Effect of Salinity and Nitrogen Fertilization Levels on Growth Parameters of Sarcocornia fruticosa, Salicornia brachiata, and Arthrocnemum macrostachyum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Harvesting Regime
2.3. Relative Water Content
2.4. Assessment of Electrical Conductivity (EC) and Total Soluble Solids (TSS)
2.5. Anthocyanin Determination
2.6. Protein Extraction and Determination of Protein Content
2.7. Oxygen Radical Absorbance Capacity (ORAC) Measurements
2.8. Data Analysis
3. Results
3.1. Fresh Shoot Biomass Accumulation
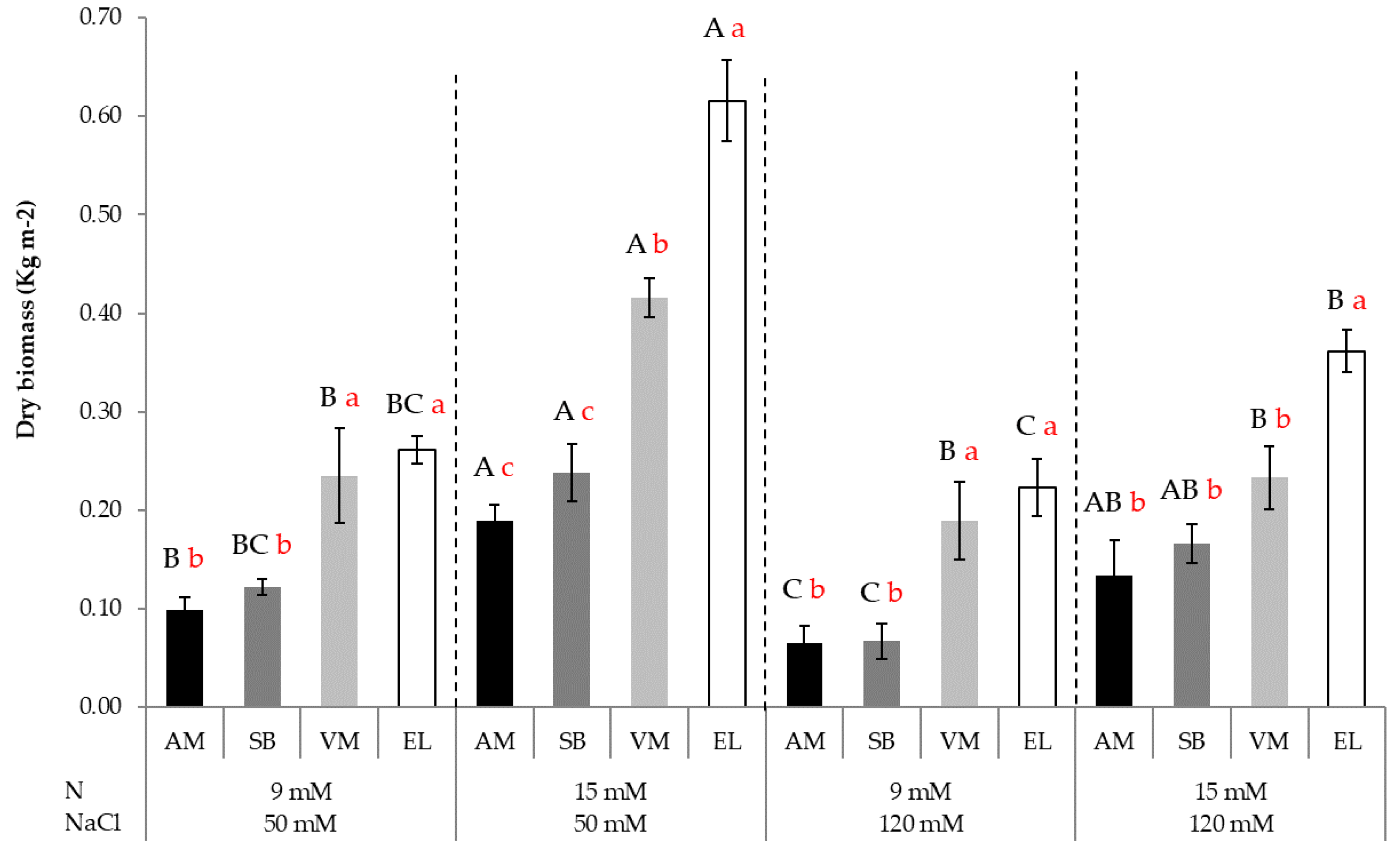
3.2. Dry Biomass Accumulation
3.3. Relative Water Content (RWC)
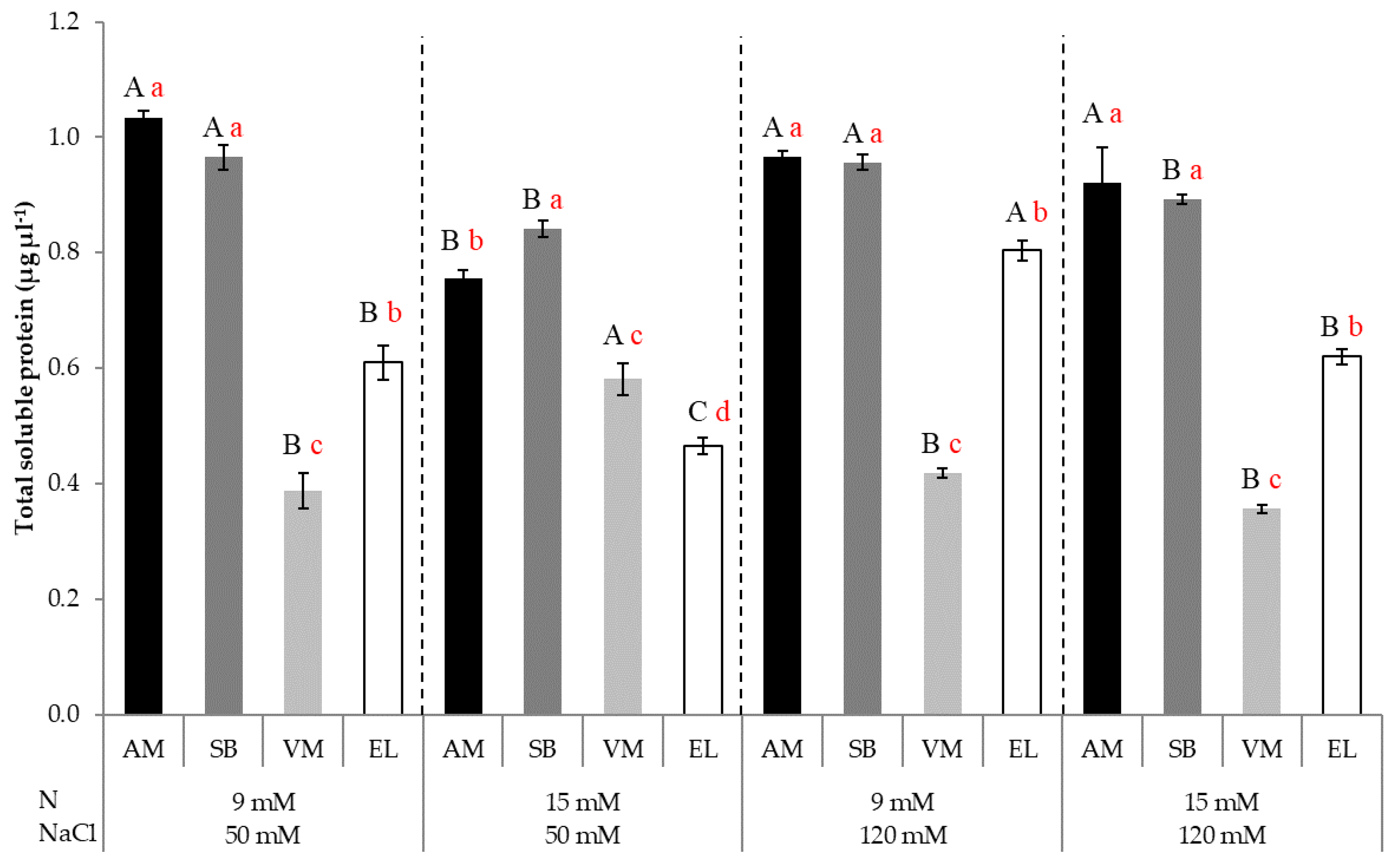
3.4. Total Soluble Protein Content
3.5. Total Soluble Solids Content
3.6. Anthocyanin Determination
3.7. Oxygen Radical Absorbance Capacity (ORAC)
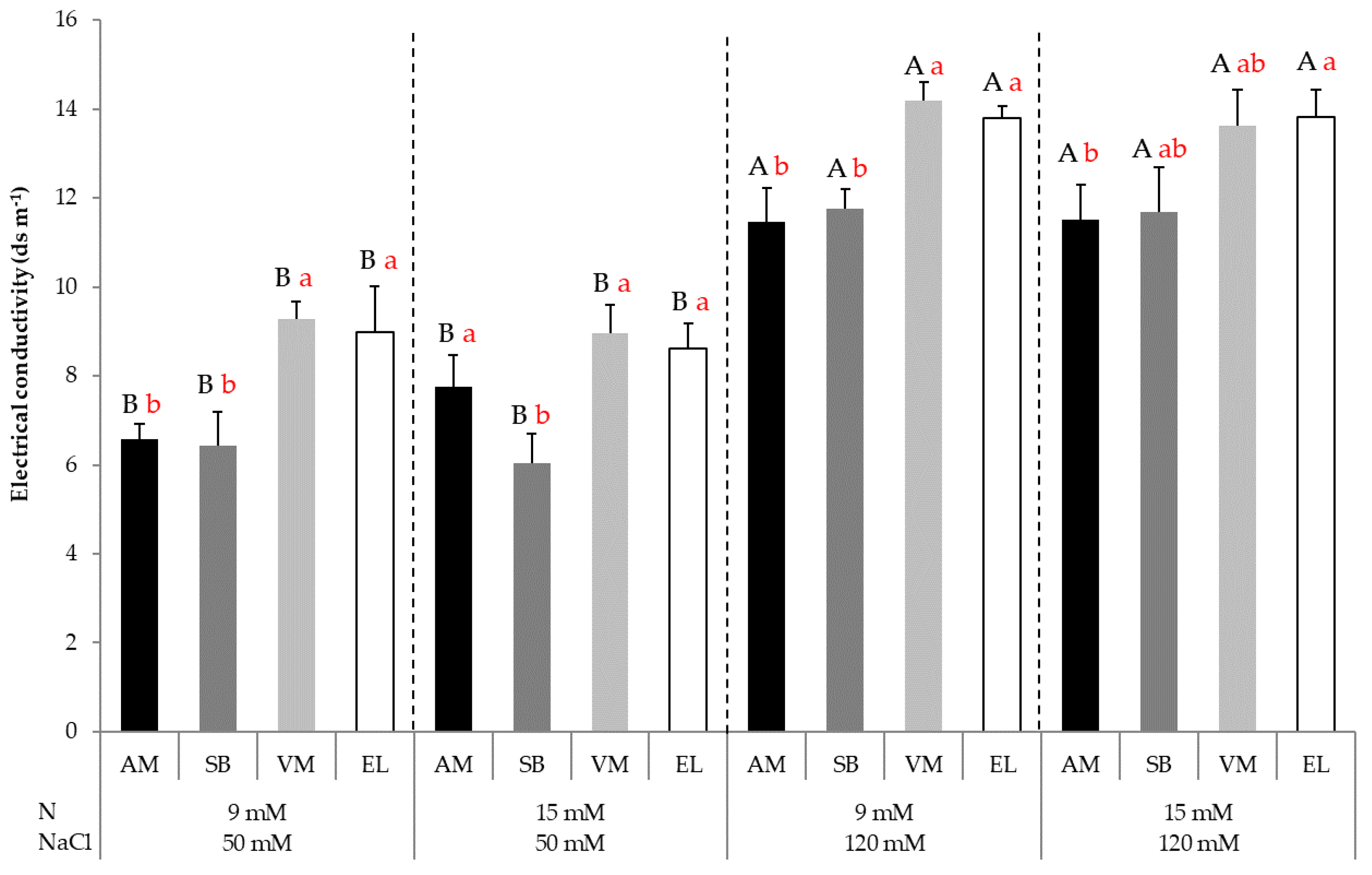
3.8. Electrical Conductivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Grese, M. Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. USA 2018, 115, E574–E583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wienhold, B.J. Soils, Land, and Food: Managing the Land during the Twenty-First Century. Soil Sci. 2003, 168, 748–749. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.J.; Hajibagheri, M.A.; Clipson, N.J.W. Halophytes. Quart. Rev. Biol. 1986, 61, 313–337. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Agudelo, A.; Carvajal, M.; del Carmen Martinez-Ballesta, M. Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change. Foods 2021, 10, 119. [Google Scholar] [CrossRef]
- Grieve, C.M.; Suarez, D.L. Purslane (Portulaca oleracea L.): A halophytic crop for drainage water reuses systems. Plant Soil 1997, 192, 277–283. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Ventura, Y.; Wuddineh, W.A.; Shpigel, M.; Samocha, T.M.; Klim, B.C.; Cohen, S.; Shemer, Z.; Santos, R.; Sagi, M. Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Sci. Hortic. 2011, 130, 510–516. [Google Scholar] [CrossRef]
- Custódio, L.; Ferreira, A.C.; Pereira, H.; Silvestre, L.; Vizetto-Duarte, C.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. The marine halophytes Carpobrotus edulis L. and Arthrocnemum macrostachyum L. are potential sources of nutritionally important PUFAs and metabolites with antioxidant, metal chelating and anticholinesterase inhibitory activities. Bot. Mar. 2012, 55, 281–288. [Google Scholar] [CrossRef]
- Stanley, O.D. Bio prospecting marine halophyte Salicornia brachiata for medical importance and salt encrusted land development. J. Coast. Dev. 2008, 11, 62–69. [Google Scholar]
- Shpigel, M.; Ben-Ezra, D.; Shauli, L.; Sagi, M.; Ventura, Y.; Samocha, T.; Lee, J.J. Constructed Wetland with Salicornia as a Biofilter for Mariculture Effluents. Aquaculture 2013, 412–413, 52–63. [Google Scholar] [CrossRef]
- Feigin, A.; Rylski, I.; Meiri, A.; Shalhevet, J. Response of melon and tomato plants to chloride-nitrate ratios in saline nutrient solutions. J. Plant Nutr. 1987, 10, 1787–1794. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in araucaria angustifolia. R. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Ventura, Y.; Mendlinger, S. Effects of suboptimal low temperature on yield, fruit appearance and quality in muskmelon (Cucumis melo L.) cultivars. J. Hortic. Sci. Biotechnol. 1999, 74, 602–607. [Google Scholar] [CrossRef]
- Laby, R.J.; Kincaid, M.S.; Kim, D.; Gibson, S.I. The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J. 2000, 23, 587–596. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Ephrath, Y.; Shpigel, M.; Sagi, M. Molybdenum as an essential element for improving total yield in seawater-grown Salicornia europaea L. Sci. Hortic. 2010, 126, 395–401. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Custódio, L.; Rodrigues, M.J.; Pereira, C.G.; Castañeda-Loaiza, V.; Fernandes, E.; Standing, D.; Neori, A.; Shpigel, M.; Sagi, M. A Review on Sarcocornia Species: Ethnopharmacology, Nutritional Properties, Phytochemistry, Biological Activities and Propagation. Foods 2021, 10, 2778. [Google Scholar] [CrossRef] [PubMed]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Comp. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Deghan, S.; Sadeghi, M.; Pöppel, A.; Fischer, R.; Lakes-Harlan, R.; Kavousi, H.R.; Vilcinskas, A.; Rahnamaeian, M. Differential inductions of phenylalanine ammonia-lyase and chalcone synthase during wounding, salicylic acid treatment, and salinity stress in safflower, Carthamus tinctorius. Biosci. Rep. 2014, 34, e00114. [Google Scholar] [CrossRef]
- Chunthaburee, S.; Sakuanrungsirikul, S.; Wongwarat, T.; Sanitchon, J.; Pattanagul, W.; Theerakulpisut, P. Changes in anthocyanin content and expression of anthocyanin synthesis genes in seedlings of black glutinous rice in response to salt stress. Asian J. Plant Sci. 2016, 15, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Chutipaijit, S.; Cha-um, S.; Sompornpailin, K. High contents of proline and anthocyanin increase protective response to salinity in Oryza sativa L. spp. indica. Aust. J. Crop Sci. 2011, 5, 1191–1198. [Google Scholar]
- Djanaguiraman, M.; Ramadass, R.; Devi, D.D. Effect of salt stress on germination and seedling growth in rice genotypes. Madras Agric. J. 2003, 90, 50–53. [Google Scholar]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Riedl, K.; Otegui, M.S.; Grotewold, E. Not all anthocyanins are born equal: Distinct patterns induced by stress in Arabidopsis. Planta 2014, 240, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Hurkman, W.J.; Fornari, C.S.; Tanaka, C.K. A comparison of the effect of salt on polypeptide and translatable mRNA in roots of a salt tolerant and salt sensitive cultivar of barley. Plant Physiol. 1989, 90, 1444–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareek, A.; Singla, S.L.; Grover, A. Salt responsive proteins/genes in crop plants. In Strategies for Improving Salt Tolerance in Higher Plants; Jaiwal, P.K., Singh, R.P., Gulati, A., Eds.; Oxford and IBH Publication Co.: New Delhi, India, 1997; pp. 365–391. [Google Scholar]
- Ali, G.; Srivastava, P.S.; Iqbal, M. Proline accumulation, protein pattern and photosynthesis in regenerants grown under NaCl stress. Biol. Plant. 1999, 42, 89–95. [Google Scholar] [CrossRef]
- Ashraf, M.; O’Leary, J.W. Changes in soluble proteins in spring wheat stressed with sodium chloride. Biol. Plant. 1999, 42, 113–117. [Google Scholar] [CrossRef]
- Singh, N.K.; Bracken, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A.; Pfankoch, F.; Regnier, F.E.; Bressan, R.A. Characterization of osmotin. A thaumatin-like protein associated with osmotic adjustment in plant cells. Plant Physiol. 1987, 85, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. In vivo total antioxidant capacity: Comparison of different analytical methods. Free Rad. Biol. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of Highbush Blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- Ben Amor, N.; Jiménez, A.; Boudabbous, M.; Sevilla, F.; Abdelly, C. Chloroplast Implication in the Tolerance to Salinity of the Halophyte Cakile maritima. Russ. J. Plant Physiol. 2020, 67, 507–514. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, A.; Kumar, N.; Lata, C.; Mann, A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J. Bio. Sci. 2021, 28, 2510–2517. [Google Scholar] [CrossRef]
- Kapoor, N.; Pande, V. Effect of salt stress on growth parameters, moisture content, relative water content and photosynthetic pigments of Fenugreek variety RMt-1. J. Plant Sci. 2015, 10, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Implications of Salt Tolerance in the Halophyte Salvadora persica under Hydroponic Culture Condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Shi, D.; Wang, D. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Zakery-Asl, M.A.; Bolandnazar, S.; Oustan, S. Effect of salinity and nitrogen on growth, sodium, potassium accumulation, and osmotic adjustment of halophyte Suaeda aegyptiaca (Hasselq.) Zoh. Arch. Agron. Soil Sci. 2014, 60, 785–792. [Google Scholar] [CrossRef]
- Ventura, Y.; Myrzabayeva, M.; Alikulov, Z.; Omarov, R.; Khozin-Goldberg, I.; Sagi, M. Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants 2014, 6, plu053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Env. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Kurmanbayeva, A.; Bekturova, A.; Srivastava, S.; Soltabayeva, A.; Asatryan, A.; Ventura, Y.; Khan, M.S.; Salazar, O.; Fedoroff, N.; Sagi, M. Higher novel L-Cys degradation activity results in lower organic-S and biomass in Sarcocornia than the related saltwort, Salicornia. Plant Physiol. 2017, 175, 272–289. [Google Scholar] [CrossRef] [Green Version]





| Treatment | Input | Leaching Fraction | |
|---|---|---|---|
| Nitrogen (mM) | NaCl (mM) | EC (ds m−1) | EC (ds m−1) |
| 9.42 | 50 | 5.87 | 8.12 ± 0.21 |
| 15.42 | 50 | 6.19 | 8.45 ± 0.22 |
| 9.42 | 120 | 11.87 | 14.76 ± 0.84 |
| 15.42 | 120 | 11.95 | 16.1 ± 0.84 |
| Harvests | Fresh Biomass (kg m2) | Total Biomass (kg m2) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | ||
| S. brachiata (SB) | 2.89 ± 0.53 | 10.38 ± 0.33 | 5.72 ± 0.09 | 7.18 ± 0.60 | 4.62 ± 0.95 | 7.20 ± 0.78 | 1.7 ± 0.10 | 39.73 ± 1.10 |
| S. fruticosa (VM) | 0.26 ± 0.07 | 1.00 ± 0.15 | 0.90 ± 0.36 | 1.00 ± 0.55 | 1.75 * | 4.70 * | 5.0 * | 14.60 ± 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisay, T.A.; Nurbekova, Z.; Oshanova, D.; Dubey, A.K.; Khatri, K.; Mudgal, V.; Mudgal, A.; Neori, A.; Shpigel, M.; Srivastava, R.K.; et al. Effect of Salinity and Nitrogen Fertilization Levels on Growth Parameters of Sarcocornia fruticosa, Salicornia brachiata, and Arthrocnemum macrostachyum. Agronomy 2022, 12, 1749. https://doi.org/10.3390/agronomy12081749
Sisay TA, Nurbekova Z, Oshanova D, Dubey AK, Khatri K, Mudgal V, Mudgal A, Neori A, Shpigel M, Srivastava RK, et al. Effect of Salinity and Nitrogen Fertilization Levels on Growth Parameters of Sarcocornia fruticosa, Salicornia brachiata, and Arthrocnemum macrostachyum. Agronomy. 2022; 12(8):1749. https://doi.org/10.3390/agronomy12081749
Chicago/Turabian StyleSisay, Tesfaye Asmare, Zhadyrassyn Nurbekova, Dinara Oshanova, Arvind Kumar Dubey, Kusum Khatri, Varsha Mudgal, Anurag Mudgal, Amir Neori, Muki Shpigel, Rajeev Kumar Srivastava, and et al. 2022. "Effect of Salinity and Nitrogen Fertilization Levels on Growth Parameters of Sarcocornia fruticosa, Salicornia brachiata, and Arthrocnemum macrostachyum" Agronomy 12, no. 8: 1749. https://doi.org/10.3390/agronomy12081749






