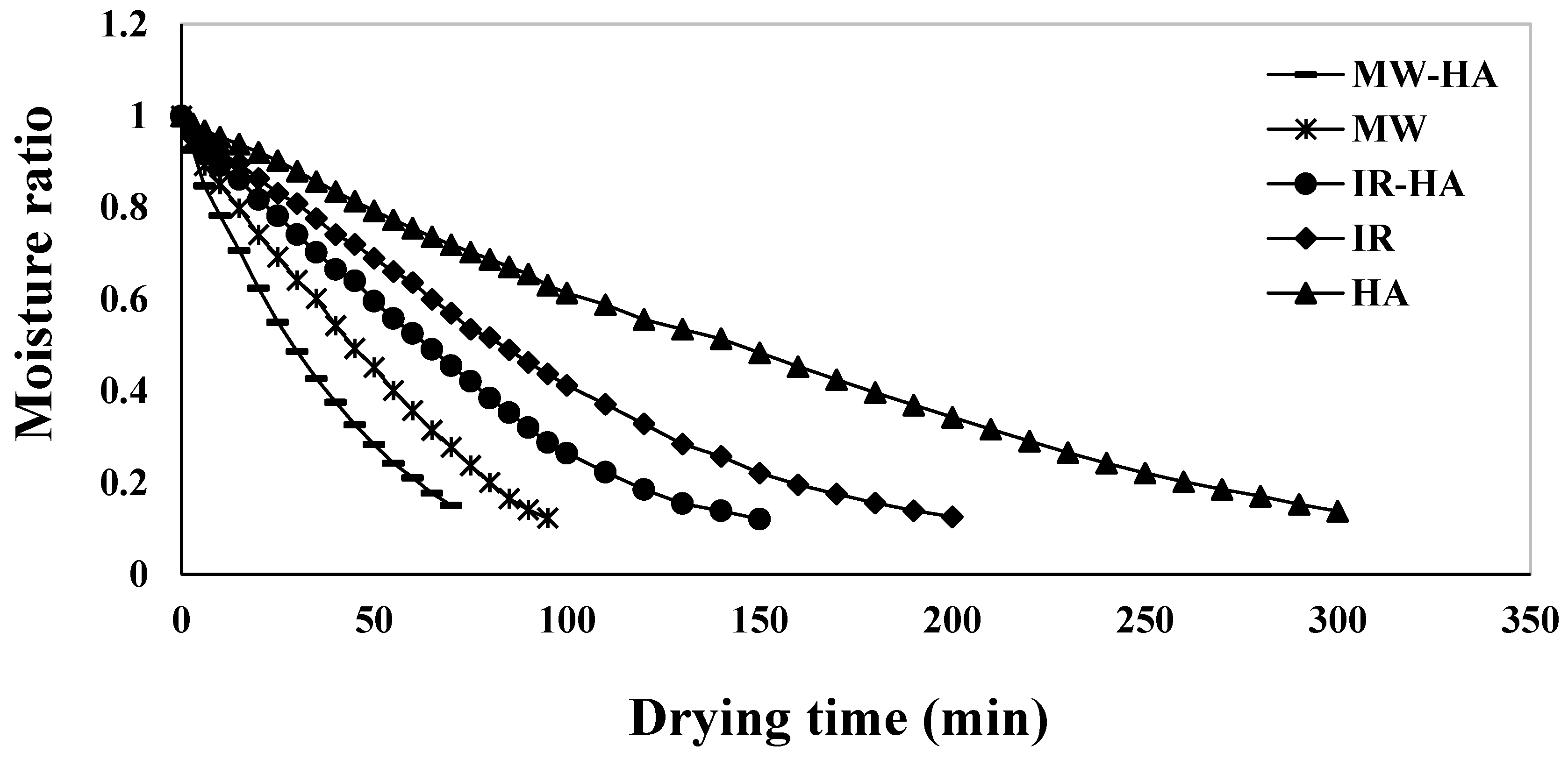
3.1. Drying Time
3.2. Effective Moisture Diffusivity
3.3. Specific Energy Consumption
3.4. Energy Efficiency
3.5. Color
3.6. Shrinkage (Sb)
3.7. Rehydration Ratio (RR)
3.8. AntiOX
3.9. TPC and TFC
3.10. pH
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Tray area (m2) | Rehydration ratio | ||
| Sample absorbance | Diameter (m) | ||
| Control absorbance | Specific energy consumption (kWh/kg) | ||
| Specific heat (kJ/kg °C) | Shrinkage (cm3) | ||
| Effective moisture diffusion coefficient (m2/s) | Drying time (min) | ||
| Energy consumed to evaporate moisture from drying samples (kJ) | Absolute temperature of drying air (K) | ||
| Thermal energy consumption (kJ) | Velocity (m/s) | ||
| Mechanical energy consumption (kJ) | Mass of removal water (kg) | ||
| Total energy input to dryer (MJ) | Weight after rehydration | ||
| Latent heat of vaporization (kJ/kg) | Initial weight of the samples (kg) | ||
| DPPH radical entrapping | Total color change | ||
| Lamp power (W) | , , | Differences between the color of the fresh and dried sample | |
| Moisture ratio | temperature difference (°C) | ||
| Initial moisture content (kg water/kg dry matter) | Air density (kg/m3) | ||
| Equilibrium moisture content (kg water/kg dry matter) | different pressure (mbar) | ||
| Moisture content at any time (kg water/kg dry matter) | Initial volume (cm3) | ||
| Mass of evaporated water from the product (kg) | Final volume (cm3) | ||
| Microwave output power (kW) | Energy efficiency (%) |
References
- Huang, J.; Zhang, M. Effect of three drying methods on the drying characteristics and quality of okra. Drying Technol. 2016, 34, 900–911. [Google Scholar] [CrossRef]
- Shewale, S.R.; Rajoriya, D.; Hebbar, H.U. Low humidity air drying of apple slices: Effect of EMR pretreatment on mass transfer parameters, energy efficiency and quality. Innov. Food Sci. Emerg. Technol. 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Osae, R.; Essilfie, G.; Alolga, R.N.; Bonah, E.; Ma, H.; Zhou, C. Drying of ginger slices—Evaluation of quality attributes, energy consumption, and kinetics study. J. Food Process. Eng. 2020, 43, e13348. [Google Scholar] [CrossRef]
- Minaei, S.; Motevali, A.; Najafi, G.; Seyedi, S.R.M. Influence of drying methods on activation energy, effective moisture diffusion and drying rate of pomegranate arils (Punica granatum). Aust. J. Crop. Sci. 2011, 6, 584–591. [Google Scholar]
- Ismail, O.; Kipcak, A.S.; Doymaz, I. Drying of okra by different drying methods: Comparison of drying time, product color quality, energy consumption and rehydration. Athens J. Sci. 2019, 6, 155–168. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Yang, H.; Wang, J.; Chen, H. Assessment of drying methods on the physiochemical property and antioxidant activity of Cordyceps militaris. Food Measure 2019, 13, 513–520. [Google Scholar] [CrossRef]
- Heshmati, M.K.; Moghaddam, A.S. Application of intermittent microwave—Convective hot air technique on quality and nutritional characteristics of dried kiwi slices. J. Food Res. 2017, 27, 111–126. (In Farsi) [Google Scholar]
- Dong, Q.; He, D.; Ni, X.; Zhou, H.; Yang, H. Comparative study on phenolic compounds, triterpenoids, and antioxidant activity of Ganoderma lucidum affected by different drying methods. Food Measure 2019. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Katsube, T.; Tsurunaga, Y.; Sugiyama, M.; Furuno, T.; Yamasaki, Y. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chem. 2009, 113, 964–969. [Google Scholar] [CrossRef]
- Wen, A.; Xie, C.; Mazhar, M.; Zhu, Y.; Zeng, H.; Qin, L.; Zhu, Y. Comparative evaluation of drying methods on kinetics, biocompounds and antioxidant activity of Bacillus subtilis-fermented dehulled adlay. Drying Technol. 2019. [Google Scholar] [CrossRef]
- Hatamnia, A.A.; Abbaspour, N.; Darvishzadeh, R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014, 145, 306–311. [Google Scholar] [CrossRef]
- Kaveh, M.; Abbaspour-Gilandeh, Y.; Chayjan, R.A.; Taghinezhad, E.; Mohammadigol, R. Mass transfer, physical, and mechanical characteristics of terebinth fruit (Pistacia atlantica L.) under convective infrared microwave drying. Heat Mass Transf. 2018, 54, 1879–1899. [Google Scholar] [CrossRef]
- Kaveh, M.; Amiri Chayjan, R.; Esna-Ashari, M. Thermal and physical properties modelling of terebinth fruit (Pistacia atlantica L.) under solar drying. Res. Agric. Eng. 2015, 61, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Duan, X.; Ren, G.; Liu, Y. Comparative study on the flavonoids extraction rate and antioxidant activity of onions treated by three different drying methods. Drying Technol. 2019, 37, 245–252. [Google Scholar] [CrossRef]
- Adiletta, G.; Alam, M.R.; Cinquanta, L.; Russo, P.; Albanese, D.; Di Matteo, M. Effect of Abrasive Pretreatment on Hot Dried Goji Berry. Chem. Eng. Trans. 2015, 44, 127–132. [Google Scholar]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G. The effectiveness of combined infrared and hot-air drying strategies for sweet Potato. J. Food Eng. 2019, 241, 75–87. [Google Scholar] [CrossRef]
- Lao, Y.; Zhang, M.; Devahastin, S.; Ye, Y. Effect of combined infrared freeze drying and microwave vacuum drying on quality of kale yoghurt melts. Drying Technol. 2019. [Google Scholar] [CrossRef]
- Patrón-Vázquez, J.; Baas-Dzul, L.; Medina-Torres, N.; Ayora-Talavera, T.; Sánchez-Contreras, A.; García-Cruz, U.; Pacheco, N. The Effect of drying temperature on the phenolic content and functional behavior of flours obtained from lemon wastes. Agronomy 2019, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Choo, C.O.; Chua, B.L.; Figiel, A.; Jałoszyński, K.; Wojdyło, A.; Szumny, A.; Łyczko, J.; Chong, C.H. Hybrid drying of Murraya koenigii leaves: Energy consumption, antioxidant capacity, profiling of volatile compounds and quality studies. Process 2020, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Tekin, Z.H.; Başlar, M.; Karasu, S.; Kilicli, M. Dehydration of green beans using ultrasound-assisted vacuum drying as a novel technique: Drying kinetics and quality parameters. J. Food Process Preserv. 2017, 41, e13227. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Lagnika, C.; Song, J.; Li, D.; Liu, C.; Jiang, N.; Zhang, M.; Duan, X. A comparative study of drying methods on physical characteristics, nutritional properties and antioxidant capacity of broccoli. Drying Technol. 2019. [Google Scholar] [CrossRef]
- Crank, J. Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975. [Google Scholar]
- Torki-Harchegani, M.; Ghanbarian, D.; Pirbalouti, A.G.; Sadeghi, M. Dehydration behaviour, mathematical modelling, energyefficiency and essentialoil yield of peppermint leaves under going microwave and hot air treatments. Rev. Energy Sustain. Renew. 2016, 58, 407–418. [Google Scholar] [CrossRef]
- Kaveh, M.; Chayjan, R.A.; Taghinezhad, E.; Sharabiani, V.R.; Motevali, A. Evaluation of specific energy consumption and GHG emissions for different drying methods (Case study: Pistacia atlantica). J. Clean. Prod. 2020, 59, 120963. [Google Scholar] [CrossRef]
- Motevali, A.; Tabatabaei, S.R.T. A comparison between pollutants and greenhouse gas emissions from operation of different dryers based on energy consumption of power plants. J. Clean. Prod. 2017, 154, 445–461. [Google Scholar] [CrossRef]
- Motevali, A.; Minaei, S.; Banakar, A.; Ghobadian, B.; Khoshtaghaza, M.H. Comparison of energy parameters in various dryers. Energy Convers. Manag. 2014, 87, 711–725. [Google Scholar] [CrossRef]
- Ghanbarian, D.; Torki-Harchegani, M.; Sadeghi, M.; Pirbalouti, A.G. Ultrasonically improved convective drying of peppermint leaves: Influence on the process time and energetic indices. Renew. Energy 2020, 153, 67–73. [Google Scholar] [CrossRef]
- Horuz, E.; Jaafar, H.J.; Maskan, M. Ultrasonication as pretreatment for drying of tomato slices in a hot air–microwave hybrid oven. Drying Technol. 2017, 35, 849–859. [Google Scholar] [CrossRef]
- Taghinezhad, E.; Kaveh, M.; Jahanbakhshi, A.; Golpour, I. Use of artificial intelligence for the estimation of effective moisture diffusivity, specific energy consumption, color and shrinkage in quince drying. J. Food Process Eng. 2020, e13358. [Google Scholar] [CrossRef]
- Roknul, A.S.M.; Zhang, M.; Law, C.L.; Mujumdar, A.S. Effects of drying methods on quality attributes of peach (Prunus persica) leather. Drying Technol. 2019, 37, 341–351. [Google Scholar] [CrossRef]
- Lin, M.; Tsai, M.; Wen, K. Supercritical fluid extraction of flavonoids from Scutellaria Radix. J. Chromatogr. A 1999, 830, 387–395. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassaova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Uni. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Beketove, E.V.; Pakhomov, V.P.P.; Nesterova, O.V. Improved method of flavonoid extraction from bird cherry fruits. J. Pharm. Chem. 2005, 39, 33–35. [Google Scholar] [CrossRef]
- Darvishi, H.; Saba, M.K.; Behroozi-Khazaei, N.; Nourbakhsh, H. Improving quality and quantity attributes of grape juice concentrate (molasses) using ohmic heating. J. Food Sci. Technol. 2020, 57, 1362–1370. [Google Scholar] [CrossRef]
- Motevali, A.; Hashemi, S.J. Investigating the drying parameters of Fijou fruit in a freeze dryer. Innov. Food Technol. 2018, 5, 699–713. (In Farsi) [Google Scholar]
- Riadh, M.H.; Ahmad, S.A.B.; Marhaban, M.H.; Soh, A.C. Infrared heating in food drying: An overview au-riadh, muhmmed hussain. Drying Technol. 2015, 33, 322–335. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Han, X.; Ni, Y.; Zhao, D.; Hao, J. Quality evaluation and drying kinetics of shitake mushrooms dried by hot air, infrared and intermittent microwave–assisted drying methods. LWT 2019, 107, 236–242. [Google Scholar] [CrossRef]
- Roknul, A.S.M.; Zhang, M.; Mujumdar, A.S.; Wang, Y. A comparative study of four drying methods on drying time and quality characteristics of stem lettuce slices (Lactuca sativa L.). Drying Technol. 2014, 32, 657–666. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Chayjan, R.A.; Kaveh, M.; Khayati, S. Modeling some thermal and physical characteristics of terebinth fruit under semi industrial continuous drying. Food Measure 2017, 11, 12–23. [Google Scholar] [CrossRef]
- Behera, G.; Sutar, P.P. Effect of convective, infrared and microwave heating on drying rates, mass transfer characteristics, milling quality and microstructure of steam gelatinized Paddy. J. Food Process Eng. 2018, 41, e12900. [Google Scholar] [CrossRef]
- Das, I.; Arora, A. Alternate microwave and convective hot air application for rapid mushroom drying. J. Food Eng. 2018, 223, 208–219. [Google Scholar] [CrossRef]
- Si, X.; Chen, Q.; Bi, J.; Wu, X.; Yi, J.; Zhou, L.; Li, Z. Comparison of different drying methods on the physical properties, bioactive compounds and antioxidant activity of raspberry powders. J. Sci. Food Agric. 2016, 96, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Kayacan, S.; Karasu, S.; Akman, P.K.; Goktas, H.; Doymaz, I.; Sagdıc, O. Effect of different drying methods on total bioactive compounds, phenolic profile, in vitro bioaccessibility of phenolic and HMF formation of persimmon. LWT 2020, 118, 108830. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Z.; Li, D.; Liu, C.; Zhang, M.; Liu, C.; Wang, D.; Niu, L. Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: Antioxidant properties, sensory quality, and energy consumption. LWT 2017, 82, 216–226. [Google Scholar] [CrossRef]
- Łechtanska, J.M.; Szadzinska, J.; Kowalski, S.J. Microwave- and infrared-assisted convective drying of green pepper: Quality and energy considerations. Chem. Eng. Process 2015, 98, 155–164. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Effects of microwave-infrared combination drying on quality of eggplants. Food Bioprocess. Technol. 2015, 8, 1198–1210. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Jahanbakhshi, A. The effect of microwave and convective dryer with ultrasound pre-treatment on drying and quality properties of walnut kernel. J. Food Process Preserv. 2019, 43, e14178. [Google Scholar] [CrossRef]
- Doymaz, I. Air-drying characteristics of tomatoes. J. Food Eng. 2008, 78, 1291–1297. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmianski, J. Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess. Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, M.; Zielinska, D. Effects of freezing, convective and microwave-vacuum drying on the content of bioactive compounds and color of cranberries. LWT 2019, 104, 202–209. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Poblete, J.; Quispe-Fuentes, I.; Uribe, E.; Bilbao-Sainz, C.; Pastén, A. Chemical and bioactive characterization of papaya (Vasconcellea pubescens) under different drying technologies: Evaluation of antioxidant and antidiabetic potential. Food Measure 2019, 13, 1980–1990. [Google Scholar] [CrossRef]
| No | Acronym | Description | Air | Power (W) | ||
|---|---|---|---|---|---|---|
| T (°C) | V (m/s) | MW | INF | |||
| 1 | HA | Convective | 65 | 1 | 0 | 0 |
| 2 | IR | Infrared | 0 | 0 | 0 | 500 |
| 3 | MW | Microwave | 0 | 0 | 450 | 0 |
| 4 | IR–HA | Infrared-assisted convective | 65 | 1 | 0 | 500 |
| 5 | MW–HA | Microwave-assisted convective | 65 | 1 | 450 | 0 |
| Equation | Equation Number | Equation | Reference |
|---|---|---|---|
| (4) | Et (HA)= Equation (4) + Equation (5) | [24] | |
| (5) | Et (MW)= Equation (7) | [25] | |
| (6) | Et (MW- HA)= Equation (4) + Equation (5) + Equation (7) | [26] | |
| (7) | Et (IR)= Equation (6) | [25] | |
| Et (IR- HA)= Equation (5) + Equation (6) | [27] |
| Equation | Equation Number | Reference | |
|---|---|---|---|
| (9) | [24] | ||
| (10) | [27] | ||
| (11) | [28] |
| S.O.V | Df | Time | Deff | SEC | ηe | Color | RR | Sb (%) |
|---|---|---|---|---|---|---|---|---|
| Drying method | 4 | 25185.00 ** | 1.01 ** | 5876.75 ** | 314.65 ** | 32.06 ** | 1.18 ** | 379.15 ** |
| error | 10 | 55 | 7.83 | 2.62 | 0.177 | 0.30 | 0.002 | 0.282 |
| CV | 4.54 | 2.97 | 2.38 | 2.75 | 3.47 | 1.98 | 1.16 |
| S.O.V | Df | TPC | TFC | AntiOX | pH |
|---|---|---|---|---|---|
| Drying method | 5 | 1459.97 ** | 179.59 ** | 1174.51 ** | 0.0095 ** |
| error | 10 | 6.32 | 0.55 | 2.62 | 0.00015 |
| CV | 1.85 | 2.40 | 1.55 | 0.31 |
| HA | IR | MW | IR–HA | MW–HA | |
|---|---|---|---|---|---|
| Drying time (min) | 300 ± 12a | 200 ± 7b | 95 ± 5d | 150 ± 9c | 70 ± 4e |
| Reduced drying time (%) | - | 33 | 68 | 50 | 76 |
| Deff (m2/s) | 5.95 × 10−10e | 2.14 × 10−9d | 4.50 × 10−9b | 2.50 × 10−9c | 5.07 × 10−9a |
| SEC (kWh/kg) | 124.33 ± 2.64a | 55.55 ± 3.09c | 23.75 ± 4.22b | 103.83 ± 2.67e | 32.35 ± 3.89d |
| ηe (%) | 5.65 ± 0.45e | 12.67 ± 0.69c | 29.64 ± 0.75a | 6.76 ± 0.42d | 21.76 ± 0.51b |
| Drying Methods | Color | Sb | RR | pH |
|---|---|---|---|---|
| HA | 20.22 ± 0.31a | 63.64 ± 2.20a | 1.72 ± 0.07e | 3.88 ± 0.009c |
| IR | 17.76 ± 0.47b | 54.49 ± 3.06b | 2.19 ± 0.10d | 3.91 ± 0.006b |
| MW | 12.20 ± 0.51e | 25.29 ± 2.46e | 3.27 ± 0.06a | 3.97 ± 0.007a |
| IR–HA | 15.21 ± 0.38c | 47.45 ± 2.01c | 2.67 ± 0.09c | 3.93 ± 0.006b |
| MW–HA | 13.29 ± 0.61d | 36.67 ± 1.59d | 3.03 ± 0.08b | 3.96 ± 0.008a |
| Drying Methods | AntiOX (%) | TPC (mg GAE/g d.w.) | TFC (mg QE/g d.w.) |
|---|---|---|---|
| Fresh | 98.81 ± 1.8a | 165.27 ± 4.04a | 43.41 ± 0.85a |
| HA | 44.04 ± 1.62f | 103.08 ± 3.39f | 23.35 ± 0.90f |
| IR | 68.59 ± 1.55e | 120.00 ± 2.94e | 23.78 ± 1.06e |
| MW | 74.01 ± 2.29d | 134.10 ± 4.09d | 30.30 ± 0.82c |
| IR–HA | 76.17 ± 1.94c | 143.59 ± 5.97c | 27.26 ± 0.96d |
| MW–HA | 94.58 ± 1.95b | 149.13 ± 4.25b | 36.83 ± 0.74b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbaspour-Gilandeh, Y.; Kaveh, M.; Fatemi, H.; Hernández-Hernández, J.L.; Fuentes-Penna, A.; Hernández-Hernández, M. Evaluation of the Changes in Thermal, Qualitative, and Antioxidant Properties of Terebinth (Pistacia atlantica) Fruit under Different Drying Methods. Agronomy 2020, 10, 1378. https://doi.org/10.3390/agronomy10091378
Abbaspour-Gilandeh Y, Kaveh M, Fatemi H, Hernández-Hernández JL, Fuentes-Penna A, Hernández-Hernández M. Evaluation of the Changes in Thermal, Qualitative, and Antioxidant Properties of Terebinth (Pistacia atlantica) Fruit under Different Drying Methods. Agronomy. 2020; 10(9):1378. https://doi.org/10.3390/agronomy10091378
Chicago/Turabian StyleAbbaspour-Gilandeh, Yousef, Mohammad Kaveh, Hamideh Fatemi, José Luis Hernández-Hernández, Alejandro Fuentes-Penna, and Mario Hernández-Hernández. 2020. "Evaluation of the Changes in Thermal, Qualitative, and Antioxidant Properties of Terebinth (Pistacia atlantica) Fruit under Different Drying Methods" Agronomy 10, no. 9: 1378. https://doi.org/10.3390/agronomy10091378








