Effects of Freezing–Thawing Processes on Net Nitrogen Mineralization in Salinized Farmland Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
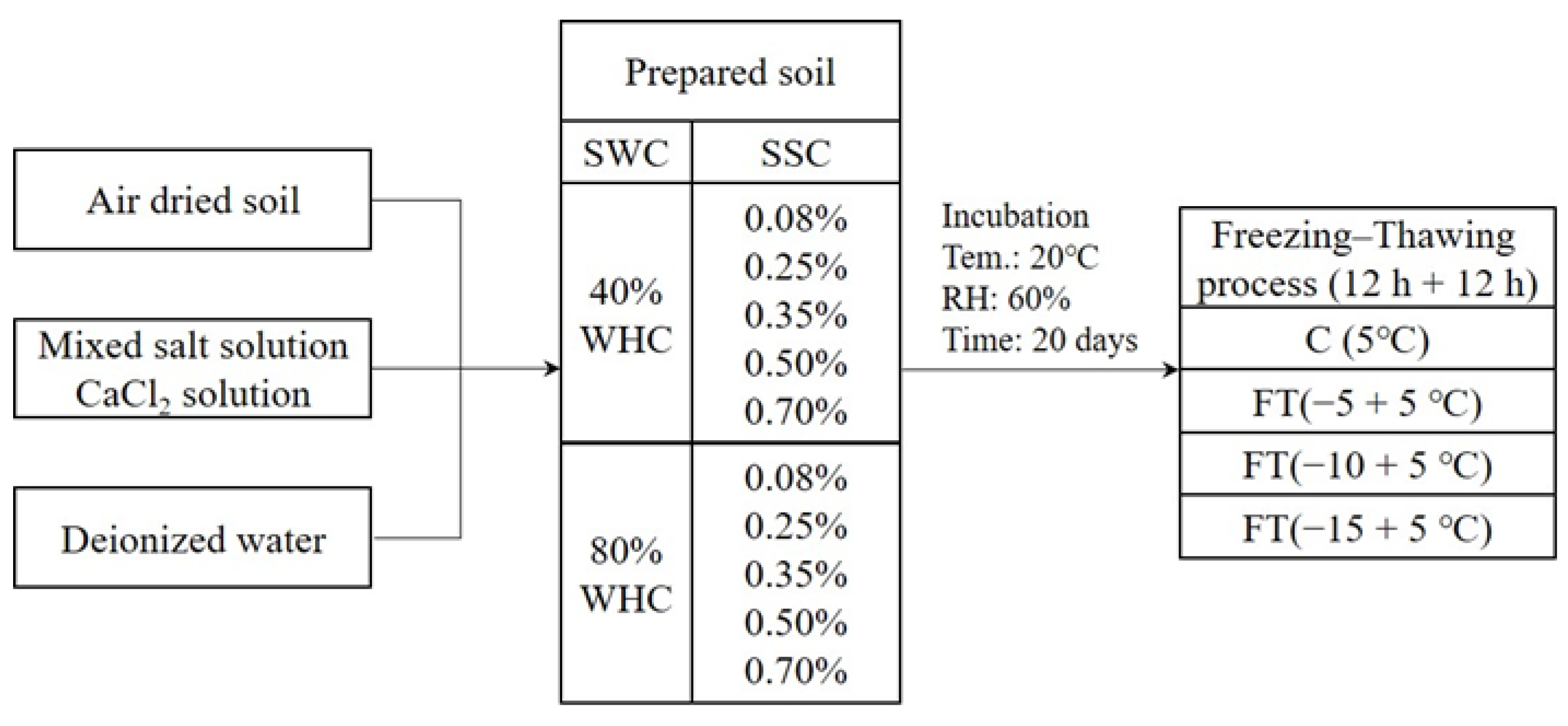
2.2. Experimental Design
2.3. Sample Analysis
2.4. Net Mineralization Rates
2.5. Statistical Analysis
3. Results
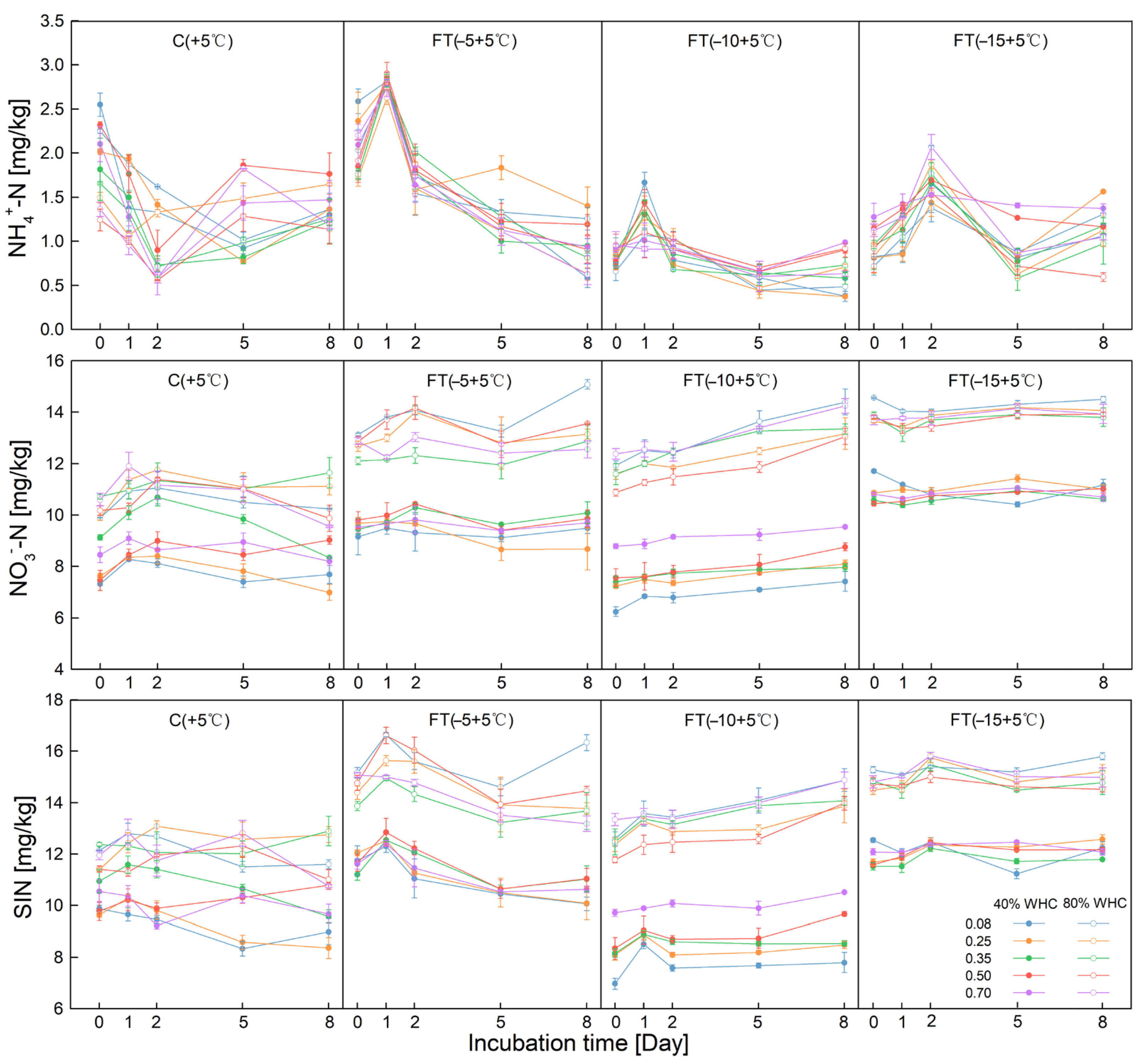
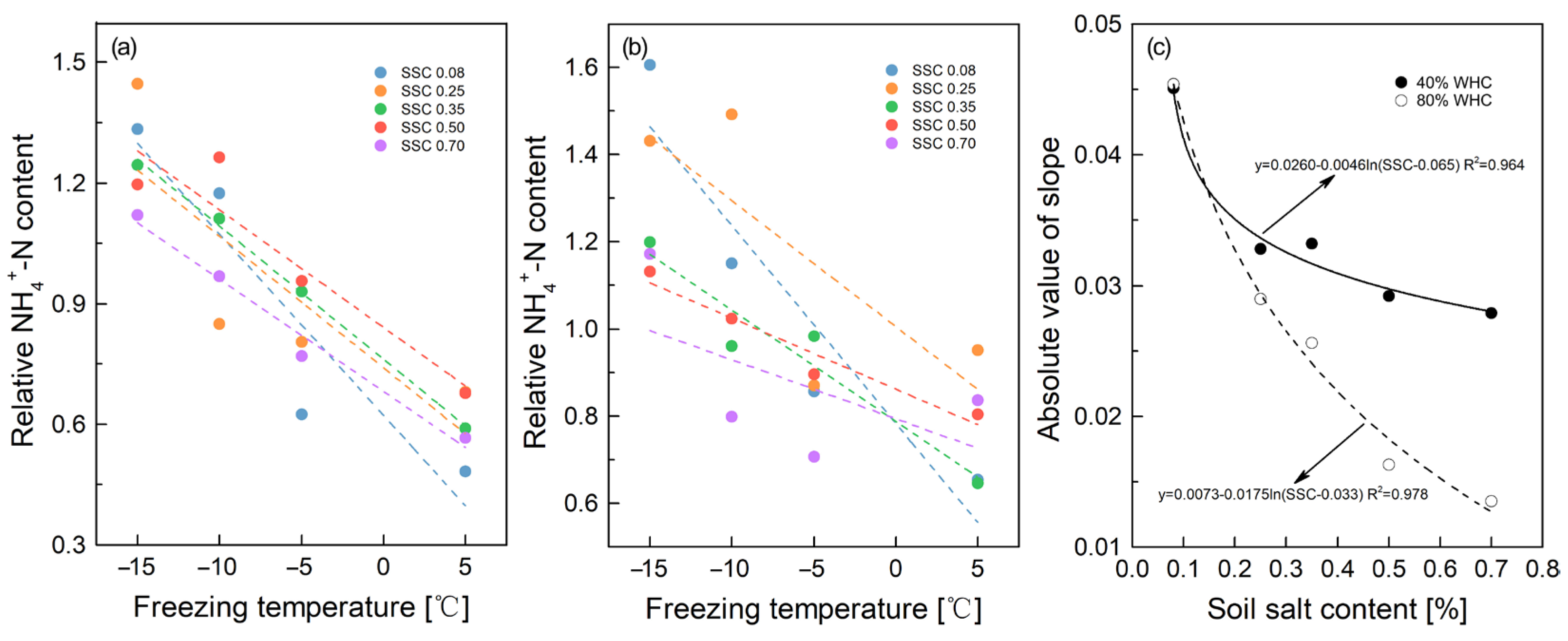
3.1. Soil Inorganic N Variation under Different SSC, FT Temperatures/Cycles, and SWC
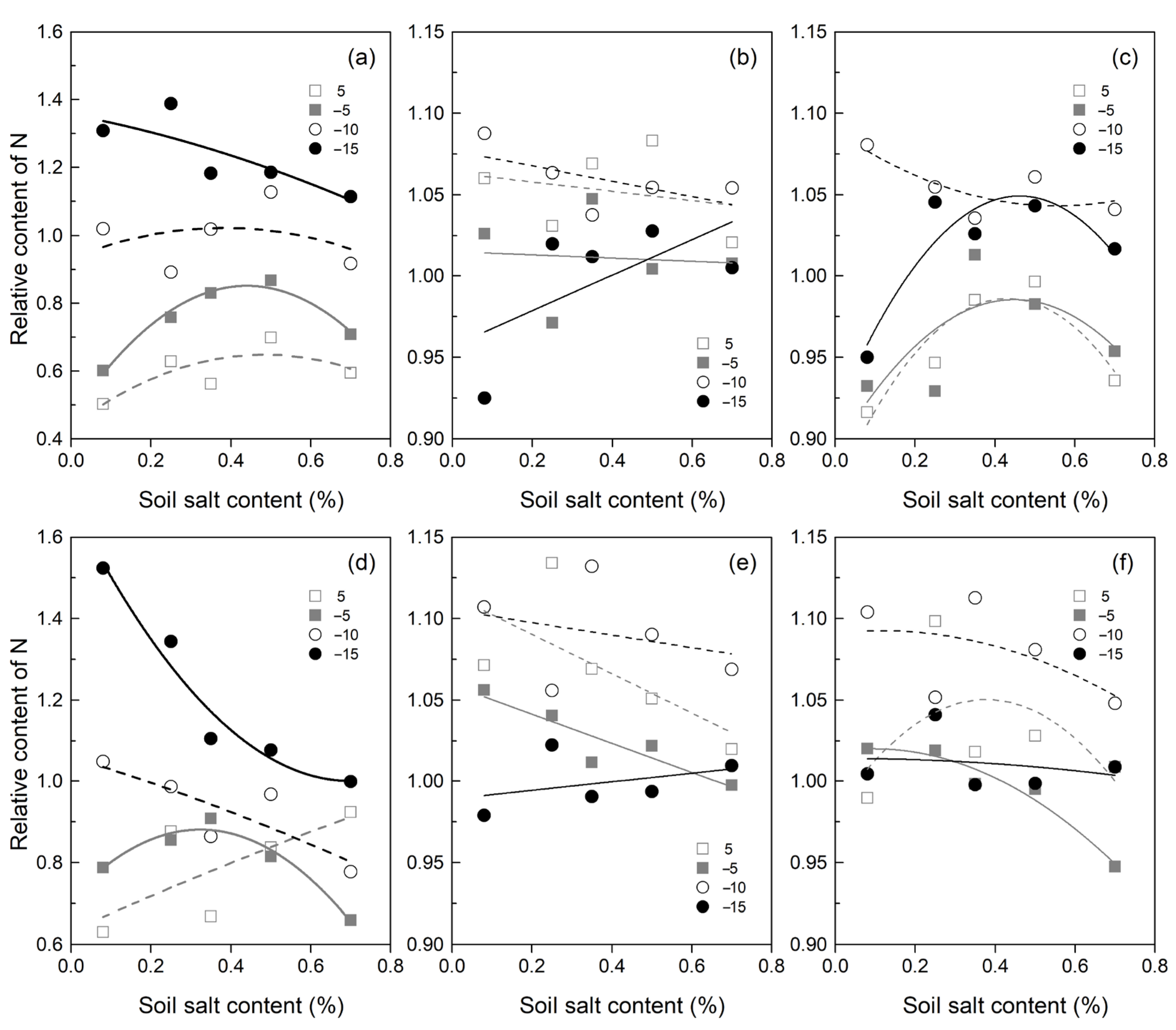
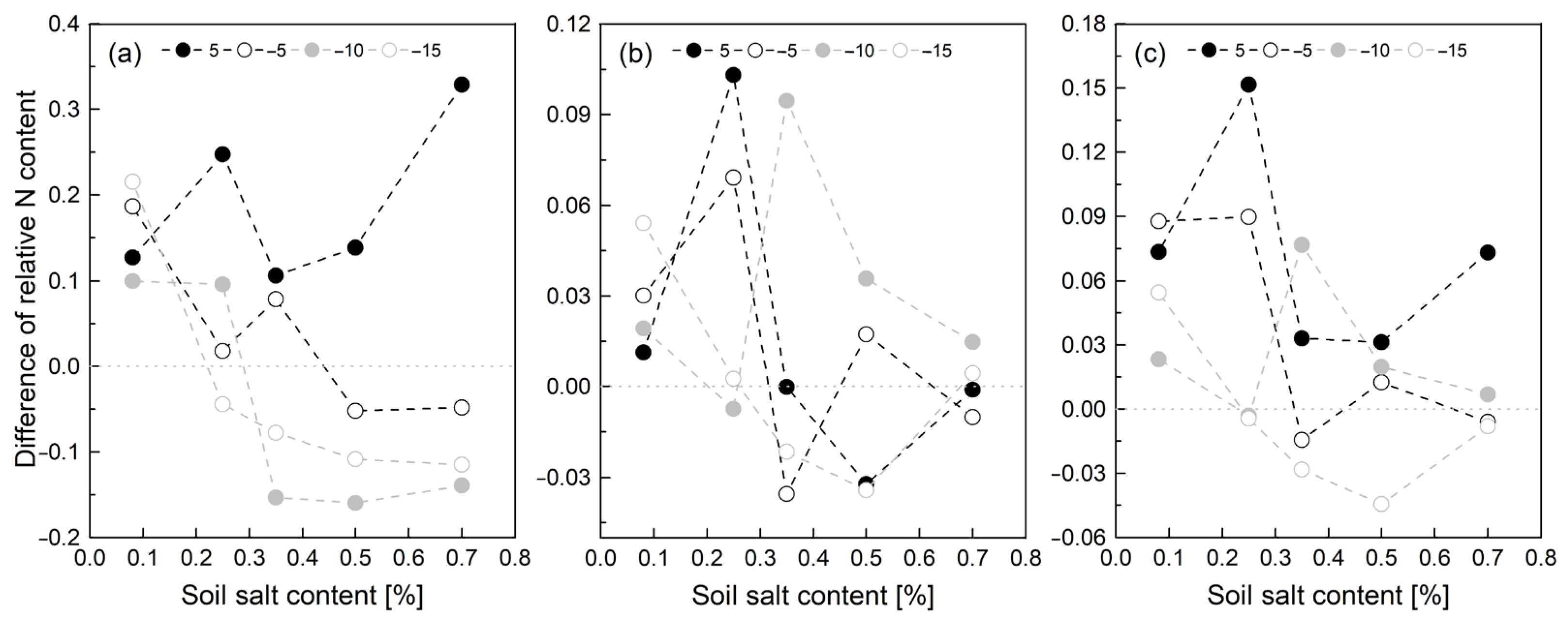
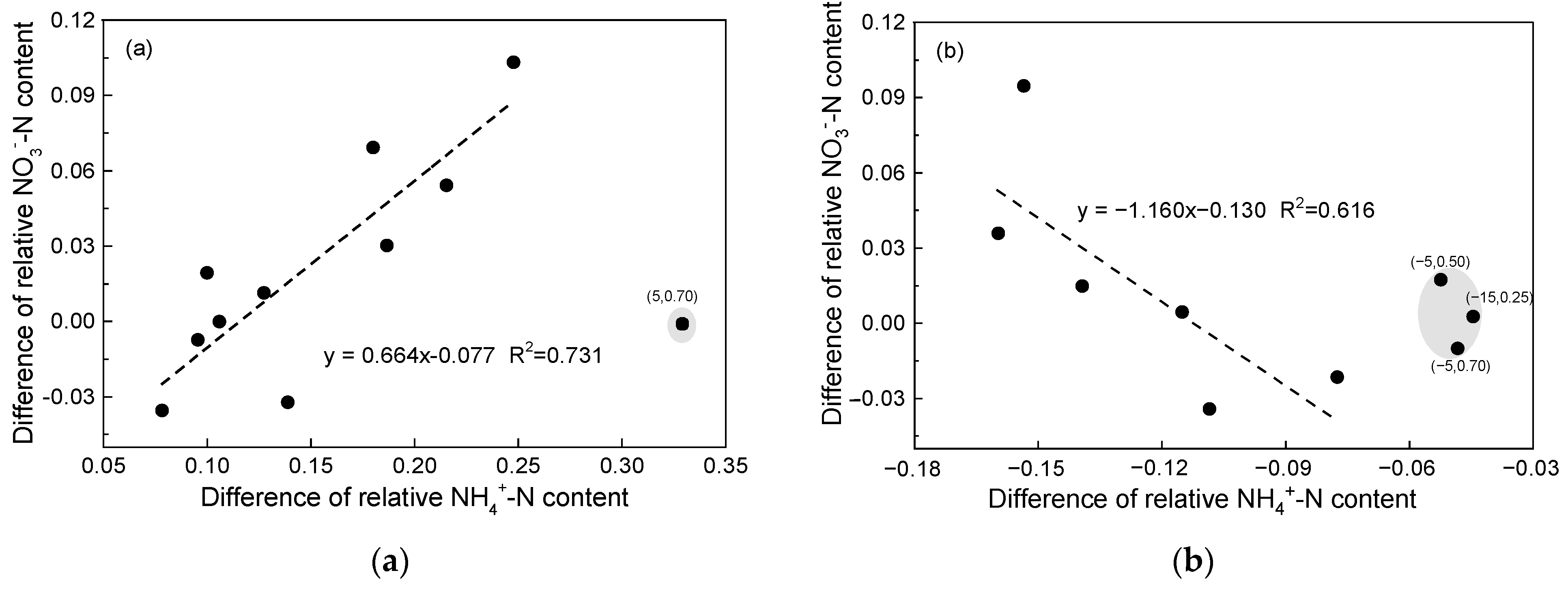
3.2. Soil Inorganic N Content Affected by SCC, FT Temperature, and SWC
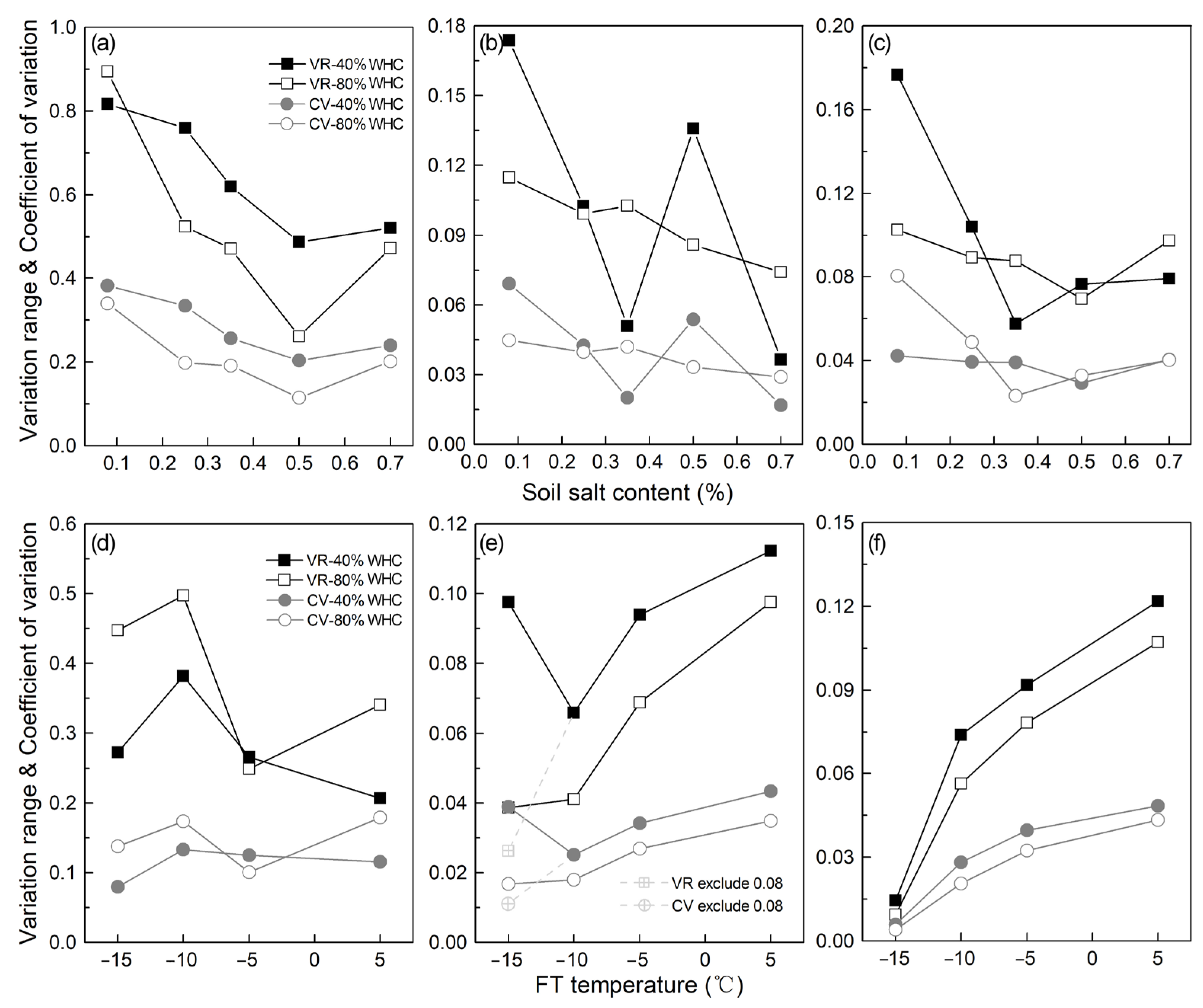
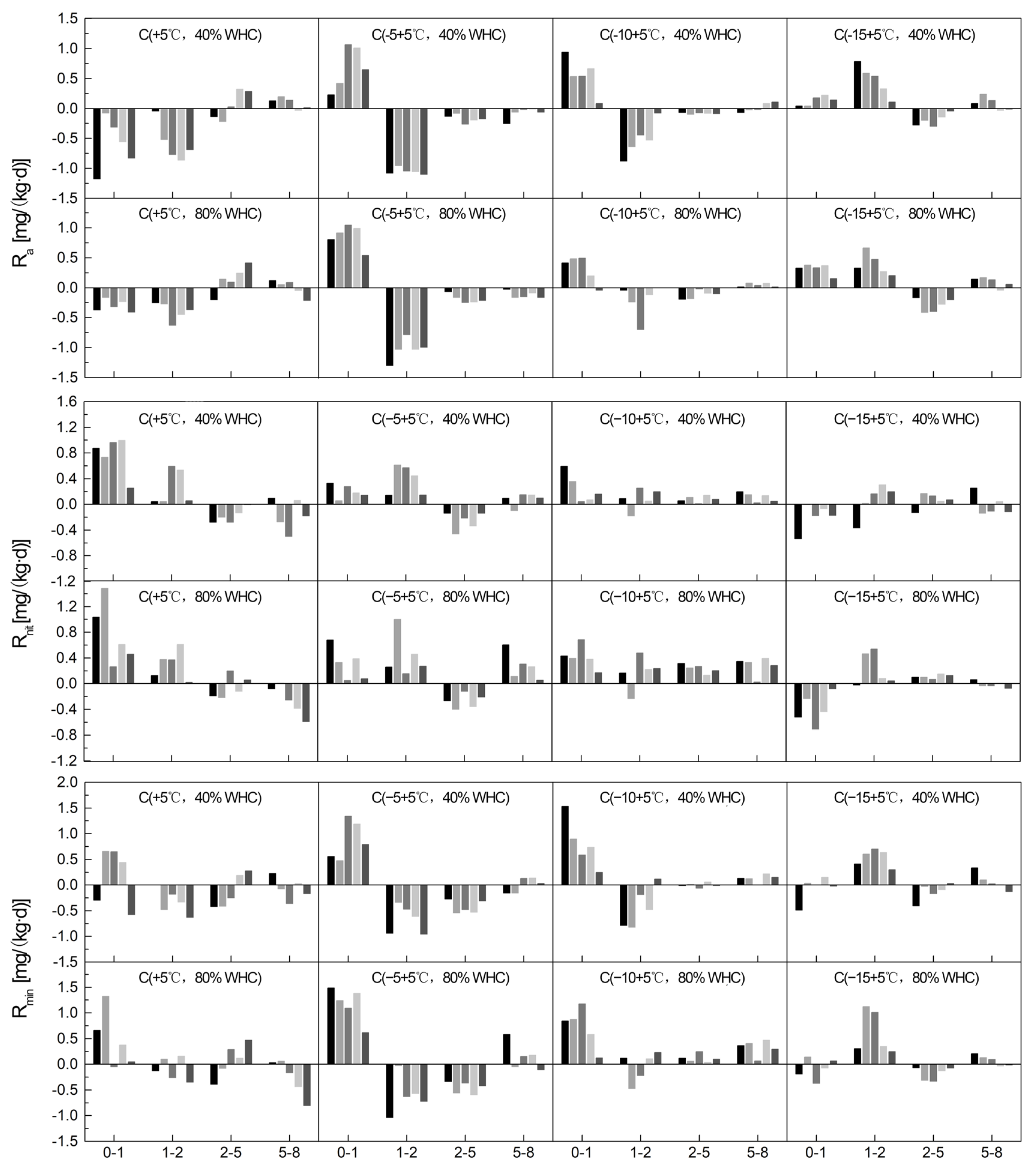
3.3. Soil Ra, Rnit, and Rmin under Different SSC, FT Temperatures/Cycles, and SWC
4. Discussion
4.1. Effects of FT Temperatures/Cycles on N Mineralization in Salinized Soil
4.2. Effects of SSC and SWC on Nitrogen Mineralization during the FT Process
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Jia, J.; Bai, J.; Gao, H.; Wang, W.; Yin, S.; Wang, D.; Han, L. Effects of salinity and moisture on sediment net nitrogen mineralization in salt marshes of a Chinese estuary. Chemosphere 2019, 228, 174–182. [Google Scholar] [CrossRef] [PubMed]
- FAO. Available online: https://www.fao.org/faostat/en/#data/RFN (accessed on 15 July 2022).
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Liu, J.; Bi, X.; Ma, M.; Jiang, L.; Du, L.; Li, S.; Sun, Q.; Zou, G.; Liu, H. Precipitation and irrigation dominate soil water leaching in cropland in Northern China. Agric. Water Manag. 2019, 211, 165–171. [Google Scholar] [CrossRef]
- Mark, D.L.; Andrew, C.V.; Edward, G.G.; Claudia, W. An improved laboratory method shows that freezing intensity increases N2O emissions. Can. J. Soil Sci. 2020, 100, 136–149. [Google Scholar]
- Yang, H.; Chen, Y.; Zhang, F. Evaluation of comprehensive improvement for mild and moderate soil salinization in arid zone. PLoS ONE 2019, 14, e224790. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, Q.; Zhao, Q.; Wu, J. Assessing the Long-Term Evolution of Abandoned Salinized Farmland via Temporal Remote Sensing Data. Remote Sens. 2021, 13, 4057. [Google Scholar] [CrossRef]
- Geng, Q.; Wu, P.; Zhao, X.; Wang, Y. Comparison of classification methods for the divisions of wet/dry climate regions in Northwest China. Int. J. Climatol. 2014, 34, 2163–2174. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Yao, N. Bias correction of the observed daily precipitation and re-division of climatic zones in China. Int. J. Climatol. 2018, 38, 3369–3387. [Google Scholar] [CrossRef]
- Liu, J.; Baulch, H.M.; Macrae, M.L.; Wilson, H.F.; Elliott, J.A.; Bergström, L.; Glenn, A.J.; Vadas, P.A. Agricultural Water Quality in Cold Climates: Processes, Drivers, Management Options, and Research Needs. J. Environ. Qual. 2019, 48, 792–802. [Google Scholar] [CrossRef]
- Wu, M.; Huang, J.; Tan, X.; Wu, J. Water, Salt and Heat Influences on Carbon and Nitrogen Dynamics in Seasonally Frozen Soils in Hetao Irrigation District, Inner Mongolia, China. Pedosphere 2019, 29, 632–641. [Google Scholar] [CrossRef]
- Bai, J.; Gao, H.; Xiao, R.; Wang, J.; Huang, C. A Review of Soil Nitrogen Mineralization as Affected by Water and Salt in Coastal Wetlands: Issues and Methods. CLEAN Soil Air Water 2012, 40, 1099–1105. [Google Scholar] [CrossRef]
- Zhou, M.; Butterbach-Bahl, K.; Vereecken, H.; Brüggemann, N. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Change Biol. 2017, 23, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y.; Shen, Z.; Yu, M.; Yu, Y.; Wang, Z.; Lv, Z.; Yu, J. Short-term effects of NaCl and Na2SO4 on nitrogen mineralization in the soil in three marshes of the Liaohe River estuary. Catena 2021, 196, 104828. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Z.; Liu, H.; Gao, M.; Zhang, Y. Microbial biomass and activity in salt affected soils under arid conditions. Appl. Soil Ecol. 2007, 35, 319–328. [Google Scholar] [CrossRef]
- Duan, M.; House, J.; Liu, Y.; Chang, S.X. Contrasting responses of gross and net nitrogen transformations to salinity in a reclaimed boreal forest soil. Biol. Fert. Soils 2018, 54, 385–395. [Google Scholar] [CrossRef]
- Wong, V.N.L.; Dalal, R.C.; Greene, R.S.B. Salinity and sodicity effects on respiration and microbial biomass of soil. Biol. Fert. Soils 2008, 44, 943–953. [Google Scholar] [CrossRef]
- Santoro, A.E. Microbial nitrogen cycling at the saltwater–freshwater interface. Hydrogeol. J. 2010, 18, 187–202. [Google Scholar] [CrossRef]
- Zeng, W.; Xu, C.; Wu, J.; Huang, J.; Ma, T. Effect of Salinity on Soil Respiration and Nitrogen Dynamics. Ecol. Chem. Eng. 2013, 20, 519–530. [Google Scholar] [CrossRef]
- Marton, J.M.; Herbert, E.R.; Craft, C.B. Effects of Salinity on Denitrification and Greenhouse Gas Production from Laboratory-incubated Tidal Forest Soils. Wetlands 2012, 32, 347–357. [Google Scholar] [CrossRef]
- Noe, G.B.; Krauss, K.W.; Lockaby, B.G.; Conner, W.H.; Hupp, C.R. The effect of increasing salinity and forest mortality on soil nitrogen and phosphorus mineralization in tidal freshwater forested wetlands. Biogeochemistry 2013, 114, 225–244. [Google Scholar] [CrossRef] [Green Version]
- Osborne, R.I.; Bernot, M.J.; Findlay, S.E.G. Changes in Nitrogen Cycling Processes Along a Salinity Gradient in Tidal Wetlands of the Hudson River, New York, USA. Wetlands 2015, 35, 323–334. [Google Scholar] [CrossRef]
- Guo, H.; Ma, L.; Liang, Y.; Hou, Z.; Min, W. Response of ammonia-oxidizing Bacteria and Archaea to long-term saline water irrigation in alluvial grey desert soils. Sci. Rep. 2020, 10, 489. [Google Scholar] [CrossRef]
- Murtaza, B.; Murtaza, G.; Sabir, M.; Owens, G.; Abbas, G.; Imran, M.; Shah, G.M. Amelioration of saline–sodic soil with gypsum can increase yield and nitrogen use efficiency in rice–wheat cropping system. Arch. Agron. Soil Sci. 2017, 63, 1267–1280. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Liu, S.; Qi, Z.; Wang, H.; Wei, Q.; Gu, Z.; Liu, X.; Hameed, F. Salinity-induced concomitant increases in soil ammonia volatilization and nitrous oxide emission. Geoderma 2020, 361, 114053. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, J.; Yao, R.; Wang, X.; Xie, W.; Zhu, W.; Liu, X.; Cao, Y.; Tao, J. Interactive effects of soil amendments (biochar and gypsum) and salinity on ammonia volatilization in coastal saline soil. Catena 2020, 190, 104527. [Google Scholar] [CrossRef]
- Song, Y.; Zou, Y.; Wang, G.; Yu, X. Altered soil carbon and nitrogen cycles due to the freeze-thaw effect: A meta-analysis. Soil Biol. Biochem. 2017, 109, 35–49. [Google Scholar] [CrossRef]
- Patel, K.F.; Tatariw, C.; MacRae, J.D.; Ohno, T.; Nelson, S.J.; Fernandez, I.J. Repeated freeze–thaw cycles increase extractable, but not total, carbon and nitrogen in a Maine coniferous soil. Geoderma 2021, 402, 115353. [Google Scholar] [CrossRef]
- Xu, X. Effect of freeze-thaw disturbance on soil C and N dynamics and GHG fluxes of East Asia forests: Review and future perspectives. Soil Sci. Plant Nutr. (Tokyo) 2022, 68, 15–26. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Wang, X.; Huo, Z. Response of soil compaction to the seasonal freezing-thawing process and the key controlling factors. Catena 2020, 184, 104247. [Google Scholar] [CrossRef]
- Kværnø, S.H.; Øygarden, L. The influence of freeze–thaw cycles and soil moisture on aggregate stability of three soils in Norway. Catena 2006, 67, 175–182. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, C.; Wang, K.; Chang, D.; Huang, J. In situ experiment on change law of soil mineral nitrogen availability in seasonal freezing agricultural areas. Trans. Chin. Soc. Agric. Eng. 2019, 35, 140–146. (In Chinese) [Google Scholar]
- Jiang, N.; Juan, Y.; Tian, L.; Chen, X.; Sun, W.; Chen, L. Soil Water Contents Control the Responses of Dissolved Nitrogen Pools and Bacterial Communities to Freeze-Thaw in Temperate Soils. Biomed Res. Int. 2020, 6867081. [Google Scholar] [CrossRef]
- Juan, Y.; Tian, L.; Sun, W.; Qiu, W.; Curtin, D.; Gong, L.; Liu, Y. Simulation of soil freezing-thawing cycles under typical winter conditions: Implications for nitrogen mineralization. J. Soil Sediments 2020, 20, 143–152. [Google Scholar] [CrossRef]
- Juan, Y.; Jiang, N.; Tian, L.; Chen, X.; Sun, W.; Chen, L. Effect of Freeze-Thaw on a Midtemperate Soil Bacterial Community and the Correlation Network of Its Members. Biomed Res. Int. 2018, 2018, 8412429. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Feng, F.; Cai, T.; Tang, S. Soil Microbial Community Response Differently to the Frequency and Strength of Freeze–Thaw Events in a Larix gmelinii Forest in the Daxing’an Mountains, China. Front. Microbiol. 2020, 11, 1164. [Google Scholar] [CrossRef]
- Sang, C.; Xia, Z.; Sun, L.; Sun, H.; Jiang, P.; Wang, C.; Bai, E. Responses of soil microbial communities to freeze–thaw cycles in a Chinese temperate forest. Ecol. Processes 2021, 10, 66. [Google Scholar] [CrossRef]
- Zhao, Q.; Tan, X.; Zeng, Q.; Zhao, H.; Wu, J.; Huang, J. Combined effects of temperature and precipitation on the spring runoff generation process in a seasonal freezing agricultural watershed. Environ. Earth Sci. 2021, 80, 490. [Google Scholar] [CrossRef]
- Wu, M.; Huang, J.; Wu, J.; Tan, X.; Jansson, P. Experimental study on evaporation from seasonally frozen soils under various water, solute and groundwater conditions in Inner Mongolia, China. J. Hydrol. 2016, 535, 46–53. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y.; Zhao, T.; Cui, L.; Mao, W.; Ye, M.; Wu, J.; Yang, J. Chemical characteristics of salt migration in frozen soils during the freezing-thawing period. J. Hydrol. 2022, 606, 127403. [Google Scholar] [CrossRef]
- Wu, M.; Tan, X.; Huang, J.; Wu, J.; Jansson, P. Solute and water effects on soil freezing characteristics based on laboratory experiments. Cold Reg. Sci. Technol. 2015, 115, 22–29. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, L.; Zhu, X.; Wu, X.; Wu, T.; Li, R.; Xie, C.; Hao, J. Review of algorithms and parameterizations to determine unfrozen water content in frozen soil. Geoderma 2020, 368, 114277. [Google Scholar] [CrossRef]
- Tan, X.; Wu, M.; Huang, J.; Wu, J.; Chen, J. Similarity of soil freezing characteristic and soil water characteristic: Application in saline frozen soil hydraulic properties prediction. Cold Reg. Sci. Technol. 2020, 173, 102876. [Google Scholar] [CrossRef]
- Gao, D.; Bai, E.; Yang, Y.; Zong, S.; Hagedorn, F. A global meta-analysis on freeze-thaw effects on soil carbon and phosphorus cycling. Soil Biol. Biochem. 2021, 159, 108283. [Google Scholar] [CrossRef]
- Rosinger, C.; Clayton, J.; Baron, K.; Bonkowski, M. Soil freezing-thawing induces immediate shifts in microbial and resource stoichiometry in Luvisol soils along a postmining agricultural chronosequence in Western Germany. Geoderma 2022, 408, 115596. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Han, L.; Cheng, L.; Lv, Y.; Fu, B.; Feng, X.; Wu, X. Shortened duration and reduced area of frozen soil in the Northern Hemisphere. Innovation 2021, 2, 100146. [Google Scholar] [CrossRef]
- Chen, X.; Jeong, S.; Park, C.; Park, H.; Joo, J.; Chang, D.; Yun, J. Different responses of surface freeze and thaw phenology changes to warming among Arctic permafrost types. Remote Sens. Environ. 2022, 272, 112956. [Google Scholar] [CrossRef]
- Hatami, S.; Nazemi, A. Compound changes in temperature and snow depth lead to asymmetric and nonlinear responses in landscape freeze–thaw. Sci. Rep. 2022, 12, 2196. [Google Scholar] [CrossRef]
- Xu, S.; Fu, Q.; Li, T.; Meng, F.; Liu, D.; Hou, R.; Li, M.; Li, Q. Spatiotemporal characteristics of the soil freeze-thaw state and its variation under different land use types—A case study in Northeast China. Agric. For. Meteorol. 2022, 312, 108737. [Google Scholar] [CrossRef]
- Jiang, Y. China’s water scarcity. J. Environ. Manag. 2009, 90, 3185–3196. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Koponen, H.T.; Martikainen, P.J. Soil water content and freezing temperature affect freeze–thaw related N2O production in organic soil. Nutr. Cycl. Agroecosystems 2004, 69, 213–219. [Google Scholar] [CrossRef]
- Larsen, K.S.; Jonasson, S.; Michelsen, A. Repeated freeze–thaw cycles and their effects on biological processes in two arctic ecosystem types. Appl. Soil Ecol. 2002, 21, 187–195. [Google Scholar] [CrossRef]
- Jiang, N.; Juan, Y.; Tian, L.; Chen, X.; Sun, W.; Chen, L. Modification of the composition of dissolved nitrogen forms, nitrogen transformation processes, and diversity of bacterial communities by freeze–thaw events in temperate soils. Pedobiologia 2018, 71, 41–49. [Google Scholar] [CrossRef]
- Smith, J.; Wagner-Riddle, C.; Dunfield, K. Season and management related changes in the diversity of nitrifying and denitrifying bacteria over winter and spring. Appl. Soil Ecol. 2010, 44, 138–146. [Google Scholar] [CrossRef]
- Wertz, S.; Goyer, C.; Zebarth, B.J.; Tatti, E.; Burton, D.L.; Chantigny, M.H.; Filion, M. The amplitude of soil freeze-thaw cycles influences temporal dynamics of N2O emissions and denitrifier transcriptional activity and community composition. Biol. Fert. Soils 2016, 52, 1149–1162. [Google Scholar] [CrossRef]
- Urakawa, R.; Shibata, H.; Kuroiwa, M.; Inagaki, Y.; Tateno, R.; Hishi, T.; Fukuzawa, K.; Hirai, K.; Toda, H.; Oyanagi, N.; et al. Effects of freeze–thaw cycles resulting from winter climate change on soil nitrogen cycling in ten temperate forest ecosystems throughout the Japanese archipelago. Soil Biol. Biochem. 2014, 74, 82–94. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Z.; Song, Z.; Chen, W.; Tian, D.; Tang, S.; Wang, X.; Wang, J.; Liu, W.; Wang, Y.; et al. Variations and controlling factors of soil denitrification rate. Glob. Change Biol. 2022, 28, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zou, Y.; Wang, G.; Yu, X. Stimulation of nitrogen turnover due to nutrients release from aggregates affected by freeze-thaw in wetland soils. Phys. Chem. Earth Parts A/B/C 2017, 97, 3–11. [Google Scholar] [CrossRef]
- Tuorto, S.J.; Darias, P.; McGuinness, L.R.; Panikov, N.; Zhang, T.; Häggblom, M.M.; Kerkhof, L.J. Bacterial genome replication at subzero temperatures in permafrost. ISME J. 2014, 8, 139–149. [Google Scholar] [CrossRef]
- Laura, R.D. Salinity and nitrogen mineralization in soil. Soil Biol. Biochem. 1977, 9, 333–336. [Google Scholar] [CrossRef]
- Mikutta, R.; Mikutta, C.; Kalbitz, K.; Scheel, T.; Kaiser, K.; Jahn, R. Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms. Geochim. Cosmochim. Acta 2007, 71, 2569–2590. [Google Scholar] [CrossRef]
- Weston, N.B.; Giblin, A.E.; Banta, G.T.; Hopkinson, C.S.; Tucker, J. The Effects of Varying Salinity on Ammonium Exchange in Estuarine Sediments of the Parker River, Massachusetts. Estuaries Coasts 2010, 33, 985–1003. [Google Scholar] [CrossRef]
- Gardner, W.S.; McCarthy, M.J.; An, S.; Sobolev, D.; Sell, K.S.; Brock, D. Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnol. Oceanogr. 2006, 51, 558–568. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, P.; Xu, G.; Li, Z.; Wang, T.; Cheng, S.; Zhang, H.; Ma, T. The effect of soil water content and erodibility on losses of available nitrogen and phosphorus in simulated freeze-thaw conditions. Catena 2018, 166, 21–33. [Google Scholar] [CrossRef]
- Gilmour, K.; Hoggarth, C.; Williams, C.; Baulch, H.M. Cold spots and cold moments: The potential for sediment freezing to depress denitrification in wetland sediments. J. Environ. Qual. 2022, 51, 990–1002. [Google Scholar] [CrossRef]
- Elliott, A.C.; Henry, H.A.L. Freeze–thaw cycle amplitude and freezing rate effects on extractable nitrogen in a temperate old field soil. Biol. Fert. Soils 2009, 45, 469–476. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Wang, S.; Zhang, J.; Cai, Z. Effects of soil moisture on gross N transformations and N2O emission in acid subtropical forest soils. Biol. Fert. Soils 2014, 50, 1099–1108. [Google Scholar] [CrossRef]
- Chen, S.; Ouyang, W.; Hao, F.; Zhao, X. Combined impacts of freeze–thaw processes on paddy land and dry land in Northeast China. Sci. Total Environ. 2013, 456–457, 24–33. [Google Scholar] [CrossRef]
- Nagare, R.; Schincariol, R.; Quinton, W.; Hayashi, M. Effects of freezing on soil temperature, freezing front propagation and moisture redistribution in peat: Laboratory investigations. Hydrol. Earth Syst. Sci. 2012, 16, 501–515. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, W.; Xu, Y.; Müller, C.; Rütting, T.; Yu, H.; Fan, J.; Zhang, J.; Zhu, T. Importance of heterotrophic nitrification and dissimilatory nitrate reduction to ammonium in a cropland soil: Evidences from a 15N tracing study to literature synthesis. Soil Biol. Biochem. 2015, 91, 65–75. [Google Scholar] [CrossRef]
- Neil, R.; David, S.; Claudia, W. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze–thaw cycles. Can. J. Soil Sci. 2013, 93, 401–414. [Google Scholar]
| pH | EC (μS/cm) | TN (mg/kg) | TC (mg/kg) | C/N | NH4+-N (mg/kg) | NO3−-N (mg/kg) | Clay (%) | Slit (%) | Sand (%) | Soil Texture |
|---|---|---|---|---|---|---|---|---|---|---|
| 8.33 ± 0.03 | 364.84 ± 14.34 | 0.82 ± 0.02 | 23.75 ± 0.59 | 28.96 | 8.30 ± 0.85 | 7.80 ± 0.55 | 6.79 ± 0.19 | 45.04 ± 0.48 | 48.17 ± 0.67 | Silty loam |
| SWC | SSC (%) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | SIN (mg/kg) | NH4+-N /SIN (%) | TN (mg/kg) | TC (mg/kg) | C/N |
|---|---|---|---|---|---|---|---|---|
| 40 % WHC | 0.08 | 1.57 ± 0.84 a | 8.69 ± 2.10 b | 10.26 ± 2.28 b | 15.20 | 0.81 ± 0.03 a | 23.50 ± 0.21 bcd | 29.01 |
| 0.25 | 1.55 ± 0.59 a | 9.77 ± 2.86 ab | 11.33 ± 2.52 ab | 14.78 | 0.79 ± 0.03 a | 23.26 ± 0.33 cd | 29.44 | |
| 0.35 | 1.19 ± 0.42 a | 8.51 ± 1.84 b | 9.70 ± 1.83 b | 12.70 | 0.81 ± 0.02 a | 23.13 ± 0.37 d | 28.73 | |
| 0.5 | 1.37 ± 0.79 a | 9.67 ± 2.52 b | 11.03 ± 2.28 ab | 12.93 | 0.81 ± 0.01 a | 23.49 ± 0.27 bcd | 29.17 | |
| 0.7 | 1.56 ± 0.92 a | 9.40 ± 0.80 b | 10.96 ± 0.95 ab | 13.84 | 0.79 ± 0.01 a | 23.15 ± 0.09 cd | 29.48 | |
| 80% WHC | 0.08 | 1.23 ± 0.51 a | 12.61 ± 1.41 a | 13.84 ± 1.67 a | 8.74 | 0.80 ± 0.06 a | 24.27 ± 0.03 ab | 30.53 |
| 0.25 | 1.15 ± 0.41 a | 11.20 ± 1.15 ab | 12.35 ± 1.35 ab | 9.24 | 0.81 ± 0.01 a | 26.15 ± 2.22 a | 32.28 | |
| 0.35 | 1.09 ± 0.29 a | 12.19 ± 0.99 a | 13.29 ± 1.01 a | 8.25 | 0.82 ± 0.02 a | 24.39 ± 0.15 a | 29.92 | |
| 0.5 | 1.21 ± 0.45 a | 11.18 ± 1.02 ab | 12.39 ± 1.45 ab | 9.49 | 0.83 ± 0.01 a | 24.43 ± 0.22 a | 29.43 | |
| 0.7 | 1.47 ± 0.48 a | 12.23 ± 1.10 a | 13.70 ± 1.02 a | 10.79 | 0.83 ± 0.01 a | 23.97 ± 0.11 abc | 29.05 |
| Factors | NH4+-N | NO3−-N | SIN | |||
|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | |
| T | 3.830 | 0.010 * | 0.133 | 0.941 | 1.771 | 0.153 |
| SSC | 31.019 | 0.000 ** | 23.837 | 0.000 ** | 12.964 | 0.000 ** |
| SWC | 3.024 | 0.083 | 0.396 | 0.529 | 4.249 | 0.040 * |
| FTC | 1.815 | 0.144 | 2.345 | 0.073 | 6.408 | 0.000 ** |
| T × SSC | 1.097 | 0.362 | 1.433 | 0.149 | 0.920 | 0.527 |
| T × SWC | 0.078 | 0.972 | 0.676 | 0.568 | 0.907 | 0.438 |
| T × FTC | 0.156 | 0.998 | 0.374 | 0.947 | 0.472 | 0.893 |
| SSC × SWC | 4.040 | 0.003 * | 22.577 | 0.000 ** | 23.520 | 0.000 ** |
| SSC × FTC | 2.876 | 0.001 * | 5.191 | 0.000 ** | 7.843 | 0.000 ** |
| SWC × FTC | 1.723 | 0.162 | 3.391 | 0.018 * | 2.882 | 0.036 * |
| T × SSC × SWC | 0.466 | 0.933 | 0.567 | 0.868 | 0.493 | 0.918 |
| T × SSC × FTC | 0.248 | 1.000 | 0.415 | 0.999 | 0.326 | 1.000 |
| T × SWC × FTC | 0.255 | 0.986 | 0.806 | 0.611 | 0.510 | 0.867 |
| SSC × SWC × FTC | 1.178 | 0.298 | 7.681 | 0.000 ** | 4.932 | 0.000 ** |
| T × SSC × SWC × FTC | 0.173 | 1.000 | 0.578 | 0.976 | 0.39 | 0.999 |
| Factors | Ra | Rnit | Rmin | |||
|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | |
| T | 224.801 | 0.000 ** | 4.190 | 0.006 * | 4.299 | 0.005 * |
| SSC | 5.949 | 0.000 ** | 3.792 | 0.005 * | 1.700 | 0.150 |
| SWC | 23.045 | 0.000 ** | 0.112 | 0.738 | 0.286 | 0.593 |
| FTC | 635.058 | 0.000 ** | 1.234 | 0.297 | 1.420 | 0.237 |
| T × SSC | 5.275 | 0.000 ** | 1.435 | 0.148 | 1.468 | 0.135 |
| T × SWC | 5.948 | 0.001 * | 0.052 | 0.984 | 0.022 | 0.996 |
| T × FTC | 518.803 | 0.000 ** | 0.339 | 0.961 | 0.171 | 0.997 |
| SSC × SWC | 2.438 | 0.047 * | 2.005 | 0.094 | 1.343 | 0.254 |
| SSC × FTC | 18.891 | 0.000 ** | 1.039 | 0.412 | 0.685 | 0.766 |
| SWC × FTC | 18.450 | 0.000 ** | 1.333 | 0.264 | 0.202 | 0.895 |
| T × SSC × SWC | 1.892 | 0.035 * | 0.426 | 0.953 | 0.305 | 0.988 |
| T × SSC × FTC | 13.511 | 0.000 ** | 0.120 | 1.000 | 0.185 | 1.000 |
| T × SWC × FTC | 16.351 | 0.000 ** | 0.364 | 0.952 | 0.152 | 0.998 |
| SSC × SWC × FTC | 5.683 | 0.000 ** | 0.998 | 0.45 | 0.552 | 0.880 |
| T × SSC × SWC × FTC | 9.379 | 0.000 ** | 0.175 | 1.000 | 0.131 | 1.000 |
| SSC | SWC = 40% WHC | SWC = 80% WHC | ||||
|---|---|---|---|---|---|---|
| Equation: Y = Relative content | R | Sig | Equation: Y = Relative Content | R | Sig | |
| 0.08 | Y = −0.0451T + 0.6226 | 0.8659 | 0.035 * | Y = −0.0454T + 0.7826 | 0.8821 | 0.030 * |
| 0.25 | Y = −0.0328T + 0.7401 | 0.6724 | 0.090 | Y = −0.029T + 1.0054 | 0.5937 | 0.115 |
| 0.35 | Y = −0.0332T + 0.7615 | 0.9975 | 0.001 ** | Y = −0.0256T + 0.7874 | 0.9206 | 0.020 * |
| 0.50 | Y = −0.0292T + 0.8411 | 0.8813 | 0.031 * | Y = −0.0163T + 0.8616 | 0.9443 | 0.014 * |
| 0.70 | Y = −0.0279T + 0.6816 | 0.9783 | 0.005 * | Y = −0.0135T + 0.7936 | 0.3240 | 0.215 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Wu, J.; Guo, C.; Zhang, J.; Wang, X.; Liu, Y.; Zhao, H.; Zhang, R. Effects of Freezing–Thawing Processes on Net Nitrogen Mineralization in Salinized Farmland Soil. Agronomy 2022, 12, 2986. https://doi.org/10.3390/agronomy12122986
Zhao Q, Wu J, Guo C, Zhang J, Wang X, Liu Y, Zhao H, Zhang R. Effects of Freezing–Thawing Processes on Net Nitrogen Mineralization in Salinized Farmland Soil. Agronomy. 2022; 12(12):2986. https://doi.org/10.3390/agronomy12122986
Chicago/Turabian StyleZhao, Qiang, Jingwei Wu, Chenyao Guo, Jifeng Zhang, Xin Wang, Yawen Liu, Hang Zhao, and Rui Zhang. 2022. "Effects of Freezing–Thawing Processes on Net Nitrogen Mineralization in Salinized Farmland Soil" Agronomy 12, no. 12: 2986. https://doi.org/10.3390/agronomy12122986






