Model Development of the Phenological Cycle from Flower to Fruit of Strawberries (Fragaria × ananassa)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Description
2.2. Plant Material and Growth Conditions
2.3. Phenological Observations
Identification of the Stages
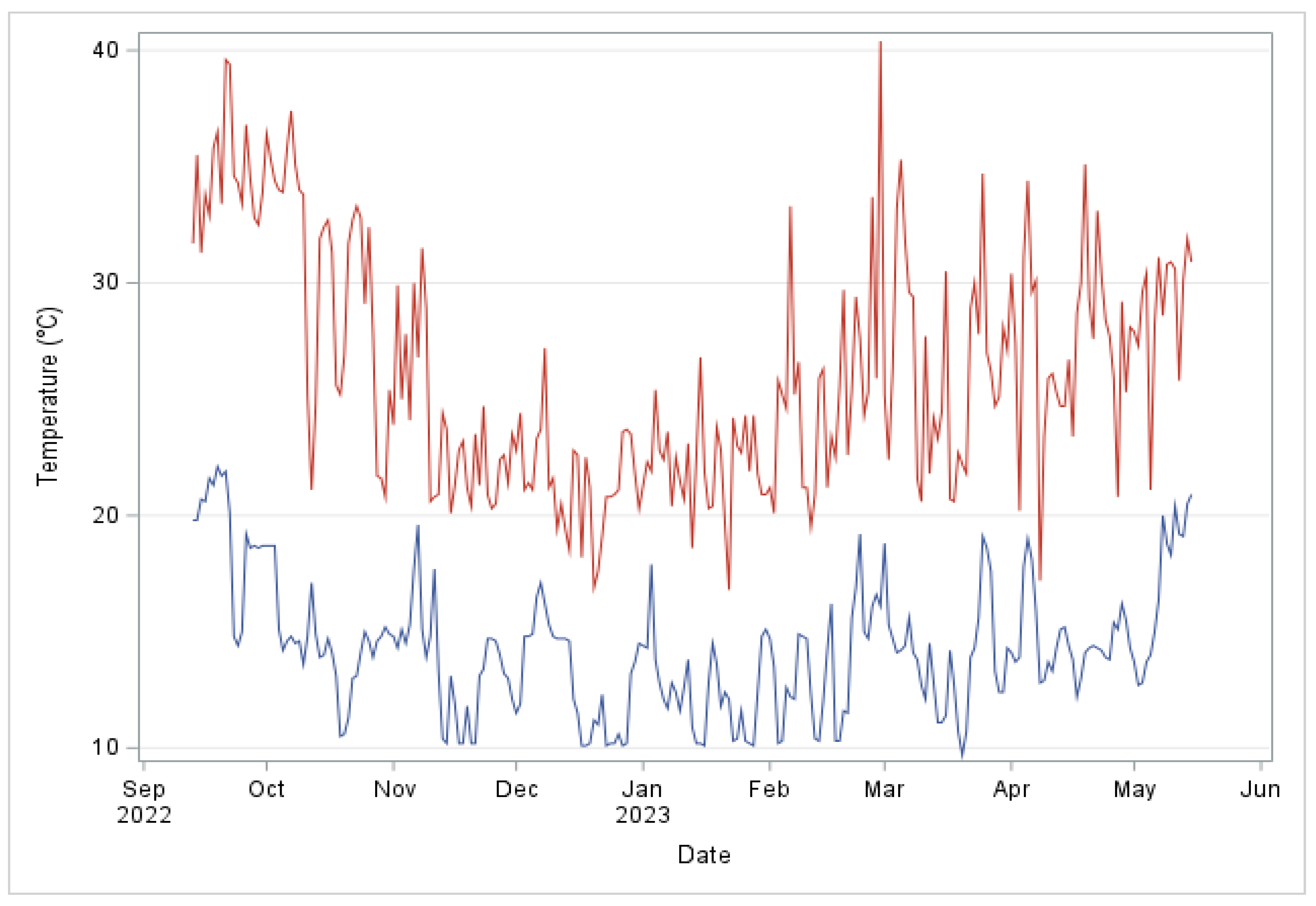
2.4. Weather Data
2.5. Data Analysis
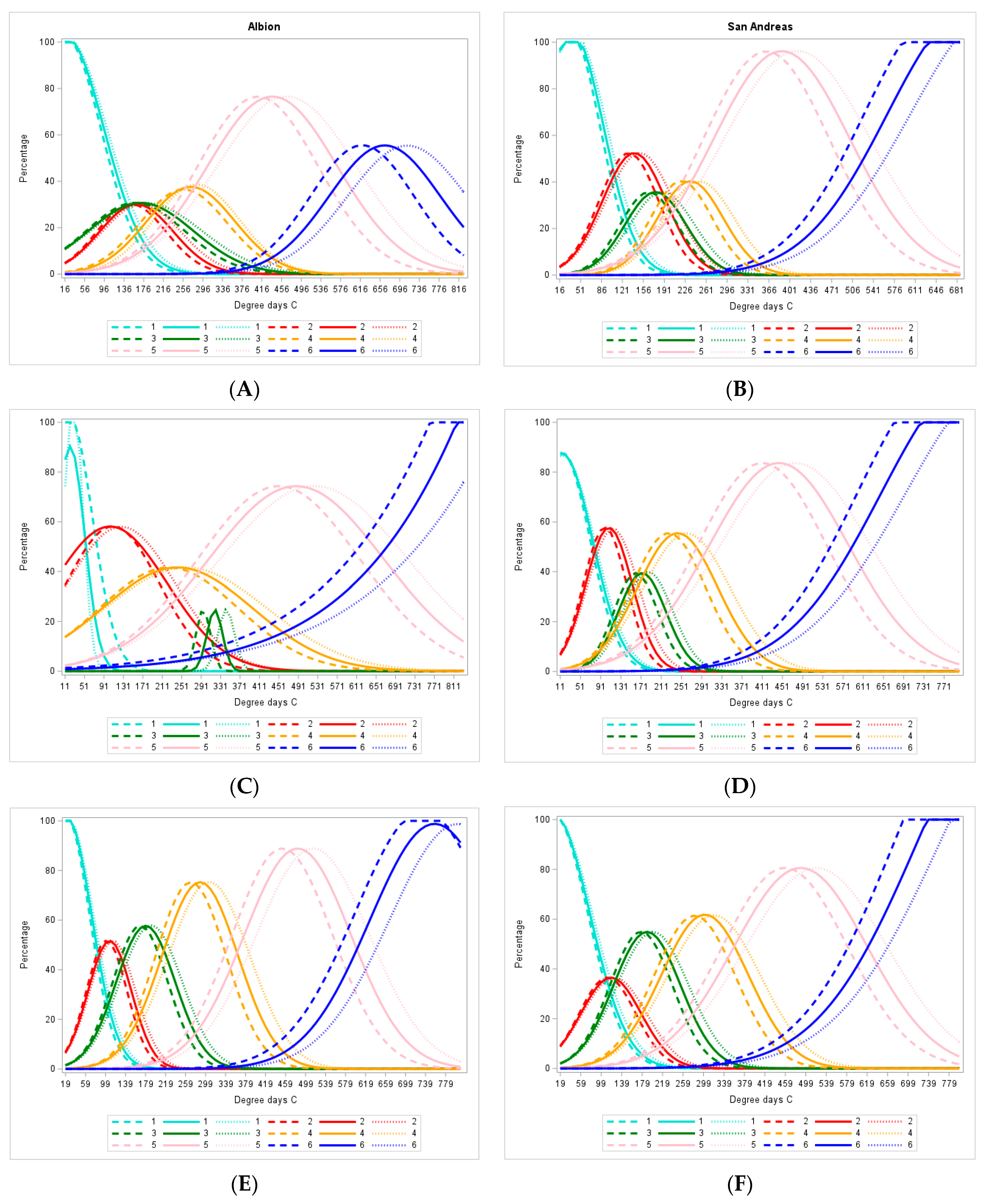
3. Results
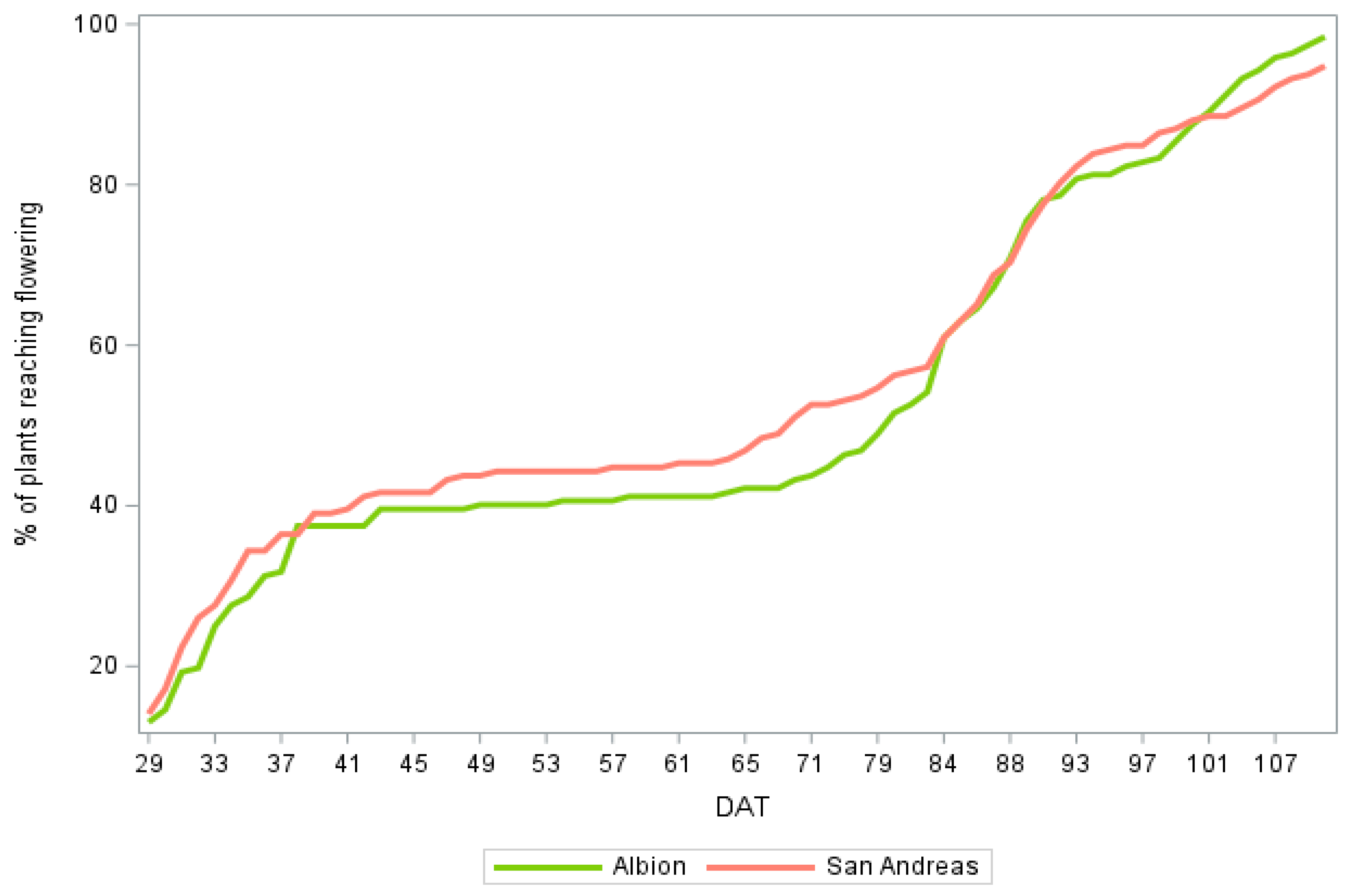
3.1. Flowering Rate of the Crop
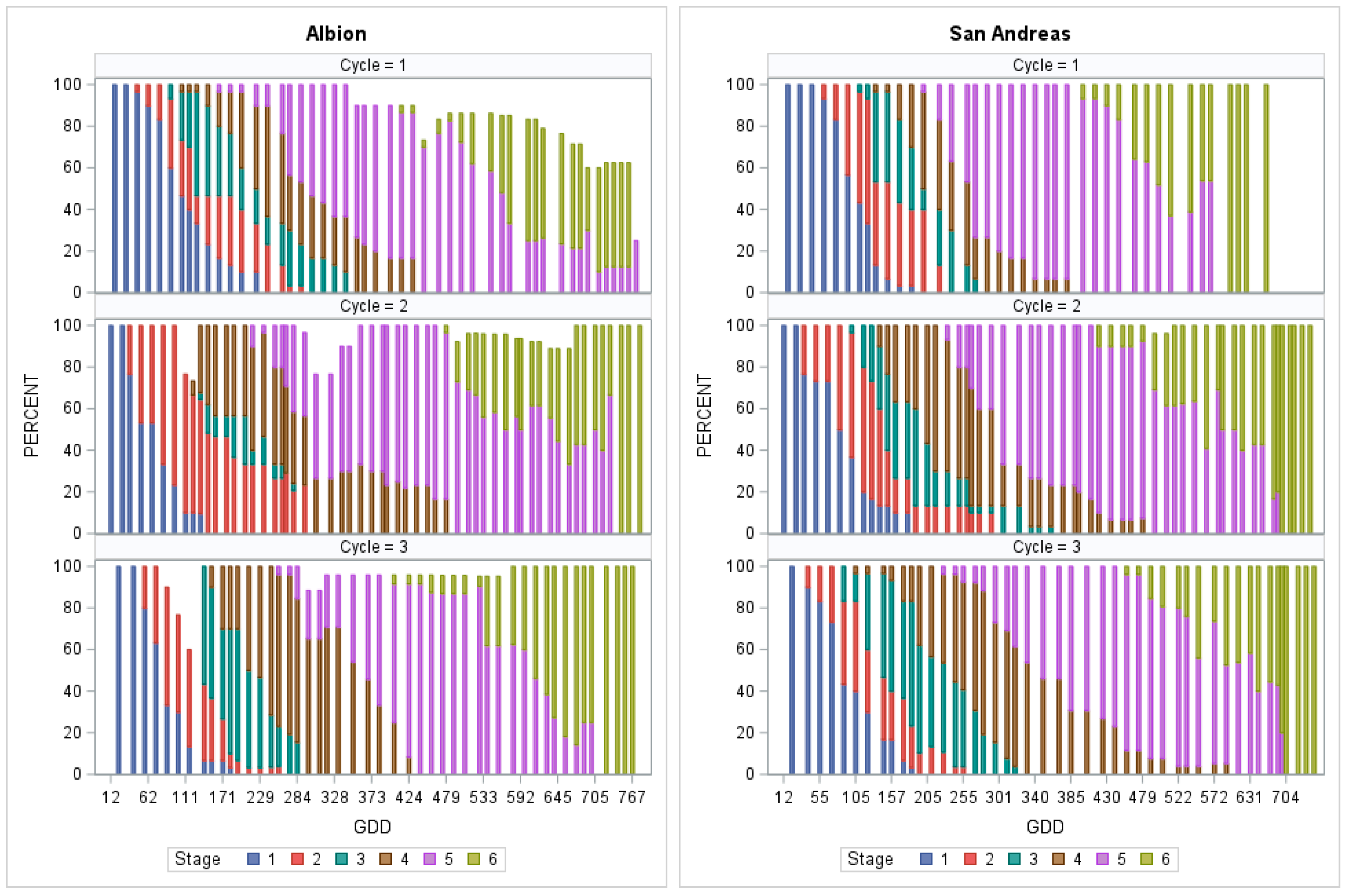
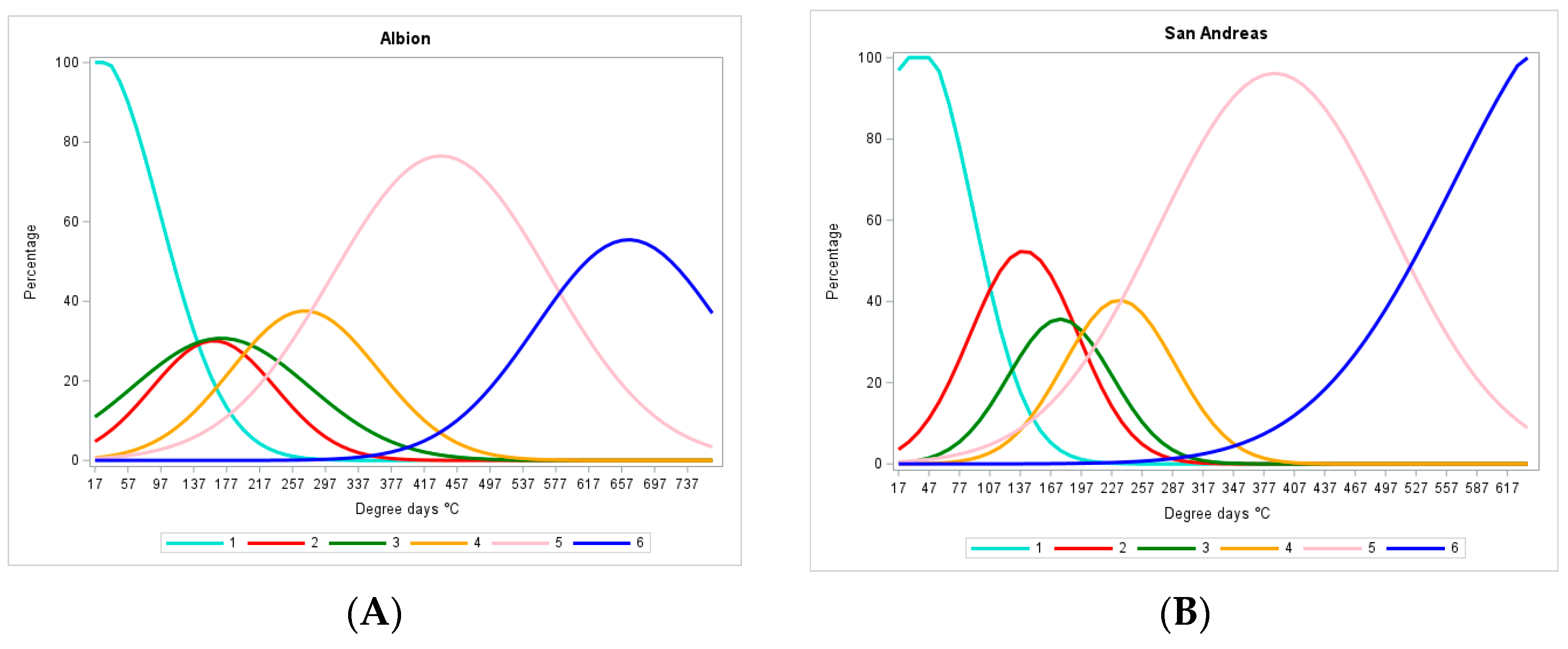
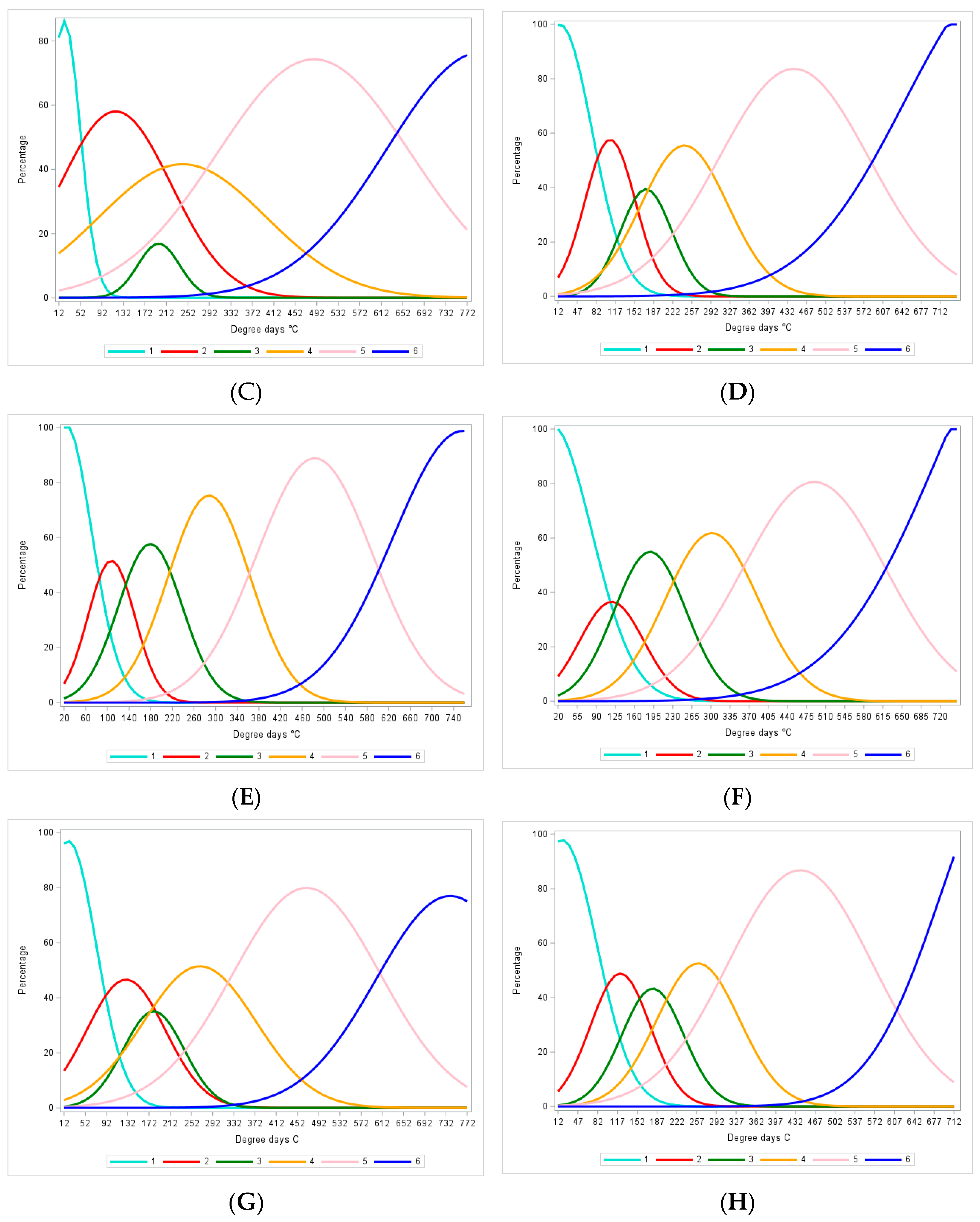
3.2. Identification of Stages
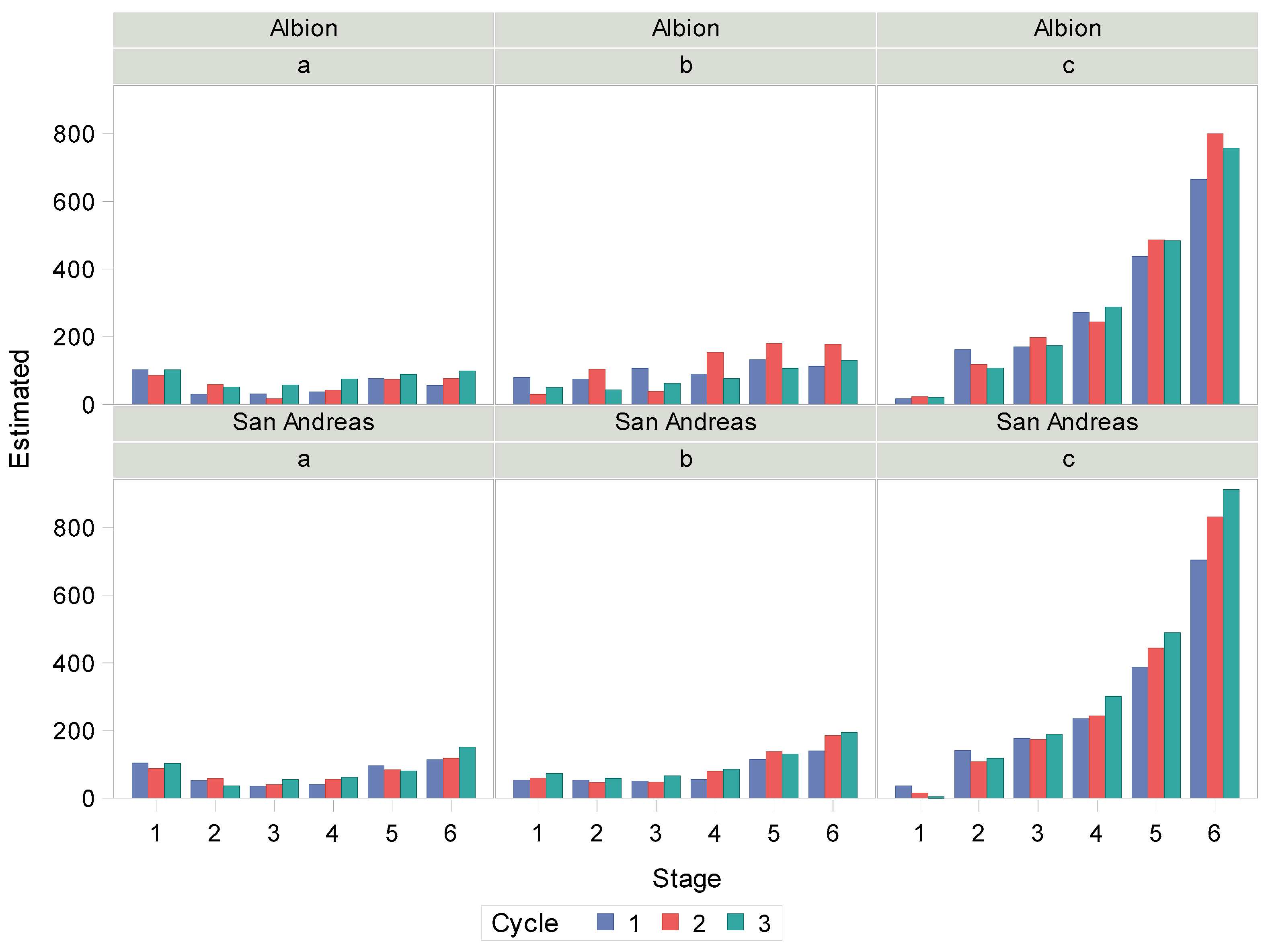
3.3. Duration of Stages
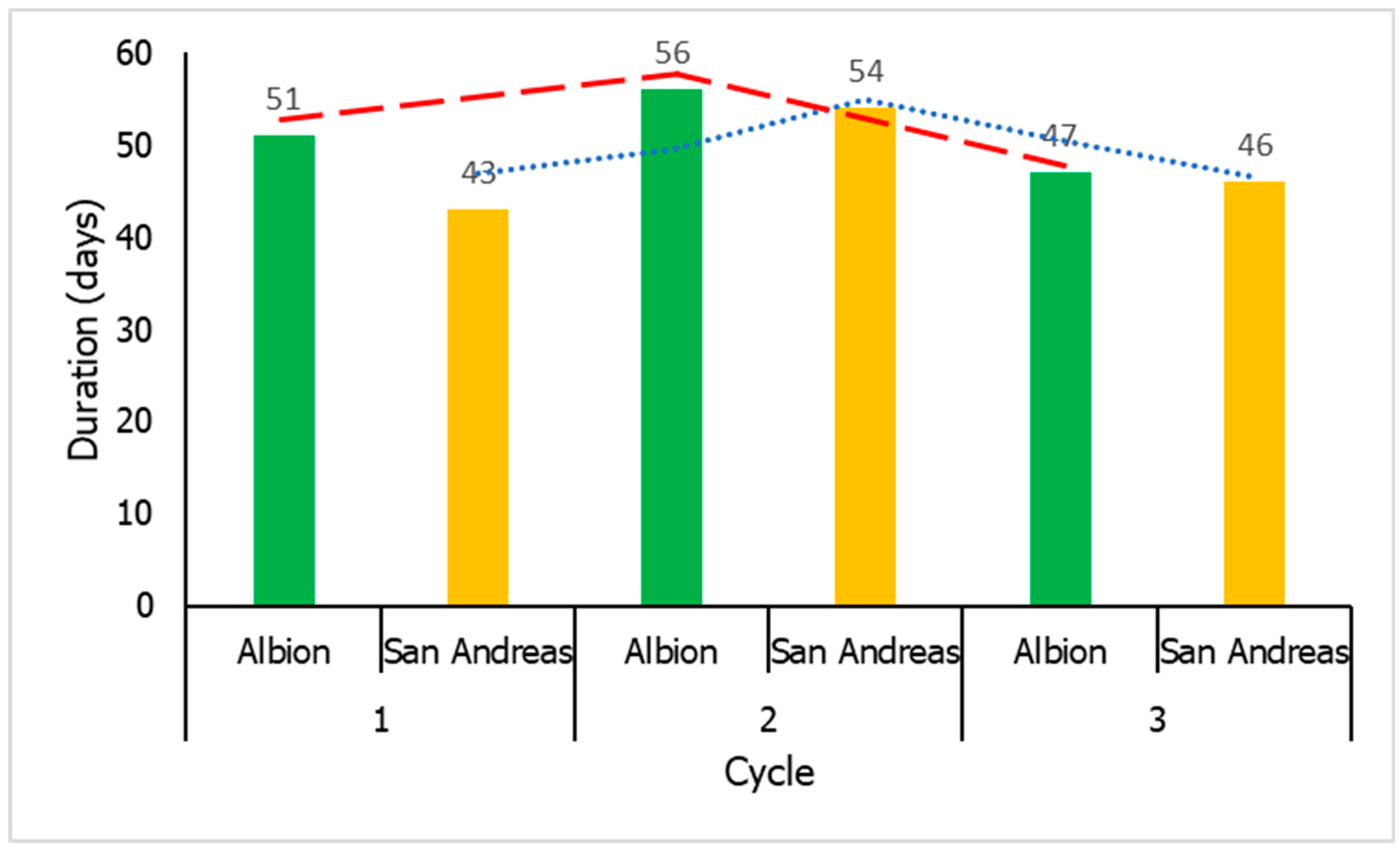
3.4. Duration of Cycle
3.5. Estimating the Parameters of the Model
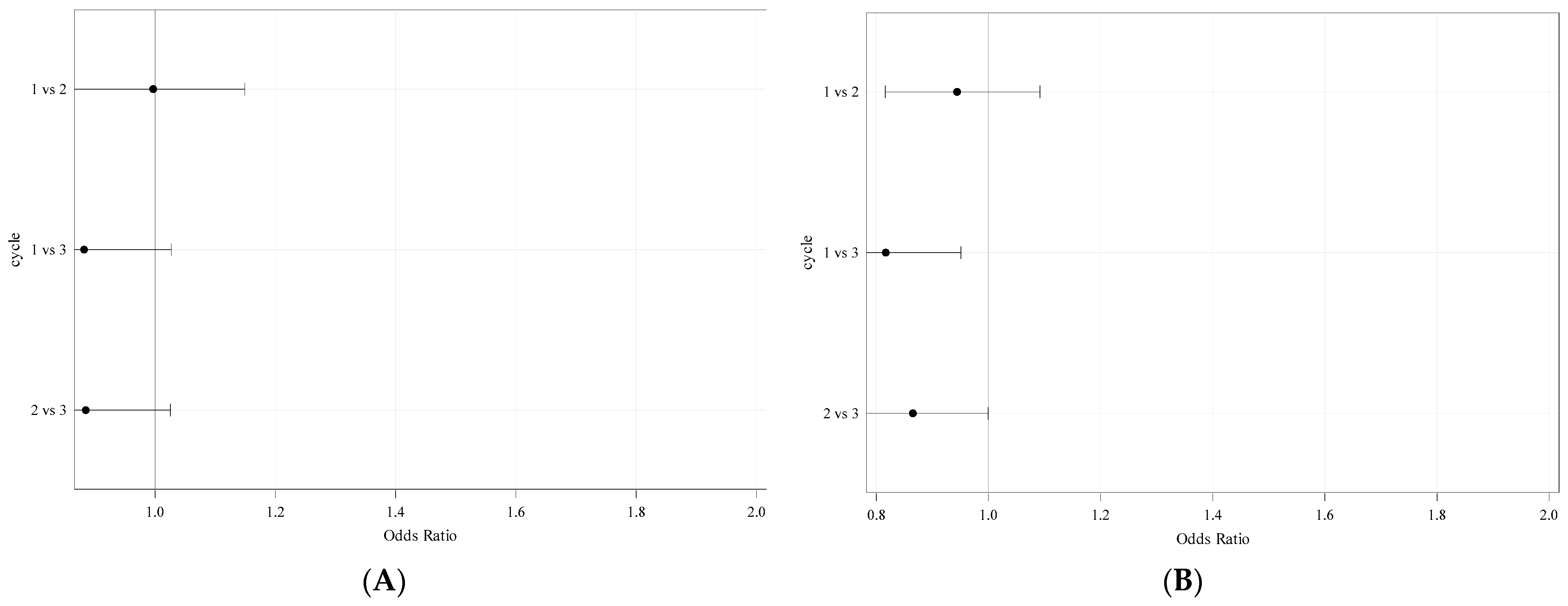
3.6. Sensitivity of the Model
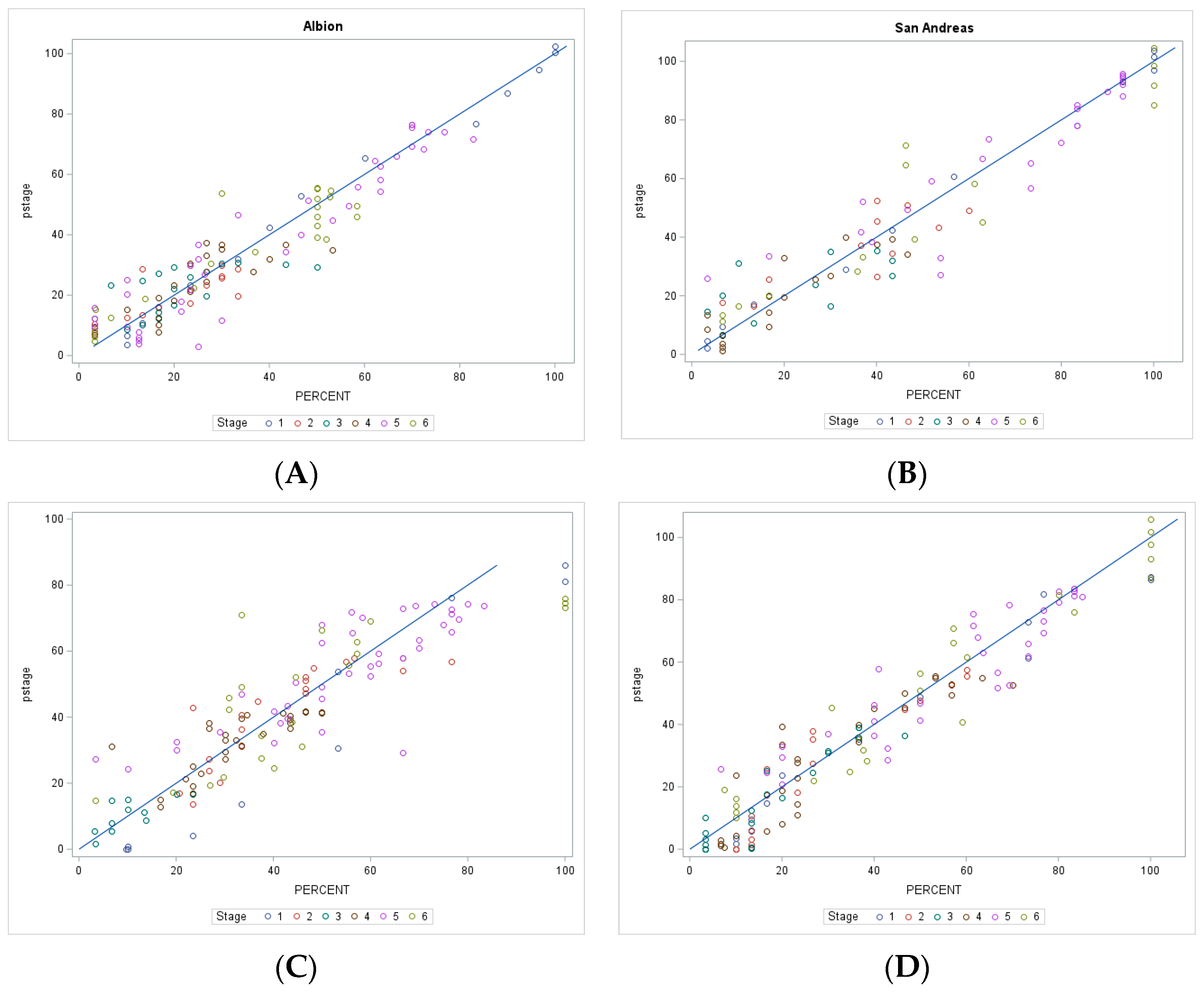
3.7. Model Evaluation
4. Discussion
4.1. Flowering Rate
4.2. Identification and Duration of Stages
4.3. Duration of Cycle
4.4. Estimating the Parameters of the Model
4.5. Model Simulations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darnell, R.; Cantliffe, D.; Kirschbaum, D.; Chandler, C. The physiology of flowering in strawberry. Hortic. Rev. 2003, 28, 325–349. [Google Scholar] [CrossRef]
- Sønsteby, A.; Roos, U.; Heide, O. Phenology, flowering, and yield performance of 13 diverse strawberry grown under Nordic field conditions. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2016, 67, 278–283. [Google Scholar] [CrossRef]
- Heide, O.; Stavang, J.; Sonsteby, A. Physiology and genetics of flowering in cultivated and wild strawberries—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 1–18. [Google Scholar] [CrossRef]
- Serce, S.; Hancock, J. The temperature and photoperiod regulation of flowering and runnering in the strawberries, Fragaria chiloensis, F. virginiana, and F. x ananassa. Sci. Hortic. 2005, 103, 167–177. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O. Long-day control of flowering in everbearing strawberries. J. Hortic. Sci. Biotechnol. 2007, 82, 875–884. [Google Scholar] [CrossRef]
- Manakasem, Y.; Goodwinm, P. Using the floral status of strawberry plants, as determined by stereomicroscopy and scanning electron microscopy to survey the phenology of commercial crops. J. Am. Soc. Hort. Sci. 1998, 123, 513–517. [Google Scholar] [CrossRef]
- Durner, E.F.; Poling, E.B. Early season yield response of selected strawberry cultivars to photoperiods and chilling in a Florida Winter production system. J. Am. Soc. Hort. Sci. 1986, 111, 53–56. [Google Scholar] [CrossRef]
- Durner, E.F.; Barden, J.A.; Himelrick, D.G.; Poling, E.B. Photoperiod and temperatura effects on flower and runner development in day-neutral, June bearing and everbearing strawberries. J. Am. Soc. Hortic. Sci. 1984, 109, 396–400. [Google Scholar] [CrossRef]
- Gude, K.; Stanley, H.; Rivard, C.L.; Cunningham, B.; Kang, Q.; Pliakoni, E.D. Quality of day-neutral strawberries grown in a high tunnel system. Sci. Hortic. 2021, 275, 109726. [Google Scholar] [CrossRef]
- Rowley, D.; Black, B.L.; Drost, D. Late-season strawberry production using day-neutral cultivars in hight-elevation hight tunnels. HortScience 2011, 46, 1480–1485. [Google Scholar] [CrossRef]
- Lopez, G.; Runkle, E. Growing your Crops above Their Base Temperature. The Basics and Beyond. Purdue University. Greenhouse Grower. 2014. Available online: https://www.purdue.edu/hla/sites/cea/wp-content/uploads/sites/15/2014/07/BasicsandBeyond-July.pdf (accessed on 21 August 2023).
- Rosa, H.T.; Walter, L.C.; Streck, N.A.; Andriolo, J.L.; da Silva, M.R.; Langner, J.A. Base temperature for leaf appearance and phyllochron of selected strawberry cultivars in a subtropical environment. Bragantia Camp. 2011, 70, 939–945. [Google Scholar] [CrossRef]
- Strand, L.L. Strawberry growth and development. In Integrated Pest Management for Strawberries; CAB International: Davis, CA, USA, 1994. [Google Scholar]
- Zeist, A.; Resende, J.; Lima, R.; Gabriel, A.; Henschel, J.; Silva, I. Phenology and agronomic components of first and second-cycle strawberry. Hortic. Bras. 2019, 37, 29–34. [Google Scholar] [CrossRef]
- Mendonca, H.; Oliveira, E.; Castoldi da Costa, R.; Nienow, A. Performance production of strawberry in environment cultivated with fig tree. Rev. Bras. Frutic. 2016, 39, e-615. [Google Scholar] [CrossRef]
- Menzel, C. No evidence of Excessive leaf production by strawberries grown in the subtropics. Agriculture 2019, 9, 197. [Google Scholar] [CrossRef]
- Doving, A.; Mage, F. Prediction of strawberry fruit yield. Acta Agric. Scand. Sect. B-Plant Soil Sci. 2010, 51, 35–42. [Google Scholar] [CrossRef]
- Krüger, E.; Josuttis, M.; Nestby, R.; Toldam-Andersen, T.; Carlen, C.; Mezzetti, B. Influence of growing conditions at different latitudes of Europe on strawberry growth performance, yield, and quality. J. Berry Res. 2012, 2, 143–157. [Google Scholar] [CrossRef]
- Geoffrey, T.; Bi, G.; Zhang, Q.; Harkess, R.; Li, T. High-tunnel production of strawberries using black and red plastic mulches. Horticulturae 2020, 6, 73. [Google Scholar] [CrossRef]
- Alavarado-Raya, H.; Vásquez-Rodríguez, J.; Ramírez-Arias, A.; Calderón-Zavala, G.; Rivera-del-Rio, R. Phenology and growing degree days of festival strawberry grown on red volcanic rock at two plant densities. Rev. Fitotec. Mex. 2021, 44, 349–356. [Google Scholar] [CrossRef]
- Martin, C.; Fountain, M.; Brown, M. Varietal and seasonal differences in the effects of commercial bumblebees on fruit quality in strawberry crops. Agric. Ecosyst. Environ. 2019, 281, 124–133. [Google Scholar] [CrossRef]
- Johnson, A.; Simpson, D. The effect of deficit irrigation on the flowering behavior of two day-neutral and one everbearing strawberry cultivar. Acta Hortic. 2014, 1049, 435–438. [Google Scholar] [CrossRef]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N. Z. J. Crop Hortic. Sci.-Taylor Fr. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.; Gómez-Merino, F. Nutrient management in strawberry: Effects on yield, quality, and plant health. In Strawberries: Cultivation, Antioxidant Properties and Health Benefits, 1st ed.; Malone, N., Ed.; Nova Science Publishers: Veracruz, Mexico, 2014; pp. 239–267. Available online: https://www.researchgate.net/publication/268037619 (accessed on 7 July 2023).
- Vervoort, M.; Meli, P.; Hanssens, J.; Craeye, S.; Pisman, M.; Smagghe, G.; Clymans, R.; Belien, T. Thrips control with predatory mites A. limonicus and A. swirskii in different strawberry cultivation systems. Acta Hortic. 2017, 1156, 833–841. [Google Scholar] [CrossRef]
- Wang, R.; Eguchi, M.; Gui, Y.; Iwasaki, Y. Evaluating the effect of light intensity on flower development uniformity in strawberry (Fragaria × ananassa) under early induction conditions in forcing culture. HortScience 2020, 55, 670–675. [Google Scholar] [CrossRef]
- Hummer, K.; Bassil, N.; Zurn, J.; Amyotte, V. Phenotypic characterization of strawberry (Fragaria × ananassa Duchesne ex Rosier) diversity collection. Plants People Planet 2022, 5, 209–224. [Google Scholar] [CrossRef]
- Hopf, A.; Boote, K.; Oh, J.; Guan, Z.; Agehara, S.; Shelia, V.; Whitaker, V.; Asseng, S.; Zhao, X.; Hoogenboom, G. Development and improvement of the CROPGRO-Strawberry model. Sci. Hortic. 2022, 291, 110538. [Google Scholar] [CrossRef]
- Whiting, M.D.; Salazar, M.R.; Hoogenboom, G. Development of bloom phenology models for tree fruits. Acta Hortic. 2015, 1068, 107–112. [Google Scholar] [CrossRef]
- Salazar, M.R.; Jones, J.W.; Chaves, B.; Cooman, A.; Fischer, G. Base temperature and simulation model for nodes appearance in Cape gooseberry (Physalis peruviana L.). Rev. Bras. Frutic. Jaboticabal 2008, 30, 862–867. [Google Scholar] [CrossRef]
- Lu, Y.; Gong, M.; Li, J.; Ma, J. Optimizing Controlled Environmental Agriculture for Strawberry Cultivation Using RL-Informer Model. Agronomy 2023, 13, 2057. [Google Scholar] [CrossRef]
- Oliveira, L.S.d.; Castoldi, R.; Martins, G.D.; Medeiros, M.H. Estimation of Strawberry Crop Productivity by Machine Learning Algorithms Using Data from Multispectral Images. Agronomy 2023, 13, 1229. [Google Scholar] [CrossRef]
- Basak, J.K.; Paudel, B.; Kim, N.E.; Deb, N.C.; Kaushalya Madhavi, B.G.; Kim, H.T. Non-Destructive Estimation of Fruit Weight of Strawberry Using Machine Learning Models. Agronomy 2022, 12, 2487. [Google Scholar] [CrossRef]
- Raj, R.; Cosgun, A.; Kuli’c, D. Strawberry Water Content Estimation and Ripeness Classification Using Hyperspectral Sensing. Agronomy 2022, 12, 425. [Google Scholar] [CrossRef]
- Labadie, M.; Denoyes, B.; Guédon, Y. Identifying phenological phases in strawberry using multiple change-point models. J. Exp. Bot. 2019, 70, 5687–5701. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Temperature responses, flowering and fruit yield of the June-bearing strawberry cultivars Florence, Frida and Korona. Sci. Hortic. 2008, 119, 49–54. [Google Scholar] [CrossRef]
- Opstad, N.; Sønsteby, A.; Myrheim, U.; Heide, O.M. Seasonal timing of floral initiation in strawberry: Effects of cultivar and geographic location. Sci. Hortic. 2011, 129, 127–134. [Google Scholar] [CrossRef]
- Zavalloni, C.; Andresen, J.A.; Flore, J.A. Phenological models of flower bud stages and fruit growth of ‘Montmorency’ sour cherry based on growing degree-day accumulation. J. Amer. Soc. Hort. Sci. 2006, 131, 601–607. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Hess, M.; Lancashire, P.; Schnock, U.; Stoß, R.; Boom, T.; et al. The BBCH system to coding the phenological growth stages of plants. Nachrichtenbl. Deut. Pflanzenschutzd 1994, 46, 141–153. [Google Scholar]
- Willmott, C.J. On the validation of models. Phys. Geogr. 1981, 2, 184–194. [Google Scholar] [CrossRef]
- Willmott, C.J. Some comments on the evaluation of model performance. Bull. Am. Meteorol. Soc. 1982, 63, 1309–1313. [Google Scholar] [CrossRef]
- Willmott, C.J.; Ackleson, S.G.; Davis, R.E.; Feddema, J.J.; Klink, K.M.; Legates, D.R.; O’Donnell, J.; Rowe, C.M. Statistics for the evaluation and comparison of models. J. Geophys. Res. Ocean. 1985, 90, 8995–9005. [Google Scholar] [CrossRef]
- Janssen, P.H.; Heuberger, P.S. Calibration of process-oriented models. Ecol. Model. 1995, 83, 55–66. [Google Scholar] [CrossRef]
- Konsin, M.; Voipio, I.; Palonen, P. Influence of photoperiod and duration of short-day treatment on vegetative growth and flowering of strawberry (Fragaria × ananassa Duch.). J. Hortic. Sci. Biotechnol. 2015, 76, 77–82. [Google Scholar] [CrossRef]
- Alvarado-Raya, H.E.; Rivera-del-Rio, R.; Ramirez-Arias, A.; Calderon-Zavala, G. Phenological description of three Mexican new cultivars of strawberry (Fragaria × ananassa Dutch.) in vegetative propagation grown on volcanic gravel. Acta. Hortic. 2019, 1265, 281–285. [Google Scholar] [CrossRef]
- Krüger, E.; Woznicki, T.L.; Heide, O.M.; Kusnierek, K.; Rivero, R.; Masny, A.; Sowik, I.; Brauksiepe, B.; Eimert, K.; Mott, D.; et al. Flowering phenology of six seasonal-flowering strawberry cultivars in a coordinated European study. Horticulturae 2022, 8, 933. [Google Scholar] [CrossRef]
- Madhuri, K.; Manju; Nidhika, T. Studies on floral biology in strawberry (Fragaria x ananassa) under hilly conditions of Uttarakhand (bearing habit, time and duration of flowering, bud development, flower characters, antesis, and dehiscence). Int. J. Agric. Sci. 2016, 8, 3177–3180. Available online: http://www.bioinfopublication.org/jouarchive.php?opt=&jouid=BPJ0000217 (accessed on 25 January 2023).
- Bethere, L.; Sile, T.; Sennikovs, J.; Bethers, U. Impacto f climate change on the timing of strawberry phenological processes in the Baltic States. Est. J. Earth Sci. 2016, 65, 48–58. [Google Scholar] [CrossRef]
- Parra-Coronado, A.; Fischer, G.; Chaves-Cordoba, B. Thermal time for reproductive phenological stages of pineapple guava (Acca sellowiana (O. Berg) Burret). Acta Biol. Colomb. 2015, 20, 163–173. [Google Scholar] [CrossRef]
- Le Miere, P.; Hadley, P.; Darby, J.; Battley, H. The effect of termal environment, planting date and crown size on growth, development and yield of Fragaria × ananassa Dutch. Cv Elsanta. J. Hortic. Sci. Biotechnol. 1998, 73, 786–795. [Google Scholar] [CrossRef]
- Cao, Z.; Qu, H. Simulation-based modelling of growth and pollination of greenhouse strawberry. arXiv 2022, arXiv:2210.14522. [Google Scholar] [CrossRef]
- McWhirt, A. Part I|Annual Plasticulture Strawberry Basics & Crop Development. Southeastern Plasticulture Strawberry School, University of Arkansas. 2021. Available online: https://www.youtube.com/watch?v=TcfUYv5rSRc&t=37s (accessed on 21 August 2023).
- Cao, Z.; He, G.; Mu, S.; Qu, H. Effects of Bee Density and Hive Distribution on Pollination Efficiency for Greenhouse Strawberries: A Simulation Study. Agronomy 2023, 13, 731. [Google Scholar] [CrossRef]
- Salazar, M.R.; Chaves-Cordoba, B.; Jones, J.W.; Cooman, A. A simple phenological and potential production model for cape gooseberry (Physalis peruviana L.). In ISHS Acta Horticulturae 718, Proceedings of the III International Symposium on Models for Plant Growth, Environmental Control and Farm Management in Protected Cultivation (HortiModel 2006); Marcelis, L.F.M., van Straten, G., Stanghellini, C., Heuvelink, E., Eds.; ISHS: Wageningen, The Netherlands, 2006. [Google Scholar]


| Cultivar | Cycle | Stage | Initial DAT | Final DAT | Initial GDD | Final GDD | Duration in DAT | Duration in GDD |
|---|---|---|---|---|---|---|---|---|
| ‘Albion’ | 1 | 1 | 42 | 55 | 17 | 222 | 13 | 205 |
| 1 | 2 | 44 | 59 | 48 | 287 | 15 | 239 | |
| 1 | 3 | 47 | 66 | 92 | 378 | 19 | 286 | |
| 1 | 4 | 48 | 73 | 108 | 471 | 25 | 363 | |
| 1 | 5 | 52 | 88 | 169 | 693 | 36 | 524 | |
| 1 | 6 | 69 | 93 | 416 | 760 | 24 | 344 | |
| 2 | 1 | 105 | 114 | 12 | 140 | 9 | 128 | |
| 2 | 2 | 107 | 126 | 37 | 294 | 19 | 257 | |
| 2 | 3 | 112 | 130 | 111 | 349 | 18 | 238 | |
| 2 | 4 | 113 | 145 | 127 | 547 | 32 | 420 | |
| 2 | 5 | 120 | 158 | 145 | 732 | 38 | 587 | |
| 2 | 6 | 140 | 161 | 479 | 776 | 21 | 297 | |
| 3 | 1 | 168 | 178 | 20 | 185 | 10 | 165 | |
| 3 | 2 | 170 | 183 | 55 | 255 | 13 | 200 | |
| 3 | 3 | 172 | 191 | 88 | 368 | 19 | 280 | |
| 3 | 4 | 176 | 194 | 157 | 424 | 18 | 267 | |
| 3 | 5 | 183 | 211 | 255 | 704 | 28 | 449 | |
| 3 | 6 | 193 | 215 | 405 | 767 | 22 | 362 | |
| ‘San Andreas’ | 1 | 1 | 42 | 53 | 17 | 185 | 11 | 168 |
| 1 | 2 | 45 | 55 | 62 | 222 | 10 | 160 | |
| 1 | 3 | 48 | 58 | 108 | 270 | 10 | 162 | |
| 1 | 4 | 50 | 66 | 138 | 378 | 16 | 240 | |
| 1 | 5 | 54 | 80 | 203 | 569 | 26 | 366 | |
| 1 | 6 | 68 | 85 | 403 | 646 | 17 | 243 | |
| 2 | 1 | 105 | 117 | 12 | 179 | 12 | 167 | |
| 2 | 2 | 107 | 126 | 37 | 294 | 19 | 257 | |
| 2 | 3 | 111 | 131 | 95 | 360 | 20 | 265 | |
| 2 | 4 | 114 | 142 | 140 | 506 | 28 | 366 | |
| 2 | 5 | 121 | 154 | 145 | 673 | 33 | 528 | |
| 2 | 6 | 136 | 159 | 412 | 746 | 23 | 334 | |
| 3 | 1 | 168 | 178 | 20 | 185 | 10 | 165 | |
| 3 | 2 | 169 | 183 | 39 | 255 | 14 | 216 | |
| 3 | 3 | 172 | 188 | 88 | 322 | 16 | 234 | |
| 3 | 4 | 173 | 204 | 105 | 593 | 31 | 488 | |
| 3 | 5 | 181 | 210 | 229 | 688 | 29 | 459 | |
| 3 | 6 | 196 | 214 | 457 | 752 | 18 | 295 |
| Cultivar | Cycle | Stage | Original | 1 °C More | 1 °C Less | |||
|---|---|---|---|---|---|---|---|---|
| Initial DAT | Initial GDD | Initial DAT | Initial GDD | Initial DAT | Initial GDD | |||
| ‘Albion’ | 1 | 1 | 42 | 17 | 42 | 17 | 42 | 17 |
| 1 | 2 | 44 | 48 | 44 | 48 | 44 | 48 | |
| 1 | 3 | 47 | 92 | 47 | 92 | 47 | 92 | |
| 1 | 4 | 48 | 108 | 48 | 108 | 49 | 108 | |
| 1 | 5 | 52 | 169 | 51 | 169 | 53 | 169 | |
| 1 | 6 | 69 | 416 | 61 | 416 | 71 | 416 | |
| 2 | 1 | 105 | 12 | 105 | 12 | 105 | 12 | |
| 2 | 2 | 107 | 37 | 107 | 37 | 107 | 37 | |
| 2 | 3 | 112 | 111 | 111 | 111 | 113 | 111 | |
| 2 | 4 | 113 | 127 | 112 | 127 | 114 | 127 | |
| 2 | 5 | 120 | 145 | 119 | 145 | 121 | 145 | |
| 2 | 6 | 140 | 479 | 138 | 479 | 143 | 479 | |
| 3 | 1 | 168 | 20 | 168 | 20 | 168 | 20 | |
| 3 | 2 | 170 | 55 | 170 | 55 | 170 | 55 | |
| 3 | 3 | 172 | 88 | 172 | 88 | 172 | 88 | |
| 3 | 4 | 176 | 157 | 175 | 157 | 177 | 157 | |
| 3 | 5 | 183 | 255 | 182 | 255 | 184 | 255 | |
| 3 | 6 | 193 | 405 | 192 | 405 | 194 | 405 | |
| San Andreas | 1 | 1 | 42 | 17 | 42 | 17 | 42 | 17 |
| 1 | 2 | 45 | 62 | 45 | 62 | 45 | 62 | |
| 1 | 3 | 48 | 108 | 48 | 108 | 49 | 108 | |
| 1 | 4 | 50 | 138 | 49 | 138 | 51 | 138 | |
| 1 | 5 | 54 | 203 | 53 | 203 | 55 | 203 | |
| 1 | 6 | 68 | 403 | 66 | 403 | 70 | 403 | |
| 2 | 1 | 105 | 12 | 105 | 12 | 105 | 12 | |
| 2 | 2 | 107 | 37 | 107 | 37 | 107 | 37 | |
| 2 | 3 | 111 | 95 | 111 | 95 | 111 | 95 | |
| 2 | 4 | 114 | 140 | 113 | 140 | 115 | 140 | |
| 2 | 5 | 121 | 145 | 120 | 145 | 123 | 145 | |
| 2 | 6 | 136 | 412 | 134 | 412 | 139 | 412 | |
| 3 | 1 | 168 | 20 | 168 | 20 | 168 | 20 | |
| 3 | 2 | 169 | 39 | 169 | 39 | 169 | 39 | |
| 3 | 3 | 172 | 88 | 172 | 88 | 172 | 88 | |
| 3 | 4 | 173 | 105 | 173 | 105 | 173 | 105 | |
| 3 | 5 | 181 | 229 | 180 | 229 | 182 | 229 | |
| 3 | 6 | 196 | 457 | 194 | 457 | 198 | 457 | |
| Cycle | RMSE ‘Albion’% | RMSE ‘San Andreas’ | ||||
|---|---|---|---|---|---|---|
| 1 | 7.33542 | 8.97607 | ||||
| 2 | 9.87164 | 7.88368 | ||||
| 3 | 5.82305 | 6.07191 | ||||
| Stage | Cycle | Cycle | ||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| 1 | 4.2213 | 17.26 | 4.7435 | 3.5633 | 8.4208 | 4.8128 |
| 2 | 7.2543 | 9.1932 | 4.4233 | 10.5214 | 8.7050 | 5.2892 |
| 3 | 9.7102 | 8.9669 | 7.7439 | 13.2335 | 6.0086 | 5.4331 |
| 4 | 7.7149 | 7.3192 | 6.1431 | 6.5211 | 8.9645 | 4.8997 |
| 5 | 8.6992 | 11.5065 | 6.9357 | 11.6447 | 9.4193 | 8.3894 |
| 6 | 8.8155 | 15.1756 | 6.8085 | 12.5538 | 8.7630 | 8.7679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Martínez, N.; Salazar-Gutiérrez, M.; Chaves-Córdoba, B.; Wells, D.; Foshee, W.; McWhirt, A. Model Development of the Phenological Cycle from Flower to Fruit of Strawberries (Fragaria × ananassa). Agronomy 2023, 13, 2489. https://doi.org/10.3390/agronomy13102489
Hernández-Martínez N, Salazar-Gutiérrez M, Chaves-Córdoba B, Wells D, Foshee W, McWhirt A. Model Development of the Phenological Cycle from Flower to Fruit of Strawberries (Fragaria × ananassa). Agronomy. 2023; 13(10):2489. https://doi.org/10.3390/agronomy13102489
Chicago/Turabian StyleHernández-Martínez, Nelda, Melba Salazar-Gutiérrez, Bernardo Chaves-Córdoba, Daniel Wells, Wheeler Foshee, and Amanda McWhirt. 2023. "Model Development of the Phenological Cycle from Flower to Fruit of Strawberries (Fragaria × ananassa)" Agronomy 13, no. 10: 2489. https://doi.org/10.3390/agronomy13102489
APA StyleHernández-Martínez, N., Salazar-Gutiérrez, M., Chaves-Córdoba, B., Wells, D., Foshee, W., & McWhirt, A. (2023). Model Development of the Phenological Cycle from Flower to Fruit of Strawberries (Fragaria × ananassa). Agronomy, 13(10), 2489. https://doi.org/10.3390/agronomy13102489





