Changes in Wheat Rhizosphere Carbon Pools in Response to Nitrogen and Straw Incorporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Site and Experimental Detail
2.2. Collection, Preparation, and Analysis of Soil and Plants
2.3. Statistical Analysis
3. Results
3.1. Labile C Pools
3.1.1. Water-Soluble Organic Carbon (WSOC)
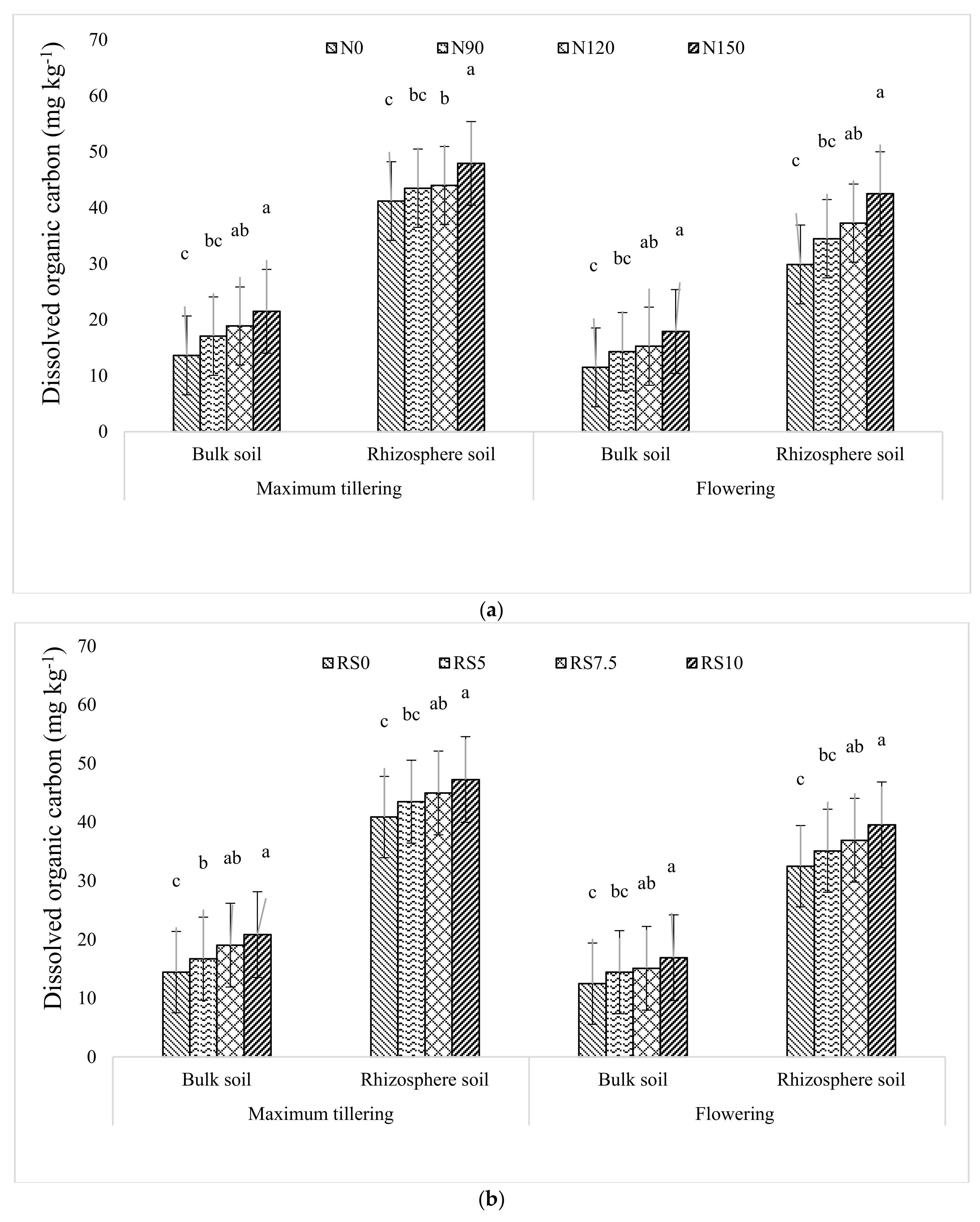
3.1.2. Dissolved Organic Carbon (DOC)
3.1.3. Dichromate Oxidized Carbon (DOXC)
3.1.4. Permanganate Oxidized Carbon (POXC)
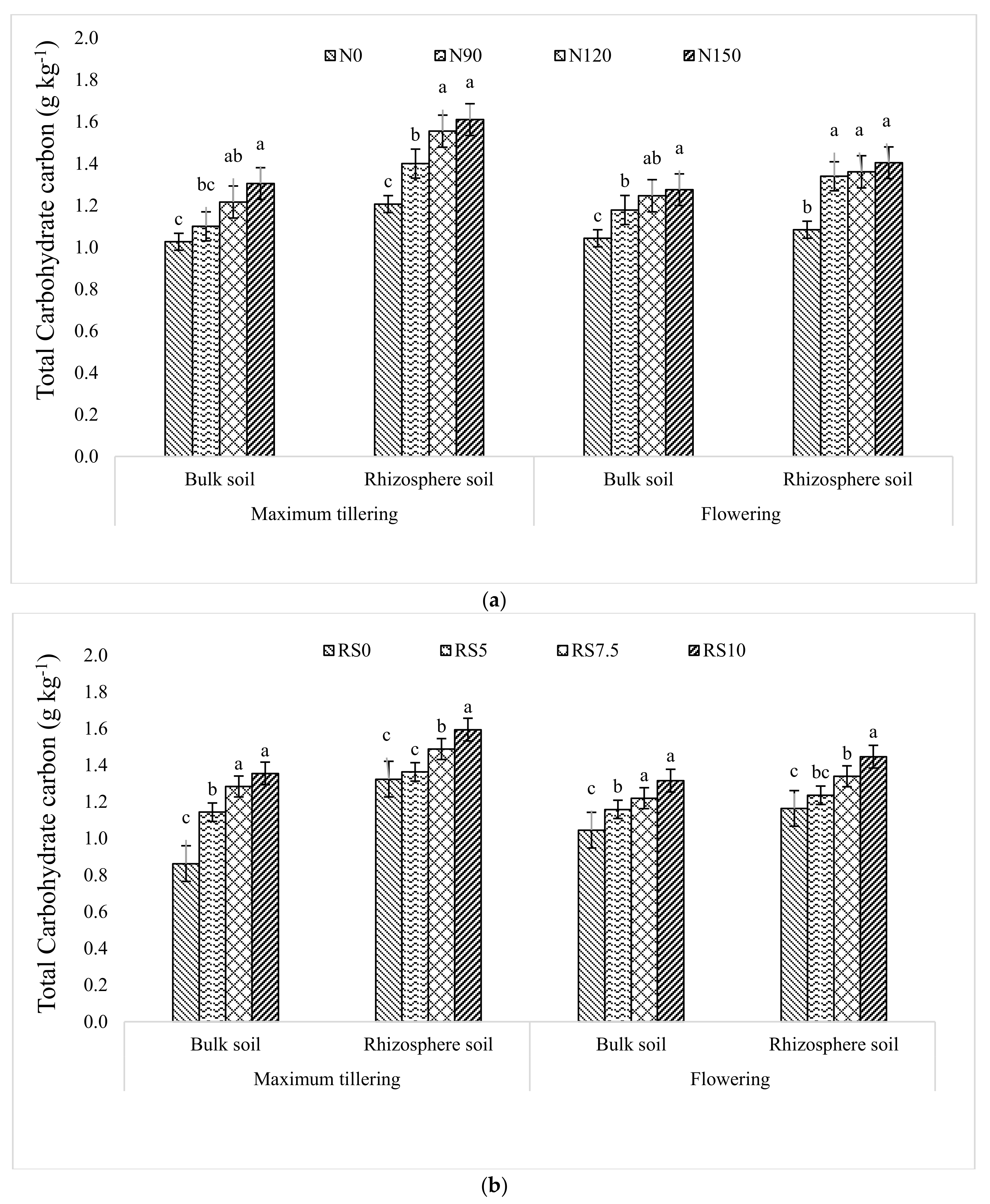
3.1.5. Total Carbohydrate Carbon (TCC)
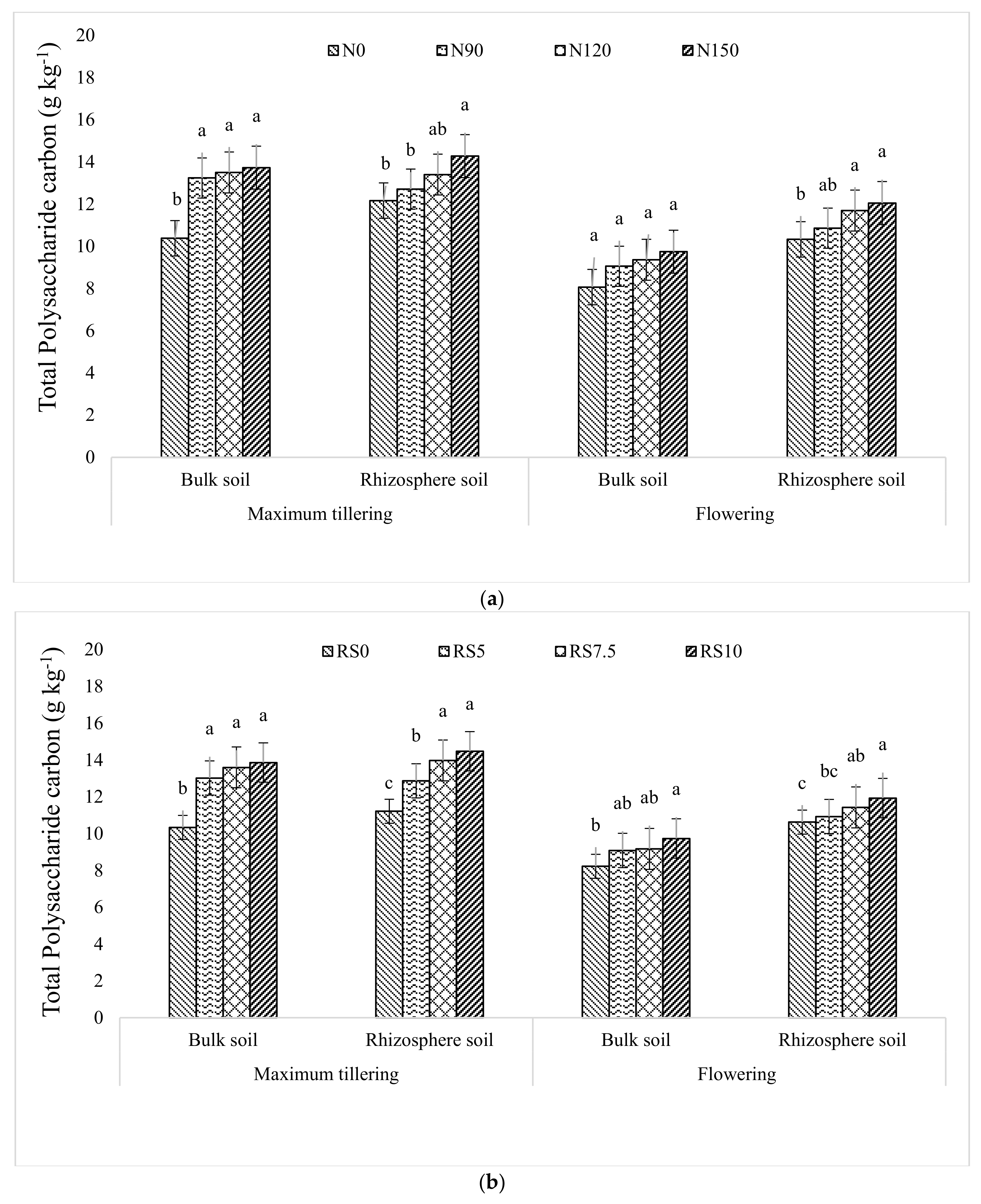
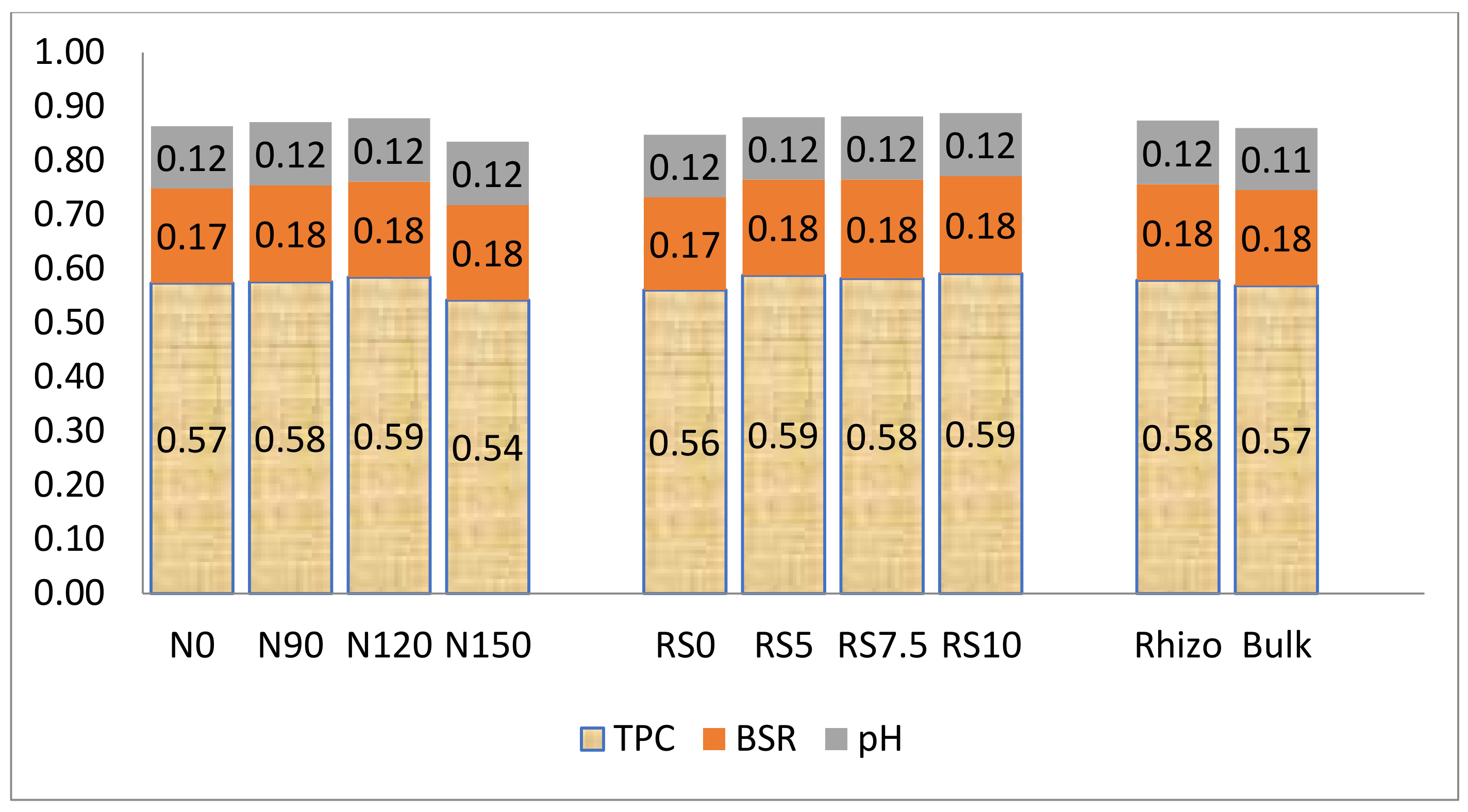
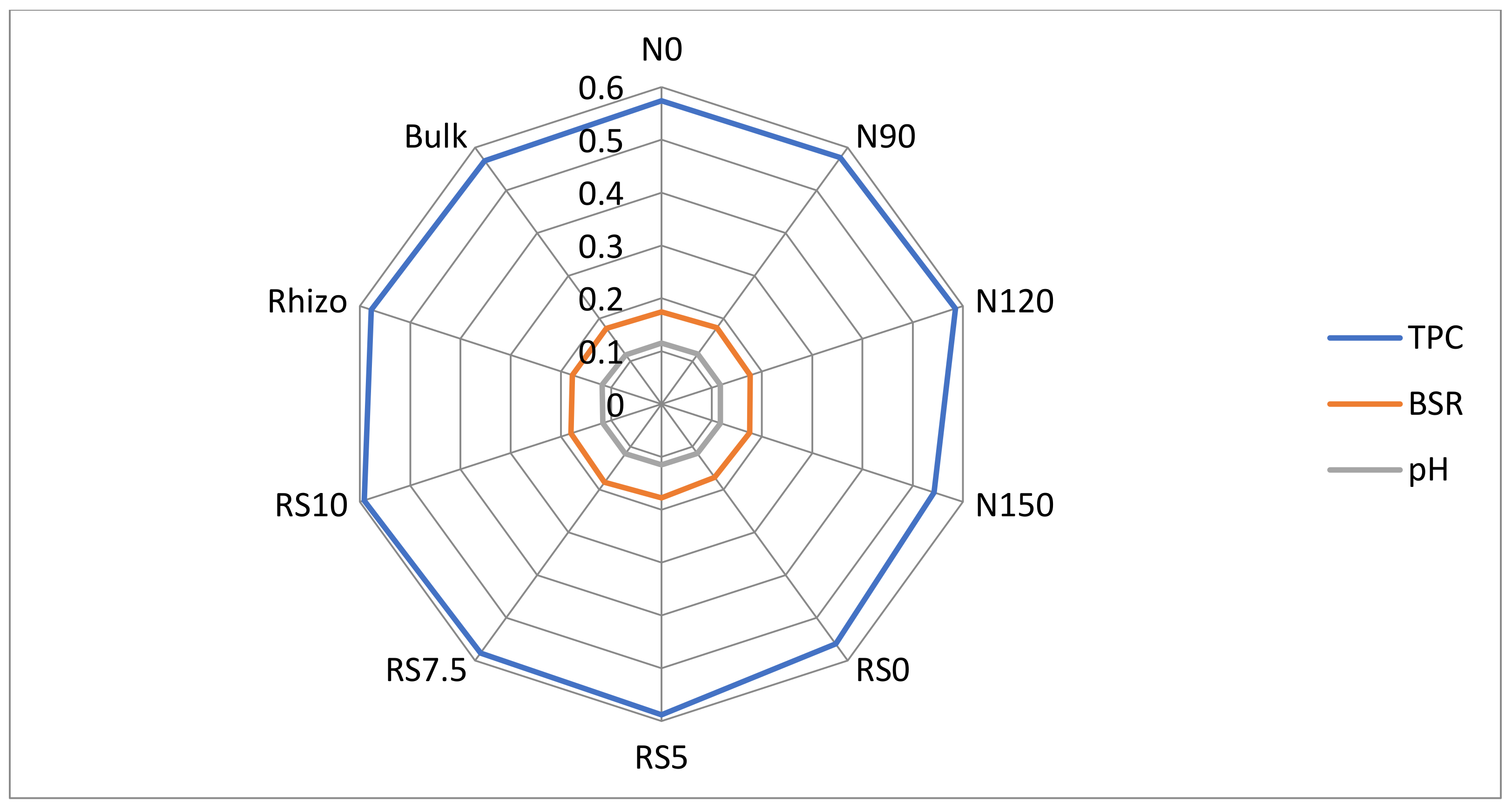
3.1.6. Total Polysaccharide Carbon (TPC)
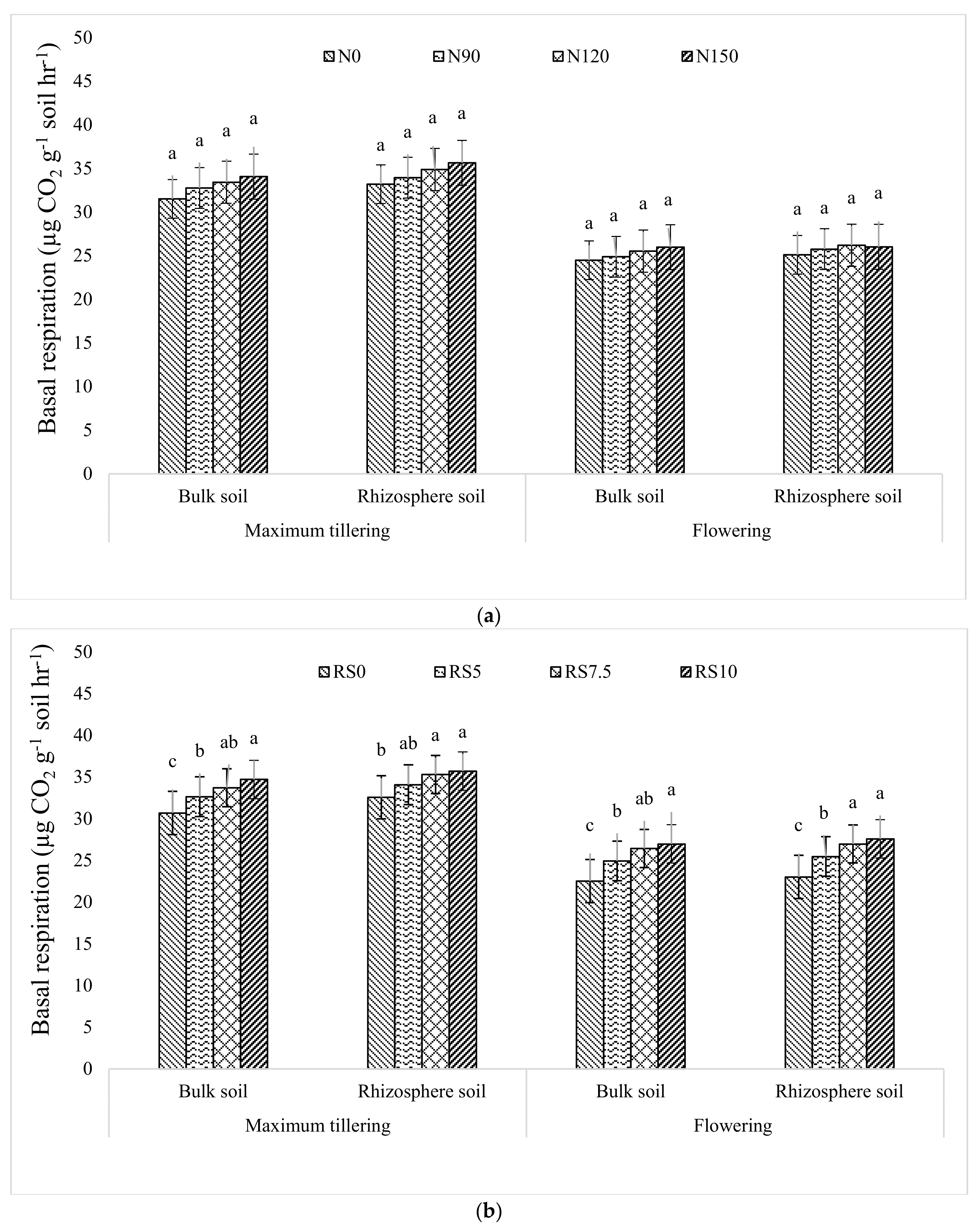
3.1.7. Basal Respiration Rate (BSR)
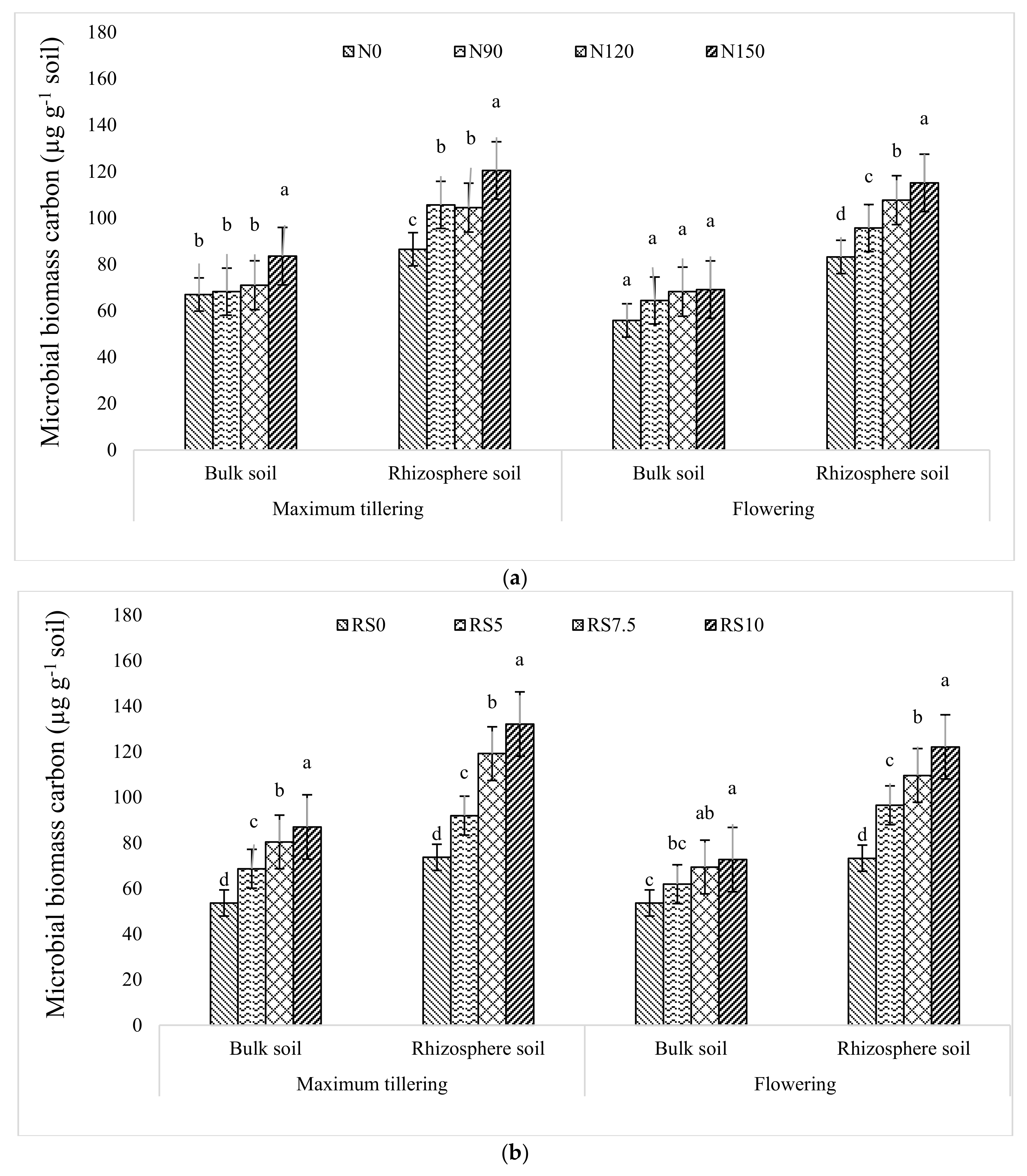
3.1.8. Microbial Biomass Carbon (MBC)
3.2. Wheat Yield and Nutrient Uptake
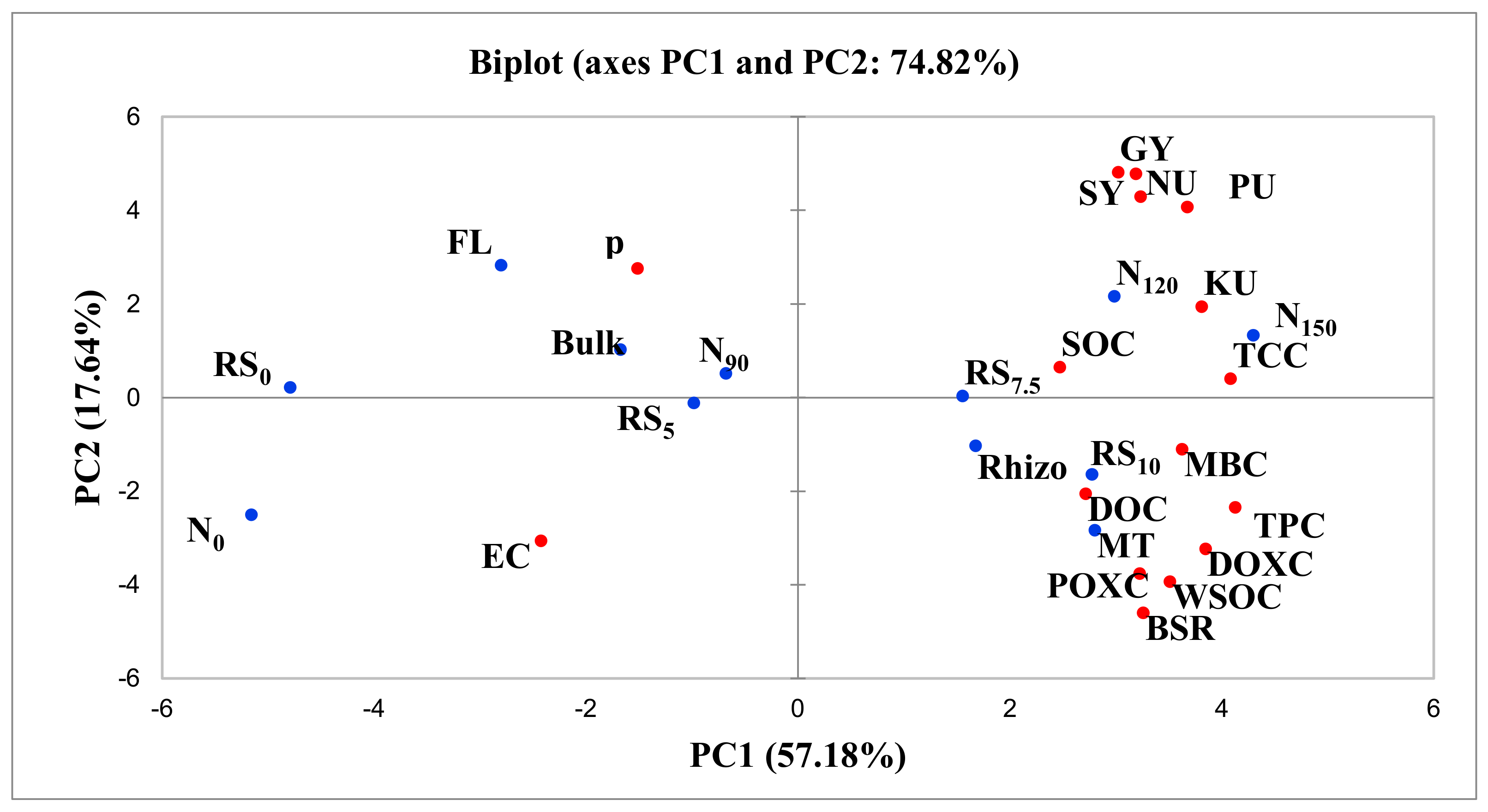
3.3. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.S.; Singh, A.K.; Sundaram, P.K. Agro-technological options for upscaling agricultural productivity in eastern Indo-Gangetic plains under impending climate change situations: A Review. J. Agrisearch 2014, 1, 55–65. [Google Scholar]
- Dhillon, B.S.; Kataria, P.; Dhillon, P.K. National food security vis-a-vis sustainability of agriculture in high crop productivity regions. Curr. Sci. 2010, 98, 33–36. [Google Scholar]
- Ladha, J.K.; Fisher, K.S.; Hossain, M.; Hobbs, P.R.; Hardy, B. Improving the Productivity of Rice-Wheat Systems of the Indo-Gangetic Plains: A Synthesis of NARS-IRRI Partnership Research; IRRI Discussion, Paper No. 40; IRRI: Los Banos, Philippines, 2000. [Google Scholar]
- Humphreys, E.; Kukal, S.S.; Christen, E.W.; Hira, G.S.; Singh, B.; Yadav, S.; Sharma, R.K. Halt in the groundwater decline in north-west India: Which crop technologies will be winner. Adv. Agron. 2010, 109, 155–217. [Google Scholar]
- Dwivedi, B.S.; Singh, V.K.; Shukla, A.K.; Meena, M.C. Optimizing dry and wet tillage for rice on a Gangetic alluvial soil: Effect on soil characteristics, water use efficiency and productivity of the rice-wheat system. Eur. J. Agron. 2012, 43, 155–165. [Google Scholar] [CrossRef]
- Singh, Y.; Sidhu, M.; Humphreys, H.S.; Thind, E.; Jat, H.S.; Blackwell, M.L.; Singh, J.V. Nitrogen management for zero till wheat with surface retention of rice residues in north-west India. Field Crops Res. 2015, 184, 183–191. [Google Scholar] [CrossRef]
- Parihar, C.M.; Yadav, M.R.; Jat, S.L.; Singh, A.K.; Kumar, B.; Pradhan, S. Long term effect of conservation agriculture in maize rotations on total organic carbon, physical and biological properties of a sandy loam soil in north-western Indo-Gangetic Plains. Soil Till. Res. 2016, 16, 116–128. [Google Scholar] [CrossRef]
- NAAS. Innovative Viable Solution to Rice Straw Burning in Rice-Wheat Cropping System through Concurrent Use of Super Straw Management System-Fitted Combines and Turbo Happy Seeder; Policy Brief No. 2; National Academy of Agricultural Sciences: New Delhi, India, 2017; p. 16. [Google Scholar]
- Singh, Y.; Sidhu, H.S. Management of cereal crop residues for sustainable rice-wheat production system in the Indo-Gangetic plains of India. Proc. Indian Natl. Sci. Acad. 2014, 80, 95–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.Q.; Tang, Z.H.; Chen, X.H.; Yu, Y.S. Burning straw, air pollution and respiratory infections in China. Am. J. Infect. Control 2014, 7, 816–817. [Google Scholar] [CrossRef]
- Sharma, S.; Vashisht, B.B.; Singh, P.; Singh, Y. Changes in soil aggregate-associated carbon, enzymatic activity and biological pools under conservation agriculture based practice in rice-wheat system. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Loveland, P.; Webb, J. Is there a critical level of organic matter in the agricultural soils of temperate regions: A review. Soil Till. Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, S.; Choudhary, O.P.; Singh, M. Tillage, green manure and residue retention improves aggregate-associated phosphorus fractions under rice-wheat cropping. Sci. Rep. 2022, 12, 7167. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar]
- Jin, K.; Sleutel, S.; Buchan, D.; De, N.S.; Cai, D.X.; Gabriels, D.; Jin, J.Y. Changes of soil enzyme activities under different tillage practices in the Chinese Loess Plateau. Soil Till. Res. 2009, 104, 115–120. [Google Scholar] [CrossRef]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizable organic carbon fractions and soil quality in an oxicpaleustalf under different pasture leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Mazumdar, S.P.; Kundu, D.K.; Saha, A.R.; Majumdar, B.; Saha, R.; Singh, A.K.; Barman, D.; Ghosh, D.; Dey, R.; Behera, M.S.; et al. Carbon and nutrient dynamics under long-term nutrient management in tropical rice-wheat-jute system. Arch. Agron. Soil Sci. 2018, 64, 1595–1607. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, B.; Ladha, J.K.; Khind, C.S.; Khera, T.S.; Bueno, C.S. Effects of residue decomposition on productivity and soil fertility in rice–wheat rotation. Soil Sci. Soc. Am. J. 2004, 68, 854–864. [Google Scholar] [CrossRef]
- Singh, V.K.; Dwivedi, B.S.; Singh, S.K.; Majumdar, K.; Jat, M.L.; Mishra, R.P.; Rani, M. Soil physical properties, yield trends and economics after five years of conservation agriculture based rice-maize system in north-western India. Soil Till. Res. 2016, 155, 133–148. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Sharma, P.C.; Kumar, V.; Yadav, A.K.; Choudhary, M.; Choudhary, V.; Gathala, M.K.; Sharma, D.K.; Jat, M.L.; et al. Assessing soil properties and nutrient availability under conservation agriculture practices in a reclaimed sodic soil in cereal-based systems of North-West India. Arc. Agron. Soil Sci. 2017, 64, 531–545. [Google Scholar] [CrossRef] [Green Version]
- Parihar, C.M.; Jat, S.L.; Singh, A.K.; Datta, A.; Parihar, M.D.; Varghese, E.; Bandyopadhyoy, K.K.; Nayak, H.S.; Kurif, B.R.; Jat, M.L. Changes in carbon pools and biological activities of a sandy loam soil under medium-term conservation agriculture and diversified cropping systems. Eur. J. Soil Sci. 2018, 69, 902–912. [Google Scholar] [CrossRef]
- Sharma, S.; Thind, H.S.; Singh, Y.; Sidhu, H.S.; Jat, M.L.; Parihar, C.M. Effects of crop residue retention on soil carbon pools after 6 years of rice–wheat cropping system. Environ. Earth Sci. 2019, 78, 296–310. [Google Scholar] [CrossRef]
- Chen, H.Q.; Hou, R.X.; Gong, Y.S.; Li, H.W.; Fan, M.S.; Kuzyakov, Y. Effects of 11 years of conservation tillage on soil organic matter fractions in wheat monoculture in Loess Plateau of China. Soil Till. Res. 2009, 106, 85–94. [Google Scholar] [CrossRef]
- Luo, G.; Ling, N.; Nannipieri, P.; Chen, H.; Raza, W.; Wang, M.; Guo, S.; Shen, Q. Long-term fertilization regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fertil. Soils 2017, 53, 375–388. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, P.; Wang, K.; Ding, R.; Yang, B.; Nie, J.; Jia, Z.; Han, Q. Effects of wheat straw incorporation on the availability of soil nutrients and enzyme activities in semiarid areas. PLoS ONE 2015, 10, e0120994. [Google Scholar] [CrossRef] [Green Version]
- Bera, T.; Sharma, S.; Thind, H.S.; Sidhu, H.S.; Jat, M.L. Soil biochemical changes at different wheat growth stages in response to conservation agriculture practices in a rice-wheat system of north-western India. Soil Res. 2017, 56, 91–104. [Google Scholar] [CrossRef]
- Saikia, R.; Sharma, S.; Thind, H.S.; Sidhu, H.S.; Singh, Y. Temporal changes in biochemical indicators of soil quality in response to tillage, crop residue and green manure management in a rice-wheat system. Ecol. Ind. 2019, 103, 383–394. [Google Scholar] [CrossRef]
- Saggar, S.; Hedley, C.B.; Salt, G.J. Soil microbial biomass, metabolic quotient, and carbon and nitrogen mineralisation in 25-year-old Pinusradiata agroforestry regimes. Aust. J. Soil Res. 2019, 9, 491–504. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, A.L. Estimation of Available Phosphorus in Soils by Extraction on with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954; Volume 939, p. 19. [Google Scholar]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, sodium and potassium. In Methods of Soil Analysis Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Singh, S.; Sharma, S. Temporal changes in rhizosphere biological soil quality indicators of wheat in response to nitrogen and straw incorporation. Trop. Ecol. 2020, 61, 328–344. [Google Scholar] [CrossRef]
- Zhang, S.R.; Wen, J.; Li, T.; Xu, X.X.; Deng, L.J.; Gong, G.S.; Hu, C. Soil carbon fractions of restored lands in Liusha River Valley Sichuan. Ecol. Eng. 2012, 40, 27–36. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lise, L. 1995. Soil carbon fractions based on their degree of oxidation and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Safarik, I.; Santruckova, H. Direct determination of total soil carbohydrate content. Plant Soil. 1992, 143, 109–114. [Google Scholar] [CrossRef]
- Lowe, L.E. Total and labile polysaccharide analysis of soils. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Canadian Society of Soil Science: Boca Raton, FL, USA, 1993; pp. 373–376. [Google Scholar]
- Anderson, J.P.E. Soil respiration. In Methods of Soil Analysis, Chemical and Microbiological Properties, Agronomy Monograph No. 9; Miller, R.H., Keeney, D.R., Eds.; ASA-SSSA Publisher: Madison, WI, USA, 1982; pp. 831–871. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass carbon. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Westerman, R.L. Soil Testing and Plant Analysis, 3rd ed.; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1990; p. 20. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter: Laboratory methods. In Methods of Soil Analysis, Part 3 Chemical Methods; Barteles, J.M., Bigham, J.M., Eds.; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- International Rice Research Institute (IRRI). IRRISTAT for Window (CD-ROM), version 4.02b; IRRI: Los Banos, Philippines, 2000.
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemometr. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Zhu, B.; Gutknecht, J.L.M.; Herman, D.J.; Keck, D.C.; Firestone, M.K.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol. Biochem. 2014, 76, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Sakagami, K.; Tanaka, H.; Hamada, R. Role of soil organic matter in stabilization of water-stable aggregates in soils under different types of land use. Soil Sci. Plant Nutr. 1998, 44, 147–155. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Meng, S.; Zheng, X.; Zhou, Z.; Han, S.; Chen, D.; Yang, Z. Effects of irrigation, fertilization and crop straw management on nitrous oxide and nitric oxide emissions from a wheat–maize rotation field in northern China. Agric. Ecosys. Environ. 2011, 140, 226–233. [Google Scholar] [CrossRef]
- Soon, Y.K.; Lupwayi, N.Z. Straw management in a cold semi-arid region: Impact on soil quality and crop productivity. Field Crops Res. 2012, 139, 39–46. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Pu, Y.; Li, T.; Xu, X.; Jia, Y.; Deng, O.; Gong, G. Dynamics of soil labile organic carbon fractions and C-cycle enzyme activities under straw mulch in Chengdu Plain. Soil Till. Res. 2016, 155, 289–297. [Google Scholar] [CrossRef]
- Soltani, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Shrivani, M.; Schulin, R. The effect of preceding crop on wheat grain zinc concentration and its relationship to total amino acids and dissolved organic carbon in rhizosphere soil solution. Biol. Fertil. Soils 2014, 50, 239–247. [Google Scholar] [CrossRef]
- Madejon, E.; Moreno, F.; Murillo, J.M.; Pelegrin, F. Soil biochemical response to long term conservation tillage under semi arid Mediterranean conditions. Soil Till. Res. 2007, 94, 346–352. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Conteh, A.; Blair, G.T.; Lefroy, R.D.B.; Whitbread, A.M. Labile organic carbon determined by permanganate oxidation and its relationships to other measurements of soil organic carbon. Hum. Subst. Environ. J. 1999, 1, 3–15. [Google Scholar]
- Kaur, T.; Brar, B.S. Organic matter, microbial biomass and enzyme activity of soils under maize-wheat cropping system. J. Soils Crops 2008, 18, 24–30. [Google Scholar]
- Rudrappa, L.; Purakayastha, T.J.; Singh, D.B.S. Long-term manuring and fertilization effects on soil organic carbon pools in a Typic Haplustept of semi-arid sub-tropical India. Soil Till. Res. 2006, 88, 180–192. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-term manure and fertilizer effects on soil organic matter fractions and microbes under a wheat-maize cropping system in northern China. Geoderma 2009, 149, 318–324. [Google Scholar] [CrossRef]
- Kumar, K.; Shukla, U.N.; Kumar, D.; Pant, A.K.; Prasad, S.K. Bio-Fertilizers for Organic Agriculture. Pop. Kheti 2013, 1, 91–96. [Google Scholar]
- Cheshire, M.V. Origin and stability of soil polysaccharides. J. Soil Sci. 1977, 28, 1–10. [Google Scholar] [CrossRef]
- Oades, J.M. Soil organic matter and structural stability: Mechanisms and implications for management. Plant Soil 1984, 76, 319–337. [Google Scholar] [CrossRef]
- Baldock, J.A.; Kay, B.D.; Schnitzer, M. Influence of cropping treatments on the monosaccharide content of the hydrolysates of a soil and its aggregate fractions. Can. J. Soil Sci. 1987, 67, 489–499. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G.; Valori, F. Microbial diversity and microbial activity in the rhizosphere. Cienc. Suelo 2007, 25, 89–97. [Google Scholar]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Nabi, G. Soil carbon and nitrogen mineralization dynamics following incorporation and surface application of rice and wheat residues. Soil Environ. 2016, 35, 207–215. [Google Scholar]
- Samuelson, L.; Mathew, R.; Stokes, T.; Feng, Y.; Aubrey, D.; Coleman, M. Soil and microbial respiration in a loblolly pine plantation in response to seven years of irrigation and fertilization. For. Ecol. Manag. 2009, 258, 2431–2438. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Kundu, S.; Srivastva, A.K.; Gupta, H.S.; Prakash, V.; Bhatt, J.C. Long term fertilization effects on soil organic carbon pools in a sandy loam soil of the Indian Himalayas. Plant Soil 2011, 341, 109–124. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Shen, Y. Comparison of soil microbial community catabolic diversity between rhizosphere and bulk soil induced by tillage or residue retention. J. Soil Sci. Plant Nutr. 2013, 13, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, P. Faunal Activities and Soil Processes: Adaptive Strategies That Determine Ecosystem Function. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: Cambridge, MA, USA, 1997; pp. 93–132. [Google Scholar]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashan, Y.; Holguin, G. Azospirillum-plant relationships: Environmental and physiological advances. Can. J. Microbiol. 1997, 43, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Saggar, S.; Yeates, G.W.; Shephard, T.G. Cultivation effects on soil biological properties, microfauna and organic matter dynamics in Eutric Gleysol and Gleyic Luvisol soils in New Zealand. Soil Till. Res. 2001, 58, 55–68. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Liang, B.C.; Drury, C.F.; Mackenzie, A.F.; McGill, W.B. Elucidation of the source and turnover of water soluble and microbial biomass carbon in agricultural soils. Soil Biol. Biochem. 2000, 32, 581–587. [Google Scholar] [CrossRef]
- Witt, C.; Cassman, K.G.; Olk, D.C. Crop rotation and residue management effects on carbon sequestration, nitrogen cycling and productivity of irrigated rice systems. Plant Soil 2000, 225, 263–278. [Google Scholar] [CrossRef]
- Wang, G.H.; Dobermann, A.; Witt, C.; Sun, Q.Z.; Fu, R.X. Performance of site-specific nutrient management for irrigated rice in southeast China. Agron. J. 2001, 93, 869–878. [Google Scholar] [CrossRef]
- Kumari, K.; Prasad, J.; Solanki, I.S.; Chaudhary, R. Long-term effect of crop residues incorporation on yield and soil physical properties under rice-wheat cropping system in calcareous soil. J. Soil Sci. Plant Nutr. 2018, 18, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Eberbach, P.L.; Humphreys, E.; Kukal, S.S. The effect of rice straw mulch on evapo-transpiration, transpiration and soil evaporation of irrigated wheat in Punjab, India. Agric. Water Manag. 2011, 98, 1847–1855. [Google Scholar] [CrossRef]
- Ram, A. Management of Sulphur Deficiency for Improving Productivity and Soil Health in Aerobic Rice (Oryza sativa L.)–Wheat (Triticum aestivum L.) Cropping System. Ph.D. Dissertation, Indian Council of Agricultural Research, New Delhi, India, 2013. [Google Scholar]
- Zhang, G.; Bai, J.; Xi, M.; Zhao, Q.; Lu, Q.; Jia, J. Soil quality assessment of coastal wetlands in the yellow river delta of China based on the minimum data set. Ecol. Ind. 2016, 66, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Hazra, G.C.; Purakayastha, T.J.; Saha, N.; Mitran, T.; Roy, S.S.; Basak, N.; Mandal, B. Establishment of critical limits of indicators and indices of soil quality in rice-rice cropping systems under different soil orders. Geoderma 2017, 292, 34–48. [Google Scholar] [CrossRef]
- Amezketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Baldrian, P.; Trogl, J.; Frouz, J.; Snajdr, J.; Valaskova, V.; Erhautova, V. Enzyme activities and microbial biomass in topsoil layer during spontaneous succession in spoil heaps after brown coal mining. Soil Biol. Biochem. 2008, 40, 2107–2115. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Carter, M.R.; Angers, D.A.; Monreal, C.M.; Ellert, B.H. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 4. [Google Scholar] [CrossRef] [Green Version]
- Dalal, R.C.; Meyer, R.J. Long-term trends in fertility of soils under continuous cultivation and cereal cropping in Southern Queensland. VII. Dynamics of nitrogen mineralization potentials and microbial biomass. Aust. J. Soil Res. 1987, 25, 461–472. [Google Scholar] [CrossRef]
- Smith, J.L.; Paul, E.A. The significance of soil microbial biomass estimations. In Soil Biochemistry; Bollag, G.M., Stotzky, G., Eds.; Marcel Dekker: New York, NY, USA, 1990; Volume 6, pp. 357–396. [Google Scholar]
- Ghoshal, S.K. Studies on Biological Attributes of Soil Quality for a Few Long-Term Fertility Experiments with Rice Base Cropping Systems. Ph.D. Thesis, Bidhan Cahndra Krishi Viswavidyalaya, Mohanpur, India, 2014. [Google Scholar]
- Shahid, M.; Nayak, A.K.; Shukla, A.K.; Tripathi, R.; Kumar, A.; Mohanty, S.; Bhattacharyya, P.; Raja, R.; Panda, B.B. Long-term effects of fertilizer and manure applications on soil quality and yields in a sub-humid tropical rice-rice system. Soil Use Manag. 2013, 29, 322–332. [Google Scholar] [CrossRef]
- Herrera, C.M.A.; Mora, J.L.; Arbelo, C.D.; Rodríguez-Rodríguez, A. Interannual variations of soil organic carbon fractions in unmanaged volcanic soils (Canary Islands, Spain). Ecol. Evol. 2012, 2, 2374–2386. [Google Scholar] [CrossRef] [PubMed]



| Soil Biochemical Properties | Method Used | Reference |
|---|---|---|
| Water-soluble carbon (WSOC) | 1:5 soil–water suspensions | [33] |
| Dissolved organic carbon (DOC) | 1:5 soil–KCl suspensions | [33] |
| Dichromate oxidized carbon (DOXC) | Oxidized with 0.07 N K2Cr2O7 under 6 N sulphuric acid | [16] |
| Permanganate oxidized carbon (POXC) | 33 mM KMnO4 | [34] |
| Total carbohydrate carbon (TCC) | By phenol method without acid hydrolysis | [35] |
| Total polysaccharide carbon (TPC) | Polysaccharide carbon digested with 12 M H2SO4 and estimated by phenol sulphuric acid method | [36] |
| Basal soil respiration (BSR) | Alkali trap method | [37] |
| Microbial biomass carbon (MBC) | CHCl3 fumigation-extraction method | [38] |
| Treatment | Grain Yield (t ha−1) | Straw Yield (t ha−1) | Total Nutrient Uptake (kg ha−1) | ||
|---|---|---|---|---|---|
| N | P | K | |||
| Nitrogen levels (kg ha−1) | |||||
| N0 | 1.64 c | 3.68 c | 23.1 c | 5.44 c | 51.4 c |
| N90 | 5.67 b | 6.03 b | 71.1 b | 17.91 b | 99.3 b |
| N120 | 6.57 a | 7.99 a | 97.1 a | 22.35 a | 136.4 a |
| N150 | 6.46 a | 8.00 a | 104.3 a | 22.55 a | 136.8 a |
| LSD (p = 0.05) | 0.54 | 0.59 | 10.1 | 2.12 | 10.4 |
| Rice residue levels (Mg ha−1) | |||||
| RS0 | 4.42 b | 5.71 b | 69.0 a | 15.35 c | 94.9 c |
| RS5 | 4.62 ab | 5.89 ab | 74.7 a | 16.99 a | 106.4 b |
| RS7.5 | 4.84 a | 6.07 a | 77.2 a | 17.96 a | 113.4 a |
| RS10 | 4.63 ab | 5.91 ab | 74.6 a | 17.95 a | 109.3 ab |
| LSD (p = 0.05) | 0.30 | 0.35 | NS | 1.41 | 6.8 |
| Soil Variables | PC1 | PC2 | PC3 | |||
|---|---|---|---|---|---|---|
| Loading Variables | Contribution of Variables (%) | Loading Variables | Contribution of Variables (%) | Loading Variables | Contribution of Variables (%) | |
| WSOC | 0.804 | 7.07 | −0.500 | 8.86 | 0.144 | 1.27 |
| DOC | 0.622 | 4.23 | −0.261 | 2.42 | −0.542 | 18.08 |
| DOXC | 0.881 | 8.48 | −0.411 | 5.97 | −0.011 | 0.01 |
| POXC | 0.739 | 5.96 | −0.478 | 8.10 | 0.269 | 4.45 |
| Total CHO | 0.935 | 9.56 | 0.052 | 0.09 | −0.009 | 0.00 |
| TPC | 0.945 | 9.77 | −0.298 | 3.15 | 0.003 | 0.00 |
| BSR | 0.746 | 6.09 | −0.585 | 12.1 | 0.123 | 0.92 |
| MBC | 0.830 | 7.53 | −0.140 | 0.70 | −0.040 | 0.10 |
| PH | −0.347 | 1.31 | 0.351 | 4.37 | 0.799 | 39.21 |
| EC | −0.556 | 3.38 | −0.389 | 5.37 | −0.122 | 0.92 |
| SOC | 0.566 | 3.50 | 0.083 | 0.24 | 0.619 | 23.57 |
| GY | 0.692 | 5.24 | 0.612 | 13.2 | −0.132 | 1.07 |
| SY | 0.730 | 5.83 | 0.608 | 13.1 | −0.194 | 2.31 |
| NU | 0.741 | 6.00 | 0.546 | 10.5 | −0.222 | 3.03 |
| PU | 0.842 | 7.74 | 0.518 | 9.50 | −0.017 | 0.02 |
| KU | 0.873 | 8.32 | 0.247 | 2.16 | 0.286 | 5.03 |
| Eigenvalue | 9.15 | 2.82 | 1.63 | |||
| Variability (%) | 57.2 | 17.6 | 10.2 | |||
| Cumulative (%) | 57.2 | 74.8 | 84.9 | |||
| PC1 Variable | TPC | TCC | DOXC | MBC |
|---|---|---|---|---|
| TPC | 1 | |||
| TCC | 0.854 ** | 1 | ||
| DOXC | 0.940 ** | 0.815 ** | 1 | |
| MBC | 0.805 ** | 0.949 | 0.775 ** | 1 |
| PC 2 variable | BSR | WSOC | POXC | |
| BSR | 1 | |||
| WSOC | 0.983 ** | 1 | ||
| POXC | 0.951 ** | 0.975 ** | 1 | |
| PC 3 variable | pH | SOC | ||
| pH | 1 | |||
| SOC | 0.271 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Singh, S.; Singh, M.; Singh, A.; Ali, H.M.; Siddiqui, M.H.; Bhattarai, D. Changes in Wheat Rhizosphere Carbon Pools in Response to Nitrogen and Straw Incorporation. Agronomy 2022, 12, 2774. https://doi.org/10.3390/agronomy12112774
Sharma S, Singh S, Singh M, Singh A, Ali HM, Siddiqui MH, Bhattarai D. Changes in Wheat Rhizosphere Carbon Pools in Response to Nitrogen and Straw Incorporation. Agronomy. 2022; 12(11):2774. https://doi.org/10.3390/agronomy12112774
Chicago/Turabian StyleSharma, Sandeep, Swarnjeet Singh, Manpreet Singh, Arshdeep Singh, Hayssam M. Ali, Manzer H. Siddiqui, and Dwarika Bhattarai. 2022. "Changes in Wheat Rhizosphere Carbon Pools in Response to Nitrogen and Straw Incorporation" Agronomy 12, no. 11: 2774. https://doi.org/10.3390/agronomy12112774







