Agrivoltaic Farming Insights: A Case Study on the Cultivation and Quality of Kimchi Cabbage and Garlic
Abstract
:1. Introduction
2. Materials and Methods
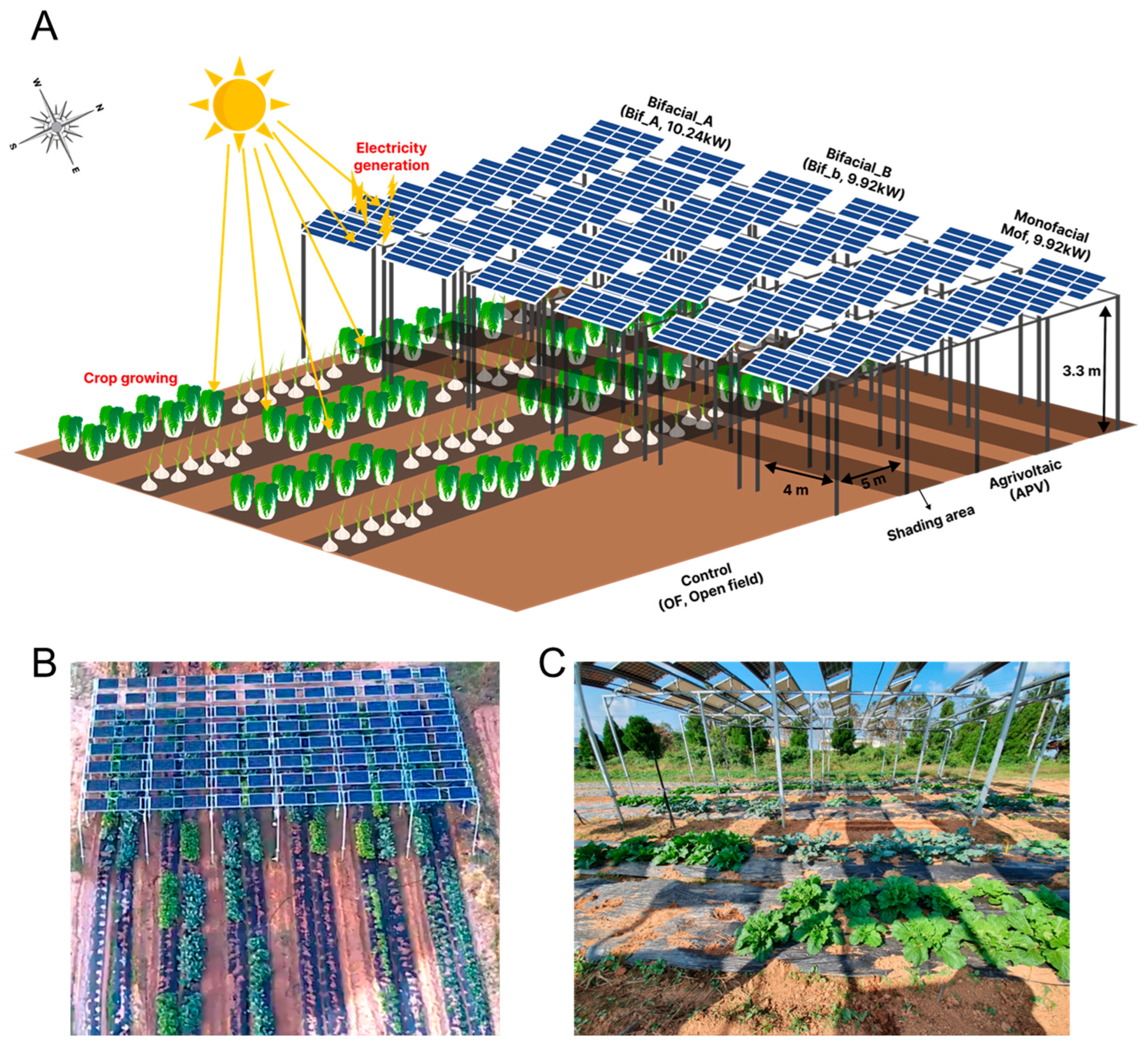
2.1. Agrivoltaic Structure and Environmental Data Collection
2.2. Crop Cultivation
2.3. Analysis of Kimchi Cabbage Glucosinolates and Hydrolysis Products
2.4. Analysis of Garlic Allicin Using Liquid Chromatograph-Mass Spectrometer (LC-MS)
2.5. Analysis of Garlic Organosulfur Compounds Using Gas Chromatograph-Mass Spectrometer (GC-MS)
2.6. Statistical and Multivariate Analysis
3. Results
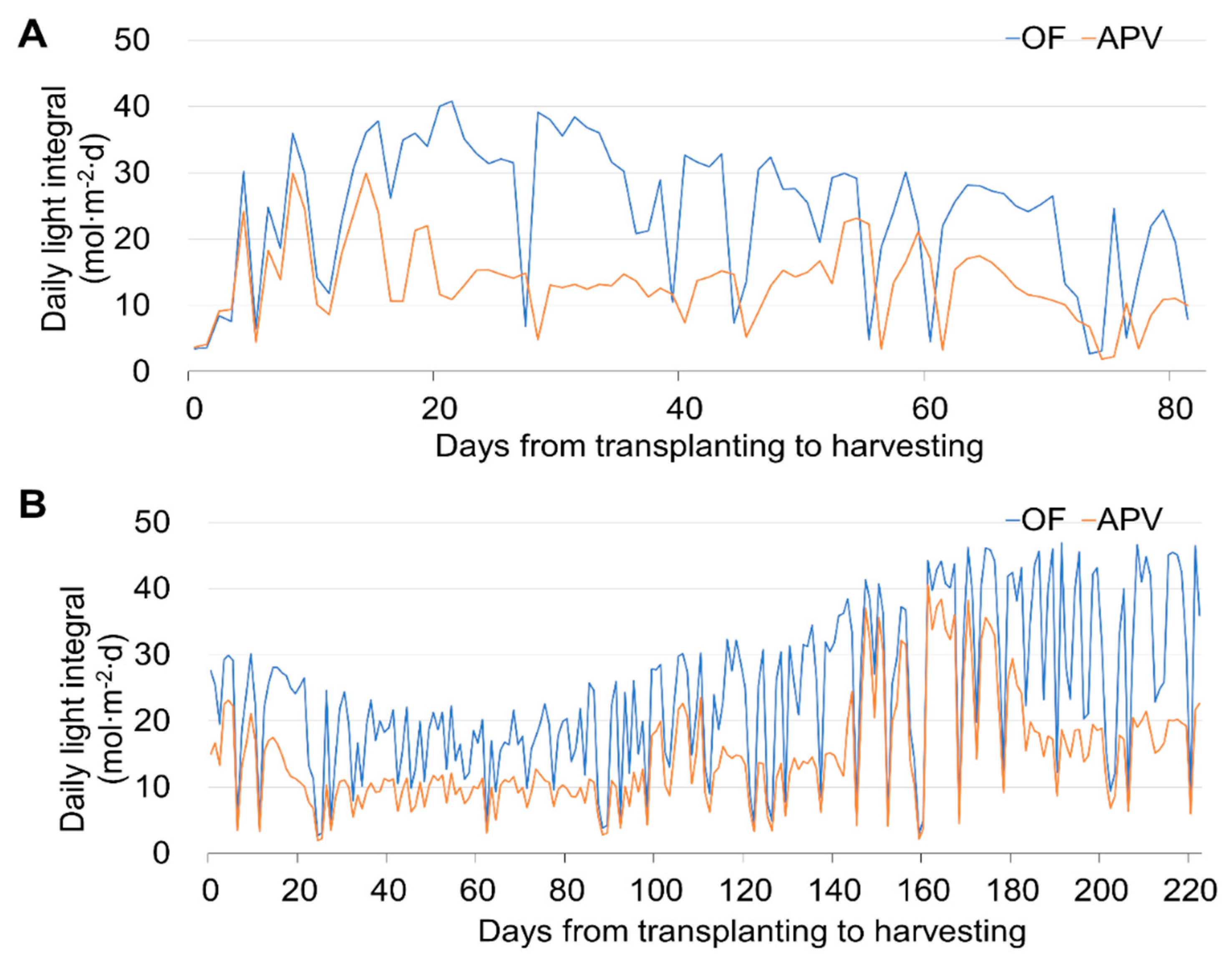
3.1. Cultivation Environments and Crop Growth
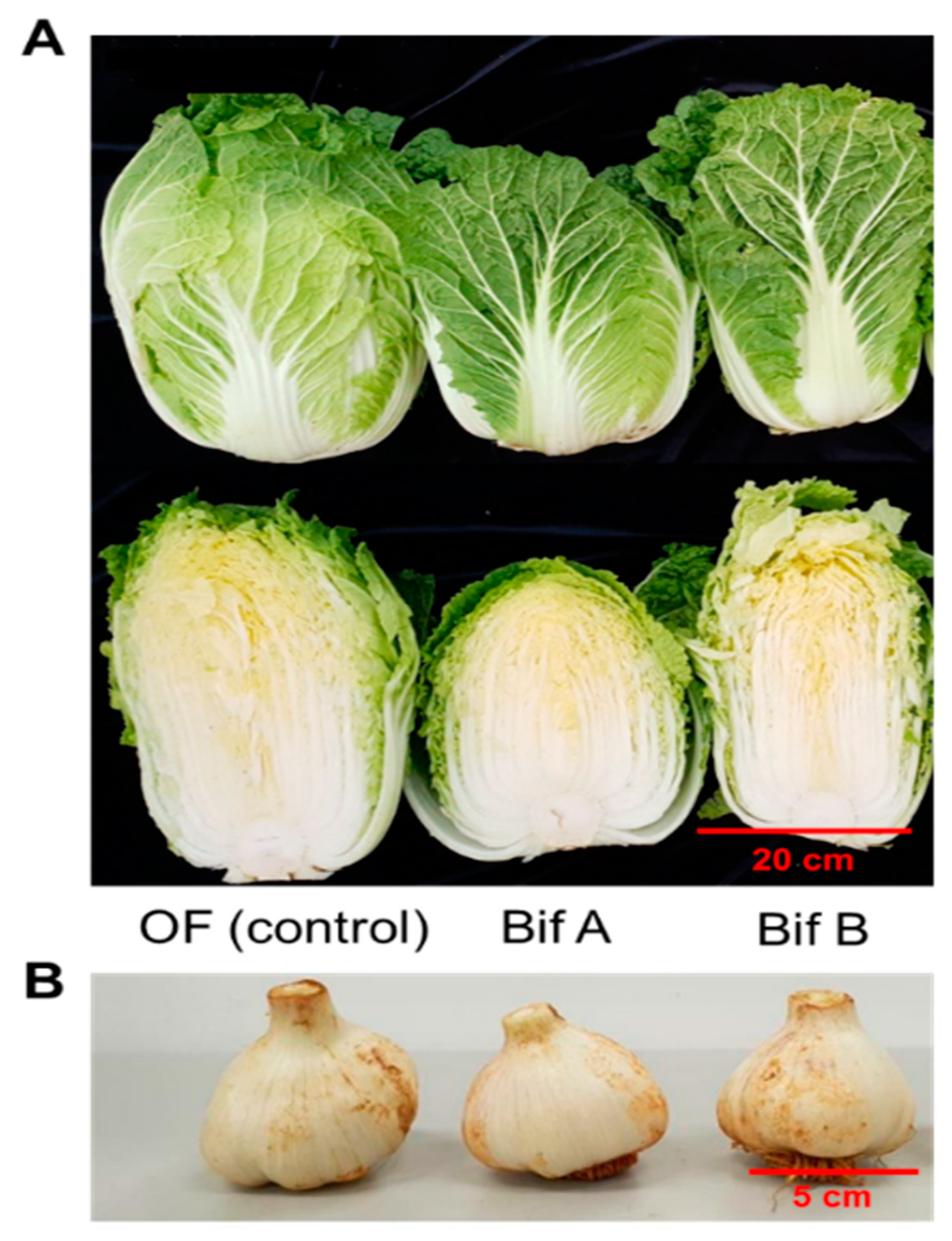
3.2. Crop Quality Determination
4. Discussion
4.1. Impact on Crop Yield and Quality
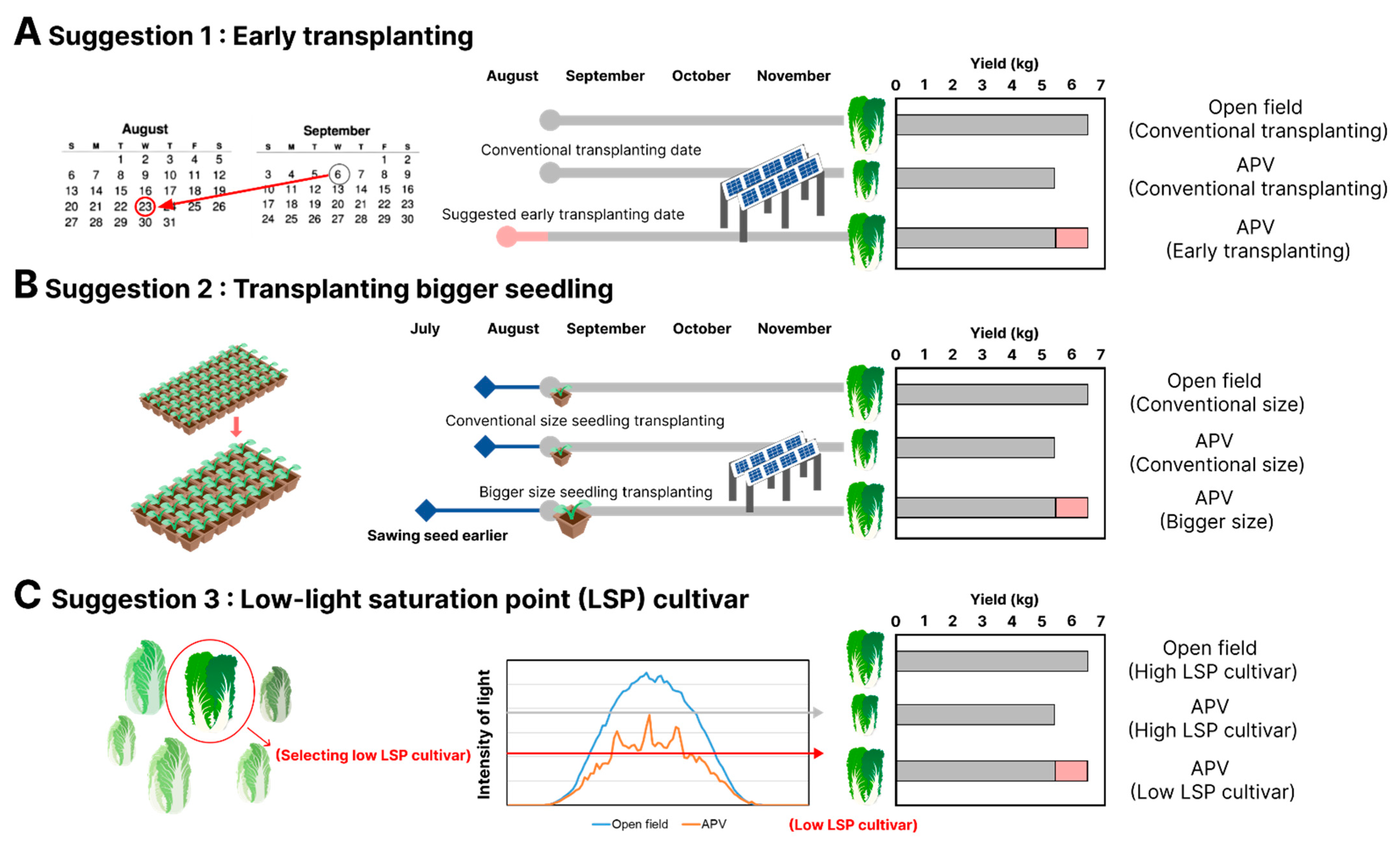
4.2. Proposed Farming Techniques
4.3. Suggestion 1: Early Transplanting Strategy
4.4. Suggestion 2: The Use of Bigger Seedlings at Transplanting to Grow over Short Periods
4.5. Suggestion 3: Cultivation of Low-Light Saturation Cultivar
5. Direction for Further Research
5.1. Research on Crop Breeding and Panel Design to Enhance Crop Photosynthetic Efficiency
5.2. Integration of AI on APV System to Improve Quality and Yield on Crop
5.3. Changing Microenvironmental Conditions and Beyond Direct Impact
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. Global Energy and Climate Model; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/global-energy-and-climate-model (accessed on 19 February 2023).
- IEA. Net Zero by 2050; IEA: Paris, France, 2021; Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 19 February 2023).
- IEA. Electricity Sector; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/electricity-sector (accessed on 19 February 2023).
- Goetzberger, A.; Zastrow, A. On the Coexistence of Solar-Energy Conversion and Plant Cultivation. Int. J. Sol. Energy 1982, 1, 55–69. [Google Scholar] [CrossRef]
- Trommsdorff, M.; Kang, J.; Reise, C.; Schindele, S.; Bopp, G.; Ehmann, A.; Weselek, A.; Högy, P.; Obergfell, T. Combining food and energy production: Design of an agrivoltaic system applied in arable and vegetable farming in Germany. Renew. Sustain. Energy Rev. 2021, 140, 110694. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Armstrong, A.; Burney, J.; Ryan, G.; Moore-O’leary, K.; Diédhiou, I.; Grodsky, S.M.; Saul-Gershenz, L.; Davis, R.; Macknick, J.; et al. Techno–ecological synergies of solar energy for global sustainability. Nat. Sustain. 2019, 2, 560–568. [Google Scholar] [CrossRef]
- Dinesh, H.; Pearce, J.M. The potential of agrivoltaic systems. Renew. Sustain. Energy Rev. 2016, 54, 299–308. [Google Scholar] [CrossRef]
- Malu, P.R.; Sharma, U.S.; Pearce, J.M. Agrivoltaic potential on grape farms in India. Sustain. Energy Technol. Assess. 2017, 23, 104–110. [Google Scholar] [CrossRef]
- Schindele, S.; Trommsdorff, M.; Schlaak, A.; Obergfell, T.; Bopp, G.; Reise, C.; Braun, C.; Weselek, A.; Bauerle, A.; Högy, P.; et al. Implementation of agrophotovoltaics: Techno-economic analysis of the price-performance ratio and its policy implications. Appl. Energy 2020, 265, 114737. [Google Scholar] [CrossRef]
- Herath, U. Consumer behavior and attitudes in purchasing vegetables. Agric. Res. Technol. Open Access J. 2019, 2, 1–7. [Google Scholar]
- Rembiałkowska, E. Quality of plant products from organic agriculture. J. Sci. Food Agric. 2007, 87, 2757–2762. [Google Scholar] [CrossRef]
- Chae, S.-H.; Kim, H.J.; Moon, H.-W.; Kim, Y.H.; Ku, K.-M. Agrivoltaic systems enhance farmers’ profits through broccoli visual quality and electricity production without dramatic changes in yield, antioxidant capacity, and glucosinolates. Agronomy 2022, 12, 1415. [Google Scholar] [CrossRef]
- Moon, H.-W.; Ku, K.-M. Impact of an agriphotovoltaic system on metabolites and the sensorial quality of cabbage (Brassica oleracea var. Capitata) and its high-temperature-extracted juice. Foods 2022, 11, 2022. [Google Scholar] [CrossRef]
- Korean Statistical Information Service “Agricultural area Survey”. Cultivated Area of Food Crops (Field). 2022. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1ET0013&conn_path=I2 (accessed on 19 February 2023).
- Melillo, J.M.; Richmond, T.; Yohe, G. Climate change impacts in the United States. Third Natl. Clim. Assess. 2014, 52, 150–174. [Google Scholar]
- NOAA National Centers for Environmental Information “Monthly Global Climate Report for Annual 2022. January 2023. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/202213 (accessed on 19 February 2023).
- Adams, R.M.; Hurd, B.H.; Lenhart, S.; Leary, N. Effects of global climate change on agriculture an interpretative review. Clim. Res. 1998, 11, 19–30. [Google Scholar] [CrossRef]
- Song, E.Y.; Moon, K.H.; Son, I.C.; Kim, C.H.; Lim, C.K.; Son, D.; Oh, S. Impact of elevating temperature in growing season on the growth and yield of Chinese cabbage at different altitudes. Korean Soc. Hortic. Sci. 2015, 33, 80. [Google Scholar]
- Kim, T.; Park, J.-Y.; Park, Y.-G. A study on factors and prospects of changes in highland vegetable acreage. Korea Rural. Econ. Inst. 2014, 192, 545–561. [Google Scholar]
- Amaducci, S.; Yin, X.; Colauzzi, M. Agrivoltaic systems to optimise land use for electric energy production. Appl. Energy 2018, 220, 545–561. [Google Scholar] [CrossRef]
- Tazawa, S. Effects of various radiant sources on plant growth (part 1). Jpn. Agric. Res. Q. 1999, 33, 163–176. [Google Scholar]
- Bischoff, K.L. Nutraceuticals Chapter 40—Glucosinolates; Academic Press: Cambridge, MA, USA, 2016; pp. 551–554. ISBN 978-0-12-802147-7. [Google Scholar]
- Bell, L.; Wagstaff, C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia). J. Agric. Food Chem. 2014, 62, 4481–4492. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Coves, S.; Soengas, P.; Velasco, P.; Fernández, J.C.; Cartea, M.E. New vegetable varieties of Brassica rapa and Brassica napus with modified glucosinolate content obtained by mass selection approach. Front. Nutr. 2023, 10, 1198121. [Google Scholar] [CrossRef]
- Qin, H.; King, G.J.; Borpatragohain, P.; Zou, J. Developing multifunctional crops by engineering brassicaceae glucosinolate pathways. Plant Commun. 2023, 4, 100565. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Laxdal, V.A.; Yu, M.; Raney, B.L. Antioxidant activity of allicin, an active principle in garlic. Mol. Cell. Biochem. 1995, 148, 183–189. [Google Scholar] [CrossRef] [PubMed]
- MOTIE. Ministry of Trade, Industry and Energy. “Act on the Promotion of the Development, Use and Diffusion of New and Renewable Energy” Act No. 13087. 28 January 2015. Available online: https://elaw.klri.re.kr/kor_service/lawView.do?hseq=33632&lang (accessed on 22 September 2023).
- Tina, G.M.; Scavo, F.B.; Merlo, L.; Bizzarri, F. Comparative analysis of monofacial and bifacial photovoltaic modules for floating power plants. Appl. Energy 2021, 281, 116084. [Google Scholar] [CrossRef]
- Korczynski, P.C.; Logan, J.; Faust, J.E. Mapping monthly distribution of daily light integrals across the contiguous united states. HortTechnology 2022, 12, 12–16. [Google Scholar] [CrossRef]
- Kim, K.-D.; Suh, J.-T.; Lee, J.-N.; Yoo, D.-L.; Kwon, M.; Hong, S.-C. Evaluation of factors related to productivity and yield estimation based on growth characteristics and growing degree days in highland kimchi cabbage. Korean J. Hortic. Sci. Technol. 2015, 33, 911–922. [Google Scholar] [CrossRef]
- Oh, S.; Moon, K.H.; Koh, S.C. Effects of different day/night temperature regimes on growth and clove development in cool-type garlic (Allium sativum L.). Korean J. Hortic. Sci. Technol. 2017, 35, 1–10. [Google Scholar] [CrossRef]
- ISO 9167-1: 1992; Determination of Glucosinolates Content-Part i: Method Using High-Performance Liquid Chromatography. International Standard Organization: Geneva, Switzerland, 1992; pp. 1–9.
- Ku, K.-M.; Kim, M.J.; Jeffery, E.H.; Kang, Y.-H.; Juvik, J.A. Profiles of glucosinolates, their hydrolysis products, and quinone reductase inducing activity from 39 arugula (Eruca sativa Mill.) accessions. J. Agric. Food Chem. 2016, 64, 6524–6532. [Google Scholar] [CrossRef]
- Nguyen, B.; Hong, H.; O’hare, T.; Wehr, J.; Menzies, N.; Harper, S. A rapid and simplified methodology for the extraction and quantification of allicin in garlic. J. Food Compos. Anal. 2021, 104, 104114. [Google Scholar] [CrossRef]
- Martín-Lagos, R.; Serrano, M.; López, R. Comparative study by gas chromatography-mass spectrometry of methods for the extraction of sulfur compounds in Allium cepa. L. Food Chem. 1992, 44, 305–308. [Google Scholar] [CrossRef]
- Faust, J.E.; Logan, J. Daily light integral: A research review and high-resolution maps of the united states. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Özdemir, Y.; Demirel, H.; Yıldırım, Y.E. Developing of dli (daily light integral) and spectrum control systems for scientific cultivation in agriculture. Int. J. Agric. For. Sci. 2016, 1, 14–17. [Google Scholar]
- Wermter, N.S.; Rohn, S.; Hanschen, F.S. Seasonal variation of glucosinolate hydrolysis products in commercial white and red cabbages (Brassica oleracea var. Capitata). Foods 2020, 9, 1682. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate and genotype to seasonal variation in the glucosinolate–myrosinase system. Ii. Myrosinase activity in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 682–690. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Yoo, M.; Lee, S.; Yoon, Y.S.; Won, M.-H.; Hwang, I.K.; Choi, J.H. Neuroprotective effects of z-ajoene, an organosulfur compound derived from oil-macerated garlic, in the gerbil hippocampal ca1 region after transient forebrain ischemia. Food Chem. Toxicol. 2014, 72, 1–7. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Organosulfur compounds from alliaceae in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 183–193. [Google Scholar] [CrossRef]
- Martín-Lagos, R.A.; Serrano, M.F.O.; Lopez, M.D.R. Determination of organic sulphur compounds in garlic extracts by gas chromatography and mass spectrometry. Food Chem. 1995, 53, 91–93. [Google Scholar] [CrossRef]
- Yoo, M.; Lee, S.; Kim, S.; Hwang, J.-B.; Choe, J.; Shin, D. Composition of organosulfur compounds from cool-and warm-type garlic (Allium sativum L.) in korea. Food Sci. Biotechnol. 2014, 23, 337–344. [Google Scholar] [CrossRef]
- Weselek, A.; Ehmann, A.; Zikeli, S.; Lewandowski, I.; Schindele, S.; Högy, P. Agrophotovoltaic systems: Applications, challenges, and opportunities. A review. Agron. Sustain. Dev. 2019, 39, 35. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Lantzke, N.; van Burgel, A. Effects of shade nets on microclimatic conditions, growth, fruit yield, and quality of eggplant (Solanum melongena. L.) A case study in carnarvon, western australia. Horticulturae 2022, 8, 696. [Google Scholar] [CrossRef]
- Gommers, C.M.; Visser, E.J.; Onge, K.R.S.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Min, S.-Y.; Kim, B.-M.; Yoon, H.-G.; Jeong, J.-H.; Oh, W. Effects of environmental changes by an agrivoltaic system on growth and quality characteristics of kimchi cabbage. Soc. People Plants Environ. 2022, 25, 659–667. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, M.K.; Youn, K.S.; No, H.K.; Han, D.C. Fermentation and quality of kimchi prepared with Chinese cabbages harvested from field and hydroponic cultivation. J. Food Sci. Nutr. 1999, 4, 241–245. [Google Scholar]
- Jo, H.; Asekova, S.; Bayat, M.A.; Ali, L.; Song, J.T.; Ha, Y.-S.; Hong, D.-H.; Lee, J.-D. Comparison of yield and yield components of several crops grown under agro-photovoltaic system in korea. Agriculture 2022, 12, 619. [Google Scholar] [CrossRef]
- Yamori, W. Chapter 12—Photosynthesis and respiration. In Plant Factory, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 197–206. [Google Scholar]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of nitrogen and sulfur fertilization on the alliin content of onions and garlic. J. Plant Nutr. 2005, 27, 1827–1839. [Google Scholar] [CrossRef]
- Seo, H.; Bae, J.-H.; Kim, G.; Kim, S.-A.; Ryu, B.H.; Han, N.S. Suitability analysis of 17 probiotic type strains of lactic acid bacteria as starter for kimchi fermentation. Foods 2021, 10, 1435. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Ciska, E.; Pawlak-Lemańska, K.; Chmielewski, J.; Borkowski, T.; Tyrakowska, B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit. Contam. 2006, 23, 1088–1098. [Google Scholar] [CrossRef]
- Koga, Y.; Yoshiga, T.; Shindo, J.-I.; Aoyama, R.; Nishimuta, K.; Koyama, R.; Miyamoto, H.; Haraguchi, T.; Ryuda, N.; Ueno, D. Identification of specific odour compounds from garlic cloves infected with the potato tuber nematode, ditylenchus destructor, using gas chromatography-olfactometry. Nematology 2021, 24, 55–63. [Google Scholar] [CrossRef]
- Cossu, M.; Sirigu, A.; Deligios, P.A.; Farci, R.; Carboni, G.; Urracci, G.; Ledda, L. Yield Response and Physiological Adaptation of Green Bean to Photovoltaic Greenhouses. Front. Plant Sci. 2021, 12, 655851. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.J.; Kim, S.K.; Lee, S.G.; Lee, H.S.; Choi, C.S. Development of growth models as affected by cultivation season and transplanting date and estimation of prediction yield in kimchi cabbage. J. Bio-Environ. Control 2017, 26, 235–241. [Google Scholar] [CrossRef]
- Kratky, B.A.; Wang, J.K.; Kubojiri, K. Effects of container size, transplant age, and plant spacing on chinese cabbage1. J. Am. Soc. Hortic. Sci. 1982, 107, 345–347. [Google Scholar] [CrossRef]
- Marsh, D.B.; Paul, K.B. Influence of container type and cell size on cabbage transplant development and field performance. HortScience 1988, 23, 310–311. [Google Scholar] [CrossRef]
- Simões, A.M.; Calouro, F.; Abrantes, E.; Sousa, E. Influence of container size and substrate mineral composition on transplant growth and yield of broccoli cv. Green duke. In Optimization of Plant Nutrition: Refereed Papers from the Eighth International Colloquium for the Optimization of Plant Nutrition, Lisbon, Portugal, 31 August–8 September 1992; Fragoso, M.A.C., Van Beusichem, M.L., Houwers, A., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 87–92. [Google Scholar]
- Polthanee, A.; Promsaena, K.; Laoken, A. Influence of low light intensity on growth and yield of four soybean cultivars during wet and dry seasons of northeast thailand. Agric. Sci. 2011, 2, 61–67. [Google Scholar] [CrossRef]
- Moon, H.-W.; Ku, K.-M. The effect of additional shading utilizing agriphotovoltaic structures on the visual qualities and metabolites of broccoli. Front. Plant Sci. 2023, 14, 1111069. [Google Scholar] [CrossRef]
- Dash, D.; Pattnaik, D.; Panda, D.; Dey, P.; Baig, M.J.; Rout, G.R.; Paikray, R.K.; Samal, K.C.; Panda, R.K.; Gupta, A.K. Effect of Low Light Stress on Leaf Chlorophyll a, b, a + b, a/b, Catalse, Peroxidase, SOD and Yield of Long duration Rice Varieties (Oryza sativa L.). Int. J. Plant Soil Sci. 2022, 34, 184–193. [Google Scholar] [CrossRef]
- Ort, D.R.; Zhu, X.; Melis, A. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol. 2011, 155, 79–85. [Google Scholar] [CrossRef]
- Walker, B.J.; Drewry, D.T.; Slattery, R.A.; VanLoocke, A.; Cho, Y.B.; Ort, D.R. Chlorophyll can be reduced in crop canopies with little penalty to photosynthesis. Plant Physiol. 2018, 176, 1215–1232. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Qu, M.; Ort, D.R.; Zhu, X.G. The impact of modifying photosystem antenna size on canopy photosynthetic efficiency—Development of a new canopy photosynthesis model scaling from metabolism to canopy level processes. Plant Cell Environ. 2017, 40, 2946–2957. [Google Scholar] [CrossRef]
- Li, Y.; Ren, B.; Gao, L.; Ding, L.; Jiang, D.; Xu, X.; Shen, Q.; Guo, S. Less chlorophyll does not necessarily restrain light capture ability and photosynthesis in a chlorophyll-deficient rice mutant. J. Agron. Crop. Sci. 2013, 199, 49–56. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crop. Res. 2017, 200, 58–70. [Google Scholar] [CrossRef]
- Sakowska, K.; Alberti, G.; Genesio, L.; Peressotti, A.; Delle Vedove, G.; Gianelle, D.; Colombo, R.; Rodeghiero, M.; Panigada, C.; Juszczak, R.; et al. Leaf and canopy photosynthesis of a chlorophyll deficient soybean mutant. Plant Cell Environ. 2018, 41, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Boyd, R.A.; Ort, D.R. The photosynthetic response of C3 and C4 bioenergy grass species to fluctuating light. Gcb Bioenergy 2022, 14, 37–53. [Google Scholar] [CrossRef]
- Dipta, S.S.; Schoenlaub, J.; Rahaman, H.; Uddin, A. Estimating the potential for semitransparent organic solar cells in agrophotovoltaic greenhouses. Appl. Energy 2022, 328, 120208. [Google Scholar] [CrossRef]
- Majumdar, D.; Pasqualetti, M.J. Dual use of agricultural land: Introducing ‘agrivoltaics’ in Phoenix Metropolitan Statistical Area, USA. Landsc. Urban Plan. 2018, 170, 150–168. [Google Scholar] [CrossRef]
- Gomez-Casanovas, N.; Mwebaze, P.; Khanna, M.; Branham, B.; Time, A.; DeLucia, E.H.; Bernacchi, C.J.; Knapp, A.K.; Hoque, M.J.; Miljkovic, N.; et al. Knowns, uncertainties, and challenges in agrivoltaics to sustainably intensify energy and food production. Cell Rep. Phys. Sci. 2023, 4, 101518. [Google Scholar] [CrossRef]
- Ort, D.R. When there is too much light. Plant Physiol. 2001, 125, 29–32. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Pavao-Zuckerman, M.A.; Minor, R.L.; Sutter, L.F.; Barnett-Moreno, I.; Blackett, D.T.; Thompson, M.; Dimond, K.; Gerlak, A.K.; Macknick, J.E.; et al. Agrivoltaics provide mutual benefits across the food–energy–water nexus in drylands. Nat. Sustain. 2019, 2, 848–855. [Google Scholar] [CrossRef]
- Hassanpour Adeh, E.; Selker, J.S.; Higgins, C.W. Remarkable agrivoltaic influence on soil moisture, micrometeorology and water-use efficiency. PLoS ONE 2018, 13, e0203256. [Google Scholar] [CrossRef]
- Bai, Z.; Jia, A.; Bai, Z.; Qu, S.; Zhang, M.; Kong, L.; Sun, R.; Wang, M. Photovoltaic panels have altered grassland plant biodiversity and soil microbial diversity. Front. Microbiol. 2022, 13, 1065899. [Google Scholar] [CrossRef]



| Crop | DTH (day) | GDD (°C) z | Humidity (%) | Solar Radiation (Wh/m2) | Precipitation (mm) |
|---|---|---|---|---|---|
| Kimchi cabbage 2019 | 89 | 1004 | 72 | 3186 | 429 |
| Kimchi cabbage 2020 | 82 | 917 | 70 | 3291 | 188 |
| Garlic 2019–2020 | 253 | 1552 | 65 | 3673 | 510 |
| Garlic 2020–2021 | 222 | 1312 | 63 | 3483 | 386 |
| Crop | Weight (kg) | APV Weight Loss (%) | |
|---|---|---|---|
| Control (OF) | APV | ||
| Kimchi cabbage 2019 z | 3.5 ± 0.4 | 2.8 ± 0.6 * | 21% |
| Kimchi cabbage 2020 y | 5.2 ± 0.6 | 4.3 ± 0.7 ns | 17% |
| Two years average | 4.4 ± 1.0 | 3.6 ± 1.0 ns | 18% |
| Crop | Weight (g) | APV Weight Loss (%) | |
|---|---|---|---|
| Control (OF) | APV | ||
| Garlic 2019–2020 z | 109.2 ± 10.5 | 86.0 ± 10.4 * | 21% |
| Garlic 2020–2021 z | 93.4 ± 11.4 | 86.5 ± 20.4ns | 7% |
| Two years average | 101.3 ± 13.2 | 86.2 ± 15.6 * | 15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, D.-Y.; Chae, S.-H.; Moon, H.-W.; Kim, H.J.; Seong, J.; Lee, M.-S.; Ku, K.-M. Agrivoltaic Farming Insights: A Case Study on the Cultivation and Quality of Kimchi Cabbage and Garlic. Agronomy 2023, 13, 2625. https://doi.org/10.3390/agronomy13102625
Ko D-Y, Chae S-H, Moon H-W, Kim HJ, Seong J, Lee M-S, Ku K-M. Agrivoltaic Farming Insights: A Case Study on the Cultivation and Quality of Kimchi Cabbage and Garlic. Agronomy. 2023; 13(10):2625. https://doi.org/10.3390/agronomy13102625
Chicago/Turabian StyleKo, Da-Yeong, Seung-Hun Chae, Hyeon-Woo Moon, Hye Joung Kim, Joon Seong, Moon-Sub Lee, and Kang-Mo Ku. 2023. "Agrivoltaic Farming Insights: A Case Study on the Cultivation and Quality of Kimchi Cabbage and Garlic" Agronomy 13, no. 10: 2625. https://doi.org/10.3390/agronomy13102625






