Soil Predisposing Factors to Fusarium oxysporum f.sp Cubense Tropical Race 4 on Banana Crops of La Guajira, Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Location and Sampling
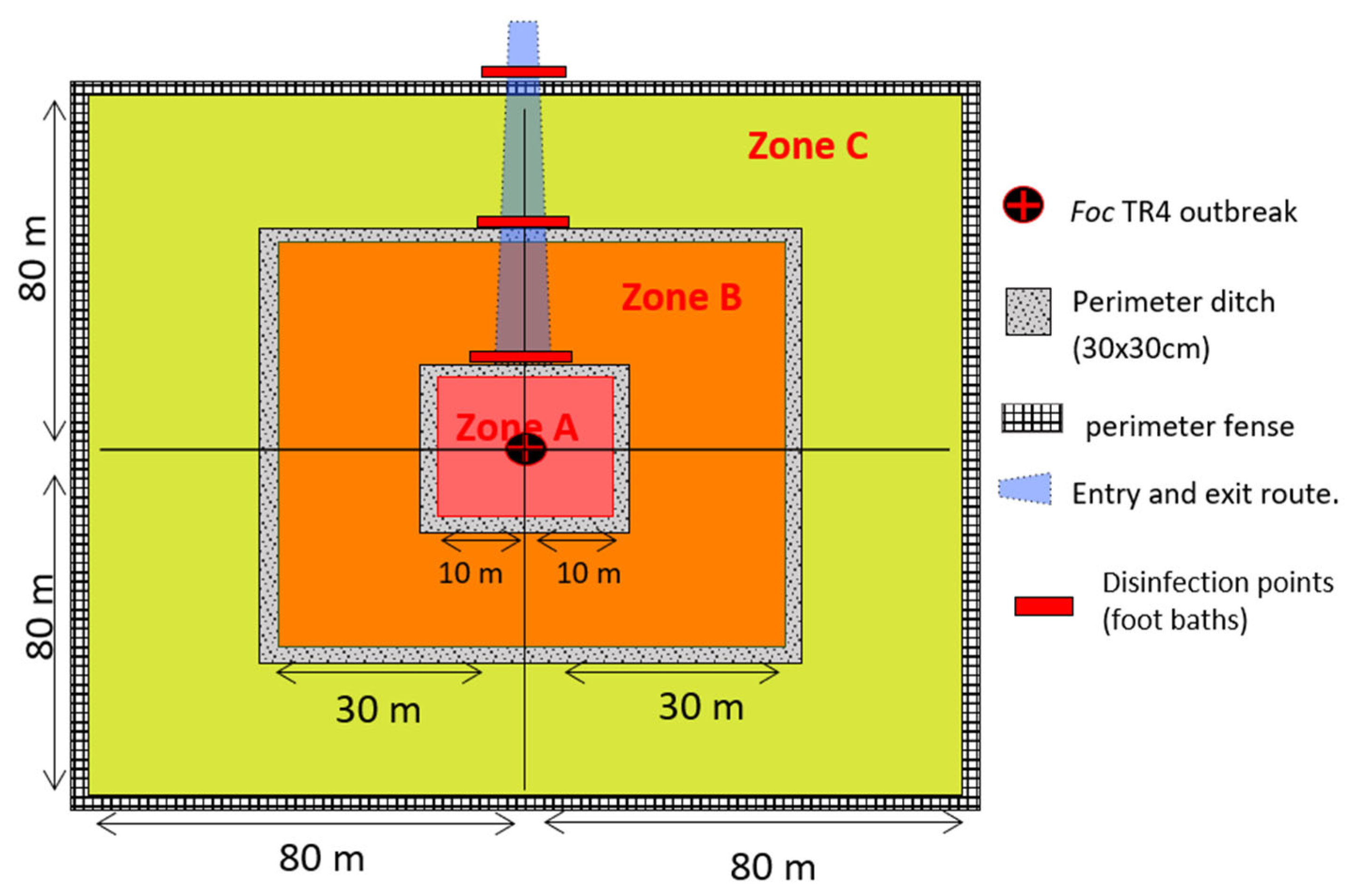
2.2. Sampling Criteria
2.3. Soil Properties
2.3.1. Physical Properties
2.3.2. Chemical Properties
2.4. Experimental Design
2.5. Analysis of the Results
2.5.1. Exploratory Analysis through Principal Component Analysis (PCA)
2.5.2. Analysis of Variance (ANOVA)
2.5.3. Correlation Analysis
2.5.4. Debiased Sparse Partial Correlation (DSPC) Algorithm
3. Results
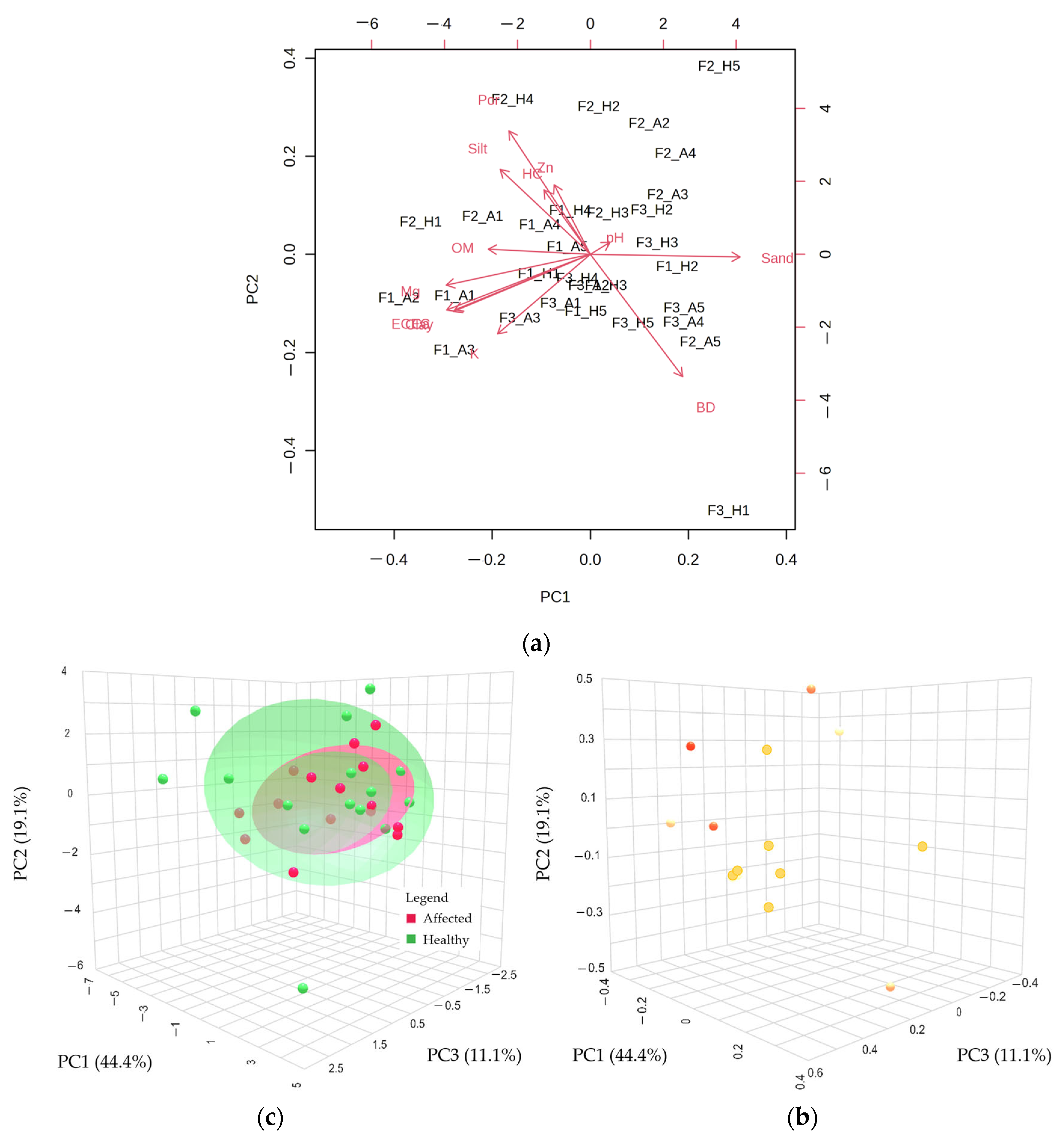
3.1. Exploratory Analysis
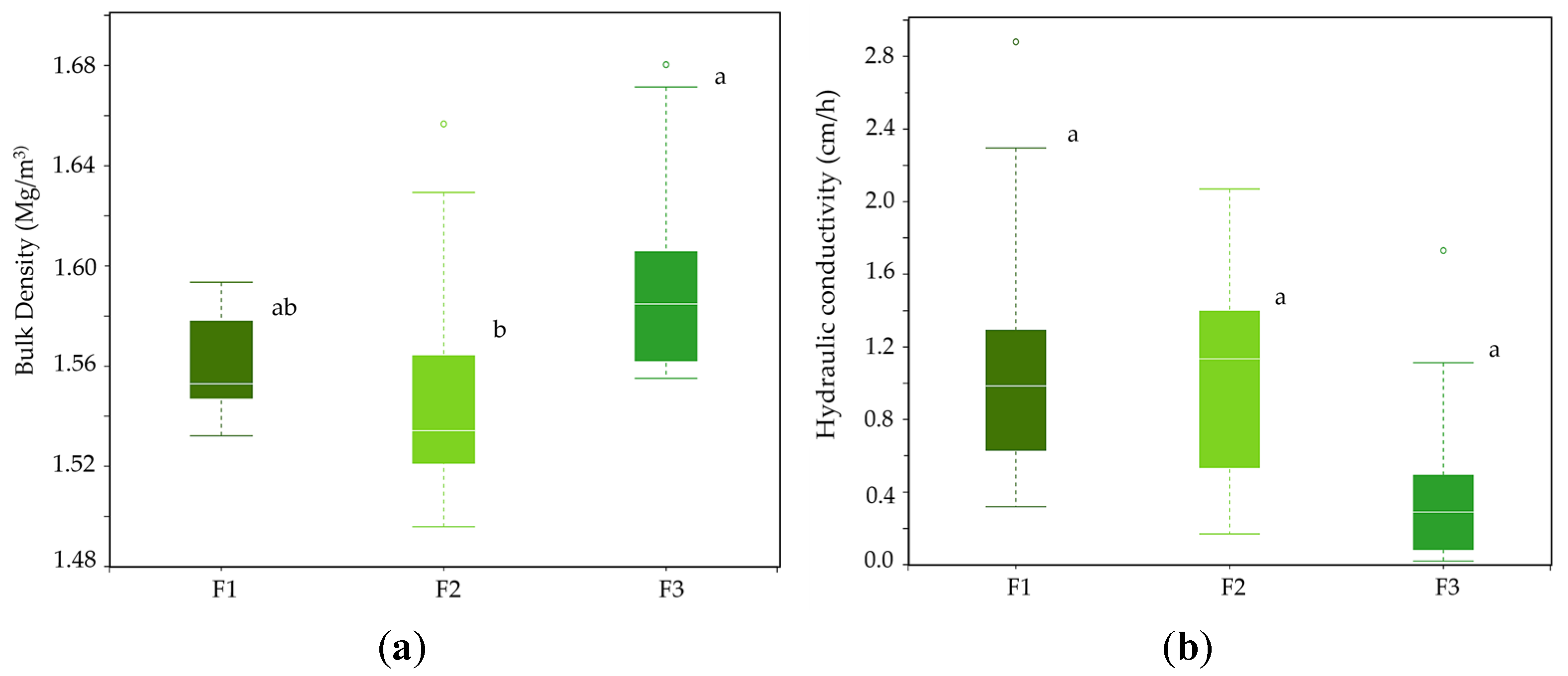
3.2. Comparative Analyses of Chemical and Physical Properties among Farms
3.3. Comparative Analyses of Chemical and Physical Properties between Healthy and Affected Plots
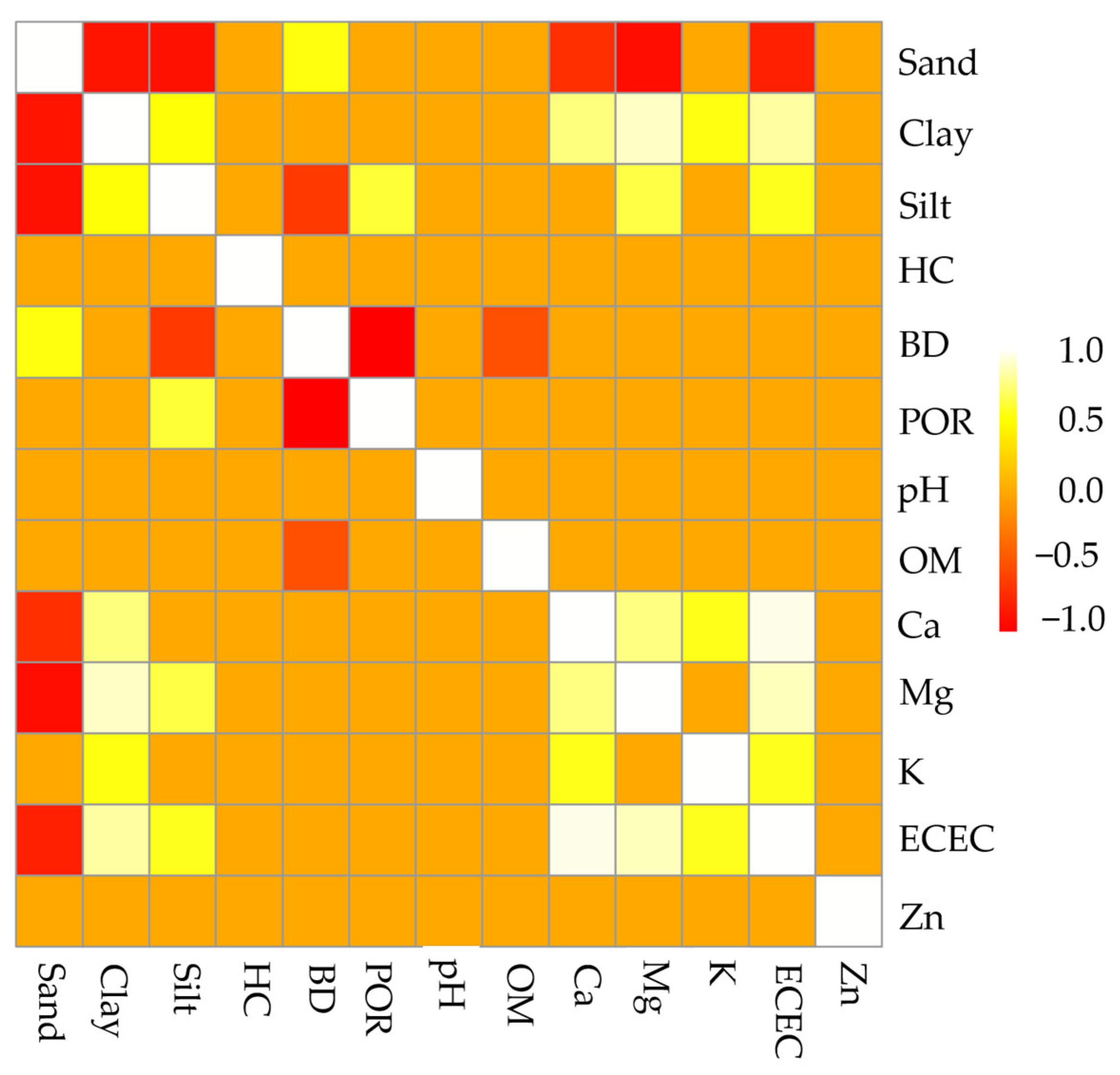
3.4. Correlations
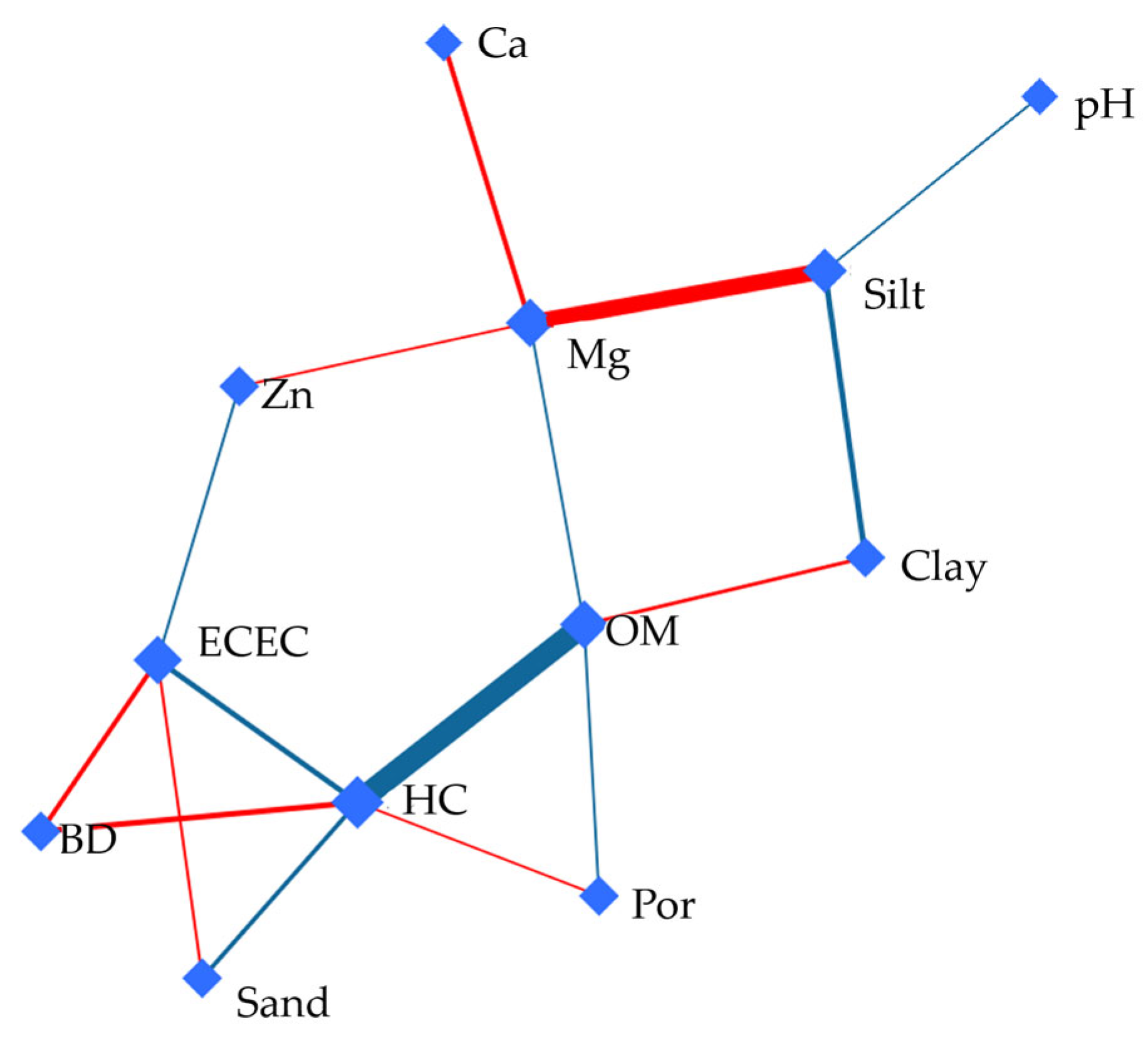
3.5. Debiased Sparse Partial Correlation (DSPC) Network
4. Discussion
4.1. Comparative Analyses of Chemical and Physical Properties among Farms
4.2. Comparative Analysis through Affected and Healthy Plots
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Code | Farm | Accumulated Incidence (%) * | Estimated Eradicated Area (ha) | Total Area of the Farm (ha) | Affected Area (%) | Cultivated Clone |
|---|---|---|---|---|---|---|
| E44001-435 | 1 | 1.2 | 1.6 | 234.0 | 0.6 | Williams (53%) Valery (47%) |
| C44001-388ab | 2 | 2.5 | 8.4 | 393.0 | 2.1 | Valery (88%) Williams (12%) |
| A44001-288 | 3 | 6.6 | 27.8 | 217.0 | 12.8 | Valery (100%) |
References
- Pegg, K.G.; Coetes, L.M.; O´Neill, W.T.; Turner, D.W. The epidemiology of Fusarium Wilt of Banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef] [PubMed]
- Pattison, A.B.; Wright, C.L.; Kukulies, T.L.; Molina, A.B. Ground cover management alters the development and alters the development of Fusarium wilt symptoms in ducasse bananas. Australas. Plant Pathol. 2014, 43, 465–476. [Google Scholar] [CrossRef]
- Teixeira, L.; Nomura, E.; Damatto, E.; Vieira, H.; Staver, C.; Dita, M. Effectiveness of soil management practices on Fusarium wilt of banana in the Ribeira Valley, Brazil. Trop. Plant Pathol. 2022, 47, 411–420. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.; Staver, C. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plan Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Panigrahi, N.; Thompson, A.; Zubelzu, S.; Knox, J. Identifying opportunities to improve management of water stress in banana production. Sci. Hortic. 2021, 276, 109735. [Google Scholar] [CrossRef]
- Felcy-Navajothy, A.; Narayanaswamy, R.; Ponniah, D.; Irudayaraj, V. Physicochemical analysis of soil in relation to Panama disease (Fusarium wilt) in banana. IJP. 2012, 5, 15–24. [Google Scholar]
- Domínguez, J.; Negrín, M.A.; Rodríguez, C.M.; Dominguez, J.; Negrin, M.; Rodriguez, C. Aggregate water-stability, particle-size, and soil solution properties in conducive and suppressive soils to Fusarium wilt of banana from Canary Islands (Spain). Soil Biol. Biochem. 2001, 33, 449–455. [Google Scholar] [CrossRef]
- Deltour, P.; França, S.C.; Pereira, O.L.; Cardoso, I.; De Neve, S.; Debode, J.; Höfte, M. Disease suppressiveness to Fusarium wilt of banana in an agroforestry system: Influence of soil characteristics and plant community. Agric. Ecosyst. Environ. 2017, 239, 173–181. [Google Scholar] [CrossRef]
- Olivares, B.; Calero, J.; Rey, J.C.; Lobo, D.; Landa, B.; Gómez, J. Correlation of banana productivity levels and soil morphological properties using regularized optimal scaling regression. Catena 2022, 208, 105718. [Google Scholar] [CrossRef]
- Segura, R.; Stoorvogel, J.; Sandoval, J. The effect of soil properties on the relation between soil management and Fusarium wilt expresión in Gros Michel banana. Plant Soil 2022, 471, 89–100. [Google Scholar] [CrossRef]
- Nasir, N.; Pittaway, P.A.; Pegg, K.G. Effect of organic amendments and solarisation on Fusarium wilt in susceptible banana plantlets, transplanted into naturally infested soil. Aust. J. Agric. Res. 2003, 54, 251–257. [Google Scholar] [CrossRef]
- Mur LA, J.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving nitrogen to the center of plant defense against pathogens. Ann. Bot. 2016, 119, 703–719. [Google Scholar] [CrossRef]
- Teixeira, L.; Heck, D.; Nomura, E.; Vieira, H.; Dita, M. Soil attributes, plant nutrition, and Fusarium wilt of banana in São Paulo, Brazil. Trop. Plan Pathol. 2021, 46, 443–454. [Google Scholar] [CrossRef]
- Furtado, E.L.; Bueno, C.J.; Luiz De Oliveira, A.; Otávio, J.; Menten, M.; Malavolta, E. Relações entre ocorrência do Mal-de-Panama em bananeira da cv. Nanicão e nutrientes no solo e nas folhas. Trop. Plant Pathol. 2009, 34, 211–215. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Chao, X.; Zhang, J.; Li, R.; Shen, Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol. Fertil. Soils 2015, 51, 553–562. [Google Scholar] [CrossRef]
- Peng, H.X.; Sivasithamparam, K.; Turner DW, W. Chlamydospore germination and Fusarium wilt of banana plantlets in suppressive and conducive soils are affected by physical and chemical factors. Soil Biol. Biochem. 1999, 31, 1363–1374. [Google Scholar] [CrossRef]
- Nadiah, F.; Mohd, A.; Termizi, M.; Baity, N. Analysis of baterial communities and physicochemical properties associated with Fusarium wilt disease of banana in Malaysia. Cient. Rep. Nat. 2022, 12, 999. [Google Scholar] [CrossRef]
- Wang, B.; Sun, M.; Yang, J.; Shen, Z.; Ou, Y.; Fu, L.; Zhao, Y.; Li, R.; Ruan, Y.; Shen, Q. Inducing banana Fusarium wilt disease suppression through soil microbiome reshaping by pineapple-banana rotation combined with biofertilizer application. Soil 2022, 8, 17–29. [Google Scholar] [CrossRef]
- Yang, J.; Ren, X.; Liu, M.; Fan, P.; Ruan, Y.; Zhao, Y.; Wang, B.; Li, R. Suppressing soil-borne Fusarium pathogens of bananas by planting different cultivars of pineapples, with comparisons of the resulting bacterial and fungal communities. Appl. Soil Ecol. 2022, 169, 104211. [Google Scholar] [CrossRef]
- Dror, B.; Amutuhaire, H.; Frenkel, O.; Jurkevitch, E.; Cytryn, E. Identification of bacterial populations and functional mechanism potentially involved in biochar-facilized antagonism of soilborne pathogen Fusarium oxysporum. Phytobiomes J. 2022, 6, 139–150. [Google Scholar] [CrossRef]
- Haddad, F.; Rocha, L.; Soares, A.; Martins, I.; Teixeira, L.; Staver, C.; Dita, M. Management of Fusarium wilt of bananas in Minas Gerais, Brazil. Acta Hortic. 2018, 1196, 137–146. [Google Scholar] [CrossRef]
- Pattison, A.; Limbaya, C.; Gervacio, T.; Notarte, A.; Jurvena, M.; Dennis, P.; Lindsay, M.; Molina, A. Integrated Management of Fusarium wilt of Banana in the Philippines and Australia. Final Report; Australian Centre for International Agricultural Research: Canberra, Australia, 2020; 91p. [Google Scholar]
- Nowembabazi, A.; Tautaya, G.; Tinzaara, W.; Karamura, E. Effect of integrated potassium nutrition on Fusarium wilt tolerance in apple banana. Afr. J. Plant Sci. 2021, 15, 257–265. [Google Scholar]
- IGAC (Instituto Geográfico Agustín Codazzi). Estudio Semidetallado de Suelos y Zonificación de Tierras en la Media y Baja Guajira. Escala 1:25.000; Imprenta Nacional Publisher: Bogota, Colombia, 2012; pp. 154–196. [Google Scholar]
- IDEAM (Instituto de Hidrología, Meteorología y Estudios Ambientales). Catálogo Nacional de Estaciones Climatológicas. Available online: https://n9.cl/spu3z (accessed on 22 September 2023).
- Dita, M.; Echegoyén, P.; Pérez-Vicente, L. Plan de Contingencia ante un Brote de la Raza 4 Tropical de Fusarium oxysporum f. sp. Cubense en un País de la Región del OIRSA; Organismo Internacional Regional de Sanidad Agropecuaria—OIRSA: San Salvador, El Salvador, 2013; 169p. [Google Scholar]
- González, H.; Gonzalez, A.; Pineda, M.; Escalante, H.; Rodríguez, G.; Soto, A. Microbiota edáfica en lotes de plátano con vigor contrastante y su relación con propiedades del suelo. Bioagro 2021, 33, 143–148. [Google Scholar]
- R Core Team. R A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Di-Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat versión 2016. Universidad Nacional de Córdoba. Available online: http://www.infostat.com.ar (accessed on 22 September 2023).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 2017, 1.2-8. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 22 September 2023).
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, G.; Deng, R.; Hu, X.; Tan, H.; Chen, Y.; Tian, Z.; Li, J. Sapatiotemporal biocontrol and rhizosphere microbiome análisis of Fusarium wilt of banana. Commun. Biol. 2023, 6, 27. [Google Scholar] [CrossRef]
- Gazolla, C.; Britto, B.; Freitas, J.; Beneduzi, A.; Eichelberger, G.; Kayser, L. Soil-plant-microbiota interaction to enhance plant growth. Rev. Bras. Cienc. Solo 2022, 46, e0210098. [Google Scholar] [CrossRef]
- Borges-Pérez, A.; Trujillo JC, I.; Gutiérrez-Jerez, F.; Angulo-Rodríguez, D. Estudio sobre el mal de Panamá em las Islas Canarias: II-Influencia de los desequilibrios nutritivos P-Zn y K-Mg del suelo, en la alteración de los mecanismos de resistencia de la platanera (Cavendish enana) al Mal de Panamá. Fruits 1983, 38, 755–758. [Google Scholar]
- Borges-Pérez, A.; Fernández Falcón, M.; Bravo Rodrigues, J.J.; Pérez-Francés, J.F.; López-Carreño, I. Enhanced resistance of banana plants (Dwarf Cavendish) to Fusarium oxysporum f. sp. cubense by controlled Zn nutrition under field conditions. Banan. Newsl. 1991, 14, 24–26. [Google Scholar]
- Hecht-Buchholz, C.; Borges-Pérez, A.; Fernández Falcón, M.; Borges, A.A. Influence of zinc nutrition on Fusarium wilt of banana—An electron microscopic investigation. Acta Hortic. 1998, 490, 277–284. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lobo, D. Desarrollo y distribución de raíces en tres clones de musáceas y su relación con las propiedades de un suelo lacustrino de la Cuenca del lago de Valencia. Rev. Fac. Agron. (LUZ) 2004, 21, 121–128. [Google Scholar]
- Rodríguez, G. Aspectos sobre la salud radical de banano en suelos de Venezuela. Prod. Agrop. 2009, 2, 46–50. [Google Scholar]
- Delgado, E.; Trejos, J.; Villalobos, N.; Martinez, G.; Lobo, D.; Rey, J.C.; Rodriguez, G.; Rosalez, F.; Pocasangre, L. Determinación de un índice de calidad y salud de suelos para plantaciones bananeras en Venezuela. Interciencia 2010, 35, 927–933. [Google Scholar]
- Gonzalez, H.; Pernía, J.; Ramirez, Y.; Gonzalez, A.; Soto, A.; Rodríguez, G.; Rodríguez, V. Desarrollo de raíces en plantas de plátano en suelos del Sur del Lago de Maracaibo. Rev. Cient. Prod. Agrop. 2018, 6, 20–28. [Google Scholar]
- González, H.; González, A.; Rodríguez, G.; León, M.; Betancourt, M. Vigor en plantas de plátano (Musa AAB cv. hartón) y su relación con características físicas, químicas y biológicas del suelo. Agron. Costarric. 2021, 45, 115–124. [Google Scholar] [CrossRef]
- Zakaria, M.; Sakimin, S.; Ismail, M.; Ahmad, K.; Kasim, S.; Baghdadi, A. Biostimulant Activity of Silicate Compounds and Antagonistic Bacteria on Physiological Growth Enhancement and Resistance of Banana to Fusarium Wilt Disease. Plants 2023, 12, 1124. [Google Scholar] [CrossRef]
- Soto, M. Bananos: Cultivo y Comercialización., 3rd ed.; Ministerio de Agricultura y Ganadería: San José, Costa Rica, 2010; pp. 157–267. [Google Scholar]
- Olivares, B.O. Fusarium Wilt of Bananas: A Threat to the Banana Production Systems in Venezuela. In Banana Production in Venezuela; The Latin American Studies Book Series; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Olivares, B.O. Evaluation of the Incidence of Banana Wilt and its Relationship with Soil Properties. In Banana Production in Venezuela; The Latin American Studies Book Series; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Zhang, J.; Christie, P.; Li, X. Organic fertilizer application and Mg fertilizer promote banana yield and quality in an Udic Ferralsol. PLoS ONE 2020, 15, e0230593. [Google Scholar] [CrossRef]
- Rodríguez-Yzquierdo, G.; Olivares, B.O.; Silva-Escobar, O.; González-Ulloa, A.; Soto-Suarez, M.; Betancourt-Vásquez, M. Mapping of the Susceptibility of Colombian Musaceae Lands to a Deadly Disease: Fusarium oxysporum f. sp. cubense Tropical Race 4. Horticulturae 2023, 9, 757. [Google Scholar] [CrossRef]
- Olivares, B.; Vega, A.; Calderón, M.A.R.; Rey, J.C.; Lobo, D.; Gómez, J.A.; Landa, B.B. Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods. Plants 2022, 11, 2070. [Google Scholar] [CrossRef]
- Olivares, B.; Rey, J.C.; Lobo, D.; Navas-Cortés, J.A.; Gómez, J.A.; Landa, B.B. Fusarium Wilt of Bananas: A Review of Agro-Environmental Factors in the Venezuelan Production System Affecting Its Development. Agronomy 2021, 11, 986. [Google Scholar] [CrossRef]
- Olivares, B.O.; Araya-Alman, M.; Acevedo-Opazo, C.; Rey, J.C.; Cañete-Salinas, P.; Kurina, F.G.; Balzarini, M.; Lobo, D.; Navas-Cortés, J.A.; Landa, B.B.; et al. Relationship Between Soil Properties and Banana Productivity in the Two Main Cultivation Areas in Venezuela. J. Soil Sci. Plant Nutr. 2020, 20, 2512–2524. [Google Scholar] [CrossRef]
- Lobo, D.; Orlando, B.; Rey, J.C.; Vega, A.; Rueda-Calderón, A. Relationships between the Visual Evaluation of Soil Structure (VESS) and soil properties in agriculture: A meta-analysis. Sci. Agropecu. 2023, 14, 67–78. [Google Scholar] [CrossRef]
- Olivares, B.O.; Rey, J.C.; Perichi, G.; Lobo, D. Relationship of Microbial Activity with Soil Properties in Banana Plantations in Venezuela. Sustainability 2022, 14, 13531. [Google Scholar] [CrossRef]
- Paredes, F.; Olivares, B.; Rey, J.; Lobo, D.; Galvis-Causil, S. The relationship between the normalized difference vegetation index, rainfall, and potential evapotranspiration in a banana plantation of Venezuela. SAINS TANAH - JSSA 2021, 18, 58–64. [Google Scholar] [CrossRef]
- Vega, A.; Olivares, B.O.; Rueda Calderón, M.A.; Montenegro-Gracia, E.; Araya-Almán, M.; Marys, E. Prediction of Banana Production Using Epidemiological Parameters of Black Sigatoka: An Application with Random Forest. Sustainability 2022, 14, 14123. [Google Scholar] [CrossRef]
- Olivares, B. Machine learning and the new sustainable agriculture: Applications in banana production systems of Venezuela. Agric. Res. Updates 2022, 42, 133–157. [Google Scholar]
- Olivares, B.; Pitti, J.; Montenegro, E. Socioeconomic characterization of Bocas del Toro in Panama: An application of multivariate techniques. Rev. Bras. De Gest. E Desenvolv. Reg. 2020, 16, 59–71. [Google Scholar]
- Montenegro, E.; Pitti-Rodríguez, J.; Olivares-Campos, B. Identification of the main subsistence crops of Teribe: A case study based on multivariate techniques. Idesia 2021, 39, 83–94. [Google Scholar] [CrossRef]
- Pitti, J.; Olivares, B.; Montenegro, E. The role of agriculture in the Changuinola District: A case of applied economics in Panama. Trop. Subtrop. Agroecosyst. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Herrera, R.M.; Hernández, Y.; Magdama, F.; Mostert, D.; Bothma, S.; Salgado, E.M.P.; Terán, D.; González, E.; Angulo, R.; Angel, L.; et al. First report of Fusarium wilt of Cavendish bananas caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 in Venezuela. Plant Dis. 2023, 1–5. [Google Scholar] [CrossRef]

| Chemical Attributes | Physical Attributes | |||||
|---|---|---|---|---|---|---|
| Plot | pH | Ca (cmol/kg) | Mg (cmol/kg) | ECEC (cmol/kg) | Sand (%) | HC (cm/h) |
| Healthy | 7.34 a | 12.25 a | 4.59 a | 17.89 a | 41.36 a | 1.08 a |
| Affected | 7.18 b | 10.09 b | 3.75 b | 14.55 b | 33.75 b | 0.63 b |
| p-value | 0.04 | 0.04 | 0.03 | 0.02 | 0.03 | 0.02 |
| % CV | 4.62 | 19.48 | 24.72 | 19.16 | 37.94 | 77.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Yzquierdo, G.; Olivares, B.O.; González-Ulloa, A.; León-Pacheco, R.; Gómez-Correa, J.C.; Yacomelo-Hernández, M.; Carrascal-Pérez, F.; Florez-Cordero, E.; Soto-Suárez, M.; Dita, M.; et al. Soil Predisposing Factors to Fusarium oxysporum f.sp Cubense Tropical Race 4 on Banana Crops of La Guajira, Colombia. Agronomy 2023, 13, 2588. https://doi.org/10.3390/agronomy13102588
Rodríguez-Yzquierdo G, Olivares BO, González-Ulloa A, León-Pacheco R, Gómez-Correa JC, Yacomelo-Hernández M, Carrascal-Pérez F, Florez-Cordero E, Soto-Suárez M, Dita M, et al. Soil Predisposing Factors to Fusarium oxysporum f.sp Cubense Tropical Race 4 on Banana Crops of La Guajira, Colombia. Agronomy. 2023; 13(10):2588. https://doi.org/10.3390/agronomy13102588
Chicago/Turabian StyleRodríguez-Yzquierdo, Gustavo, Barlin Orlando Olivares, Antonio González-Ulloa, Rommel León-Pacheco, Juan Camilo Gómez-Correa, Marlon Yacomelo-Hernández, Francisco Carrascal-Pérez, Elías Florez-Cordero, Mauricio Soto-Suárez, Miguel Dita, and et al. 2023. "Soil Predisposing Factors to Fusarium oxysporum f.sp Cubense Tropical Race 4 on Banana Crops of La Guajira, Colombia" Agronomy 13, no. 10: 2588. https://doi.org/10.3390/agronomy13102588







