Comparative Study of the Priming Effect of Abscisic Acid on Tolerance to Saline and Alkaline Stresses in Rice Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Stress Treatment and ABA Application
2.3. Measurement of Seedling Growth
2.4. Measurement of Na+ and K+ Contents
2.5. Measurement of the Chlorophyll Content
2.6. Measurement of Membrane Injury (MI) and Malondialdehyde (MDA) Contents
2.7. Measurement of Superoxide Anion Radical (O2·−) and Hydrogen Peroxide (H2O2) Levels
2.8. Measurement of Antioxidant Enzyme Activities
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Statistical Analyses
3. Results
3.1. ABA Priming Increased Rice Seedling Survival under Both Saline and Alkaline Conditions
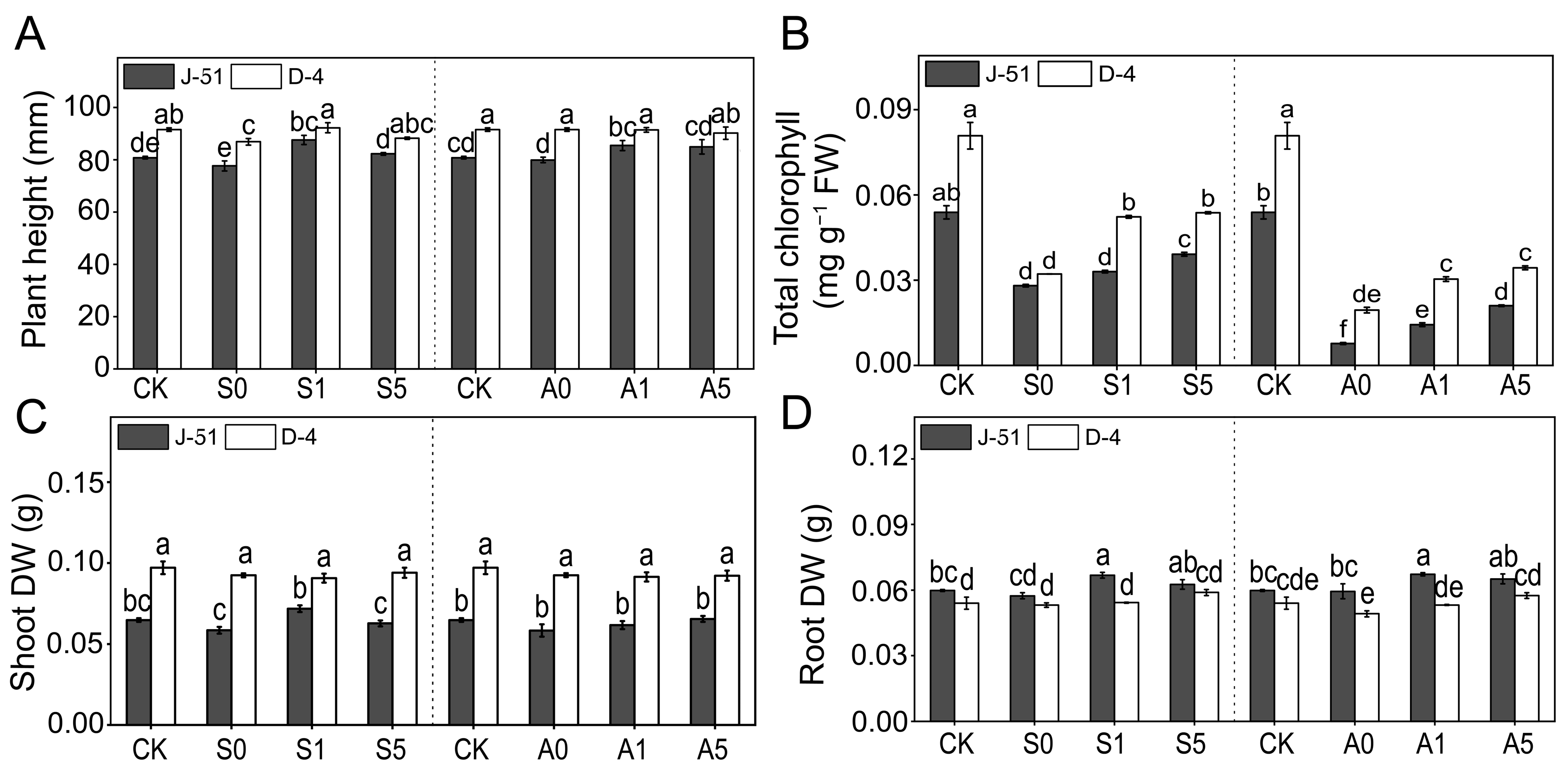
3.2. ABA Priming Enhanced Rice Seedling Growth under Saline and Alkaline Stresses
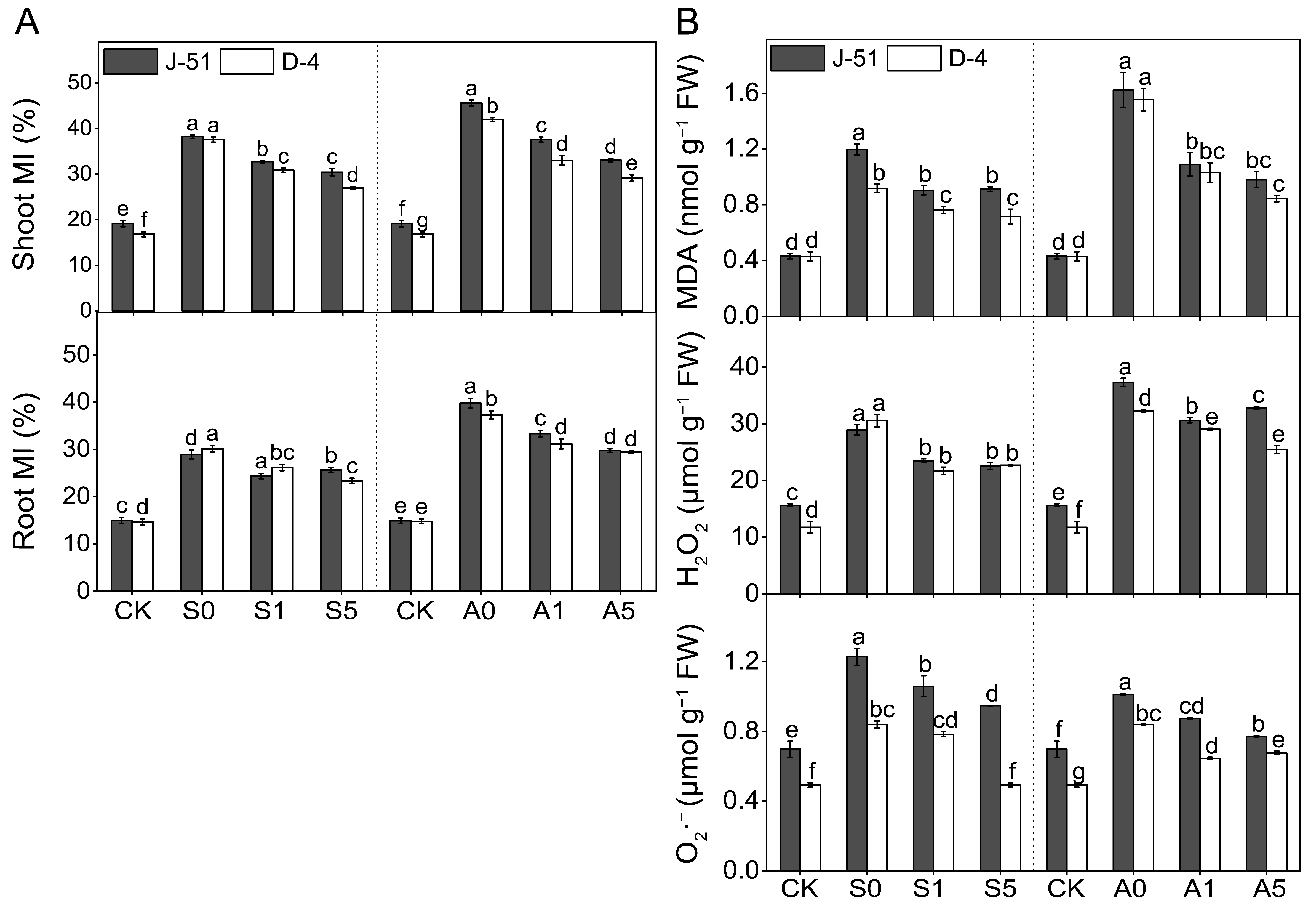
3.3. ABA Priming Mediated Ion Homeostasis in Rice Seedlings under Saline and Alkaline Stresses
3.4. ABA Priming Mitigated Plasma Membrane Damage and Reduced MDA and ROS Accumulation under Saline and Alkaline Stresses
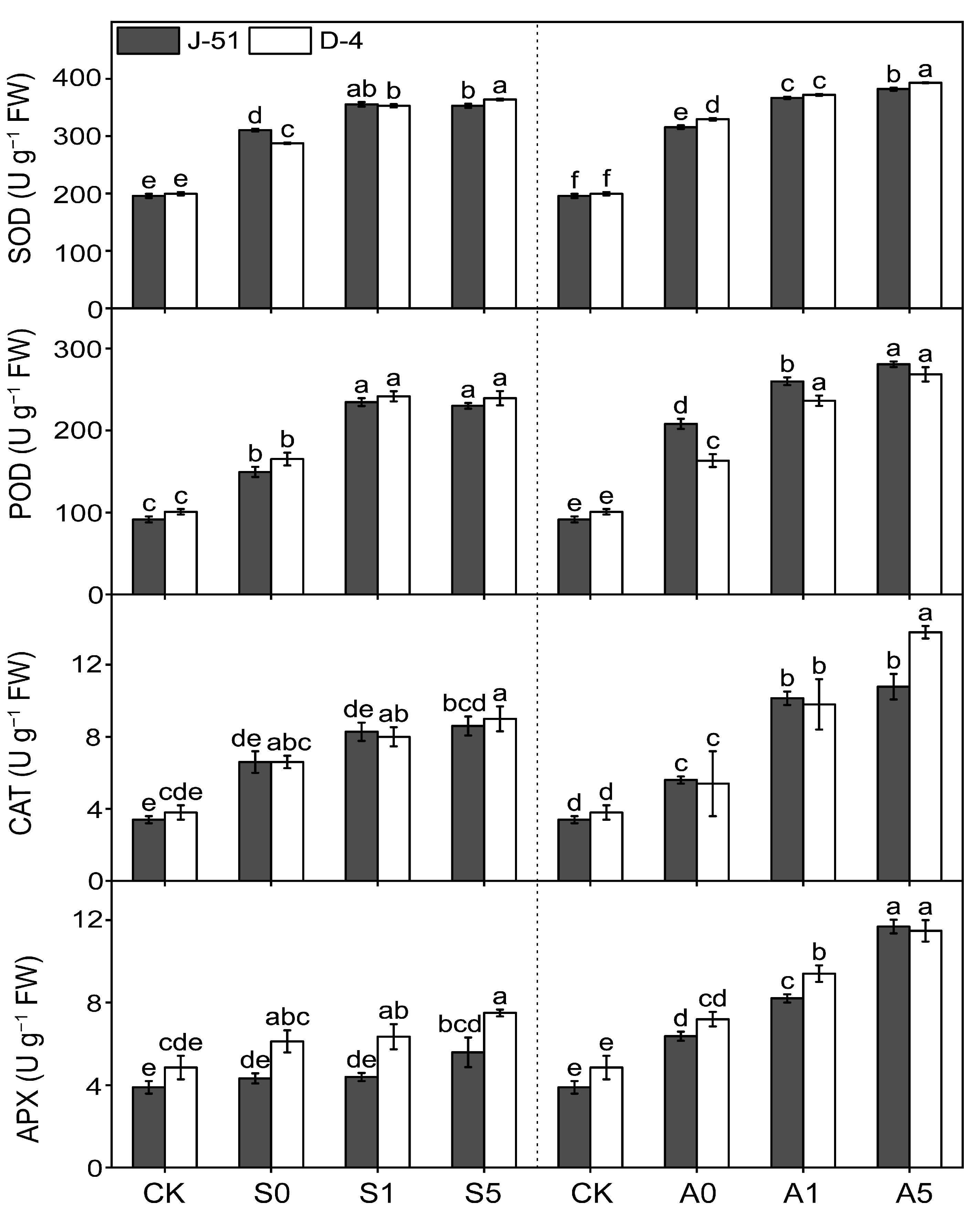
3.5. ABA Priming Alleviated Oxidative Damage by Modulating the Activities of Antioxidant Enzymes under Saline and Alkaline Stresses
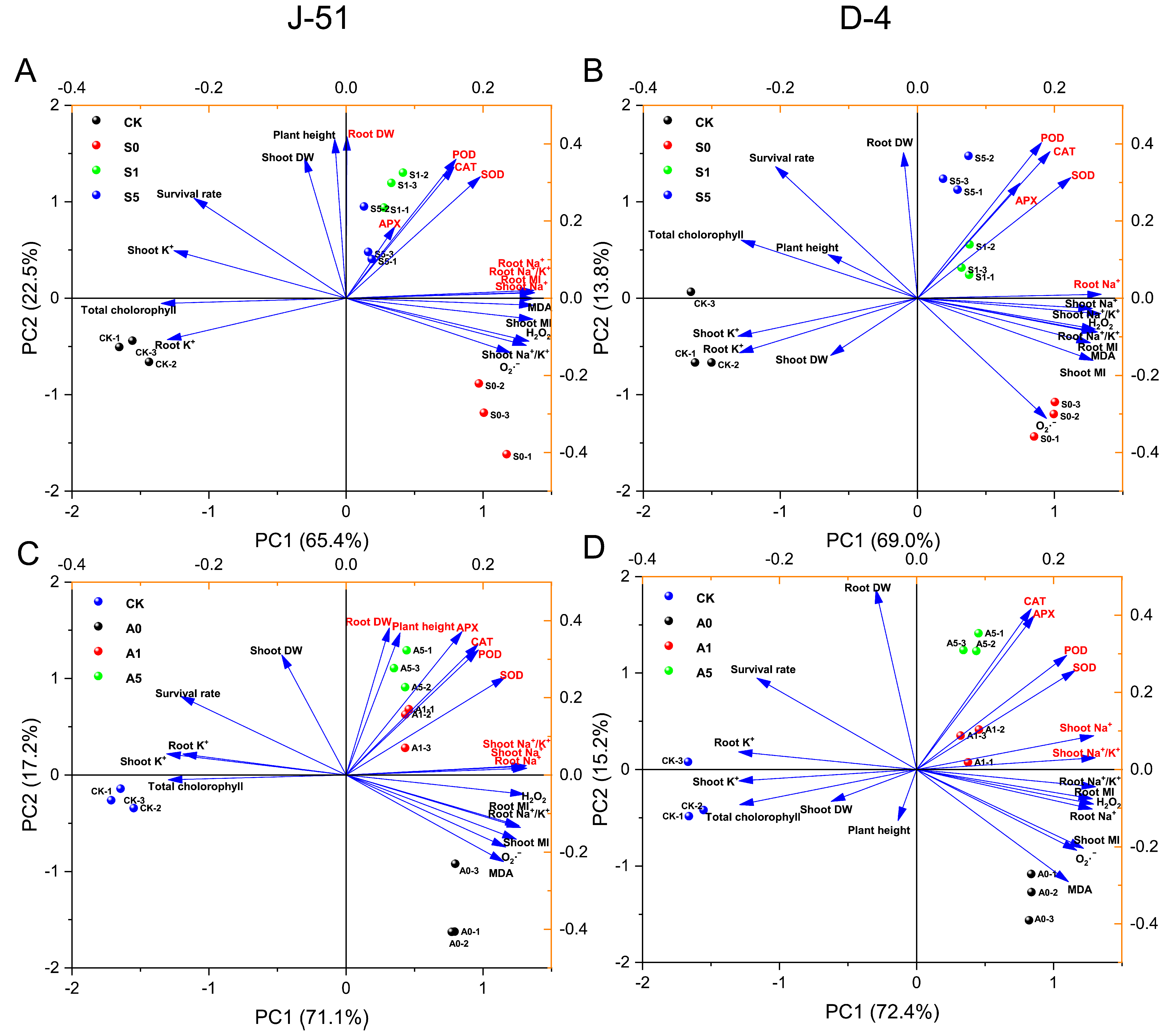
3.6. Multivariate Statistical Analysis of the Rice Seedling Response to ABA Application under Saline and Alkaline Stresses
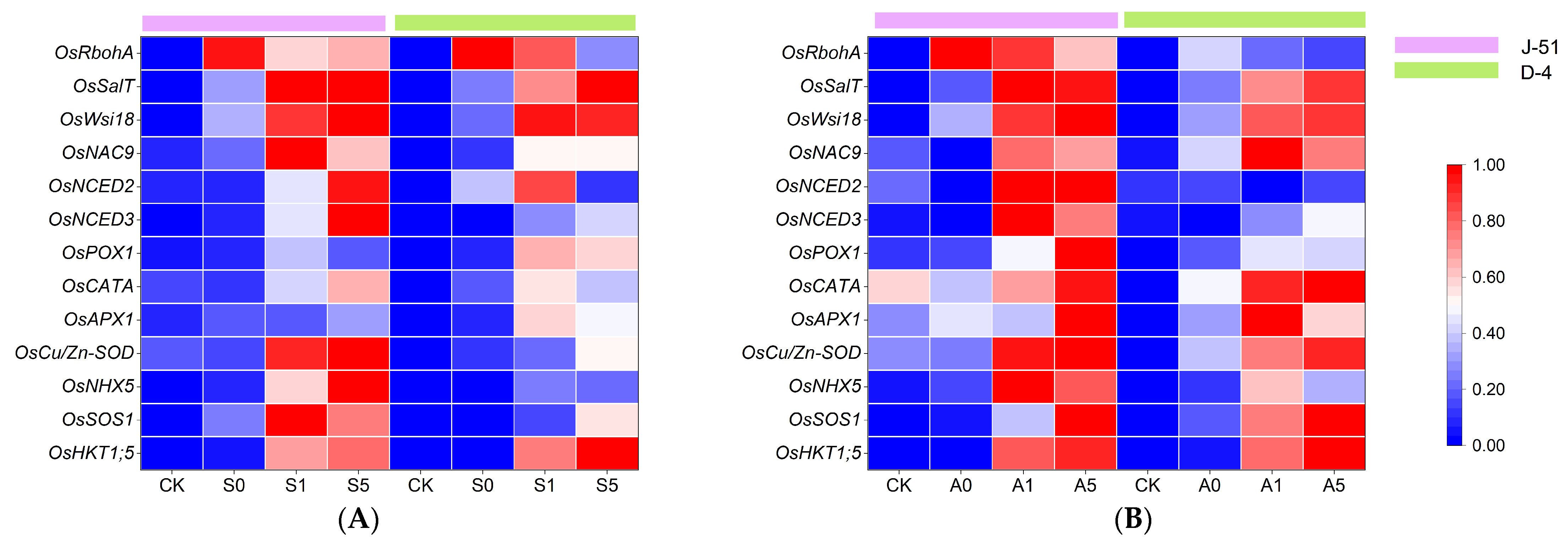
3.7. ABA Priming Mediated the Expression of Relevant Genes under Saline and Alkaline Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-Q.; Tan, Y.Z.; Zhai, H.; Du, Y.P. Evaluation of salt resistance mechanisms of grapevine hybrid rootstocks. Sci. Hortic. 2019, 243, 148–158. [Google Scholar] [CrossRef]
- Ahmad, R.; Anjum, M.A. Physiological and molecular basis of salinity tolerance in fruit crops. In Fruit Crops; Elsevier: Amsterdam, The Netherlands, 2020; pp. 445–464. [Google Scholar]
- FAO. Status of the World’s Soil Resources; FAO: Rome, Italy, 2015. [Google Scholar]
- Liu, L.; Wang, B.D. Protection of halophytes and their uses for cultivation of saline-alkali soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef]
- Lv, B.S.; Li, X.W.; Ma, H.Y.; Sun, Y.; Wei, L.X.; Jiang, C.J.; Liang, Z.W. Differences in growth and physiology of rice in response to different saline-alkaline stress factors. Agron. J. 2013, 105, 1119–1128. [Google Scholar] [CrossRef]
- Ma, H.Y.; Liang, Z.W. Effects of different soil pH and soil extracts on the germination and seedling growth of Leymus chinensis. Chin. Bull. Bot. 2007, 24, 181–188. [Google Scholar]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Summart, J.; Thanonkeo, P.; Panichajakul, S.; Prathepha, P.; McManus, M.T. Effect of salt stress on growth, inorganic ion and proline accumulation in Thai aromatic rice, Khao Dawk Mali 105, callus culture. Afr. J. Biotechnol. 2010, 9, 145–152. [Google Scholar]
- James, R.A.; Munns, R.; von Caemmerer, S.; Trejo, C.; Miller, C.; Condon, T. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl- in salt-affected barley and durum wheat. Plant Cell Environ. 2006, 29, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, A.; Zhang, W.H. Comparative studies on tolerance of rice genotypes differing in their tolerance to moderate salt stress. BMC Plant Biol. 2017, 17, 141. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Liu, Y.Q.; Dun, H.R.; Yin, X.X.; Cui, Y.N.; Chai, W.W.; Song, X.; Flowers, T.J.; Wang, S.M. SsHKT1;1 is coordinated with SsSOS1 and SsNHX1 to regulate Na+ homeostasis in Suaeda salsa under saline conditions. Plant Soil. 2020, 449, 117–131. [Google Scholar] [CrossRef]
- Gong, Z.; Chen, W.; Bao, G.; Sun, J.; Ding, X.; Fan, C. Physiological response of Secale cereale L. seedlings under freezing-thawing and alkaline salt stress. Environ. Sci. Pollut. Res. Int. 2020, 27, 1499–1507. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Zhang, Z.; Yu, X.; Gao, Z.; Wang, Y.; Wang, J.; Li, Z.; Mu, C. Salinity-alkalinity tolerance in wheat: Seed germination, early seedling growth, ion relations and solute accumulation. Afr. J. Agric. Res. 2012, 7, 467–474. [Google Scholar]
- Zhang, H.; Huo, Y.; Xu, Z.; Guo, K.; Wang, Y.; Zhang, X.; Xu, N.; Sun, G. Physiological and proteomics responses of nitrogen assimilation and glutamine/glutamine family of amino acids metabolism in mulberry (Morus alba L.) leaves to NaCl and NaHCO3 stress. Plant Signal Behav. 2020, 15, 1798108. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gene 2017, 11, 106–111. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.L.; Zhang, R.X.; Yuan, H.Y.; Wang, M.M.; Yang, H.Y.; Ma, H.Y.; Liu, D.; Jiang, C.J.; Liang, Y.W. Root damage under alkaline stress is associated with reactive oxygen species accumulation in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wang, S.; Shen, T.; Wang, Q.; Chen, C.; Xia, J.; Jing, M. Calcium/calmodulin-dependent protein kinase OsDMI3 positively regulates saline-alkaline tolerance in rice roots. Plant Signal Behav. 2020, 15, 1813999. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Flowers, T.J.; Wang, S.M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil. 2009, 326, 45–60. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Wang, Z.; Liang, Z.; Wang, M.; Liu, M.; Suarez, D.L. Interactive effects of pH, EC and nitrogen on yields and nutrient absorption of rice (Oryza sativa L.). Agric. Water Manag. 2017, 194, 48–57. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, X.; Chen, X.; Wang, J.; Wang, D.; Wang, S.; Guo, L.; Rui, C.; Zhang, Y.; Malik, W.A.; et al. Cotton transcriptome analysis reveals novel biological pathways that eliminate reactive oxygen species (ROS) under sodium bicarbonate (NaHCO3) alkaline stress. Genomics 2021, 113, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Baranova, E.N.; Gulevich, A.A. Asymmetry of Plant Cell Divisions under Salt Stress. Symmetry 2021, 13, 1811. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Ganapati, R.K.; Naveed, S.A.; Zafar, S.; Wang, W.; Xu, J. Saline-alkali tolerance in rice: Physiological response, molecular mechanism, and QTL identification and application to breeding. Rice Sci. 2022, 29, 412–434. [Google Scholar] [CrossRef]
- Grattan, S.; Zheng, L.; Shannon, M.C.; Roberts, S.R. Rice is more sensitive to salinity than previously thought. Calif. Agric. 2002, 56, 189–198. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, F.; Xie, P.; Sun, S.; Qiao, X.; Tang, S.; Chen, C.; Yang, S.; Mei, C.; Yang, D.; et al. A Gγ protein regulates alkaline sensitivity in crops. Science 2023, 379, eade8416. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Shi, L.; Yan, C.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef]
- Duan, G.; Liu, M.; Liang, Z.; Wang, M.; Yang, H.; Xu, Y.; Yu, T.; Jin, Y.; Hu, J.; Liu, J. Amendments of severe saline-sodic paddy land: Optimal combination of Phosphogypsum, farmyard fertilizer, and wood peat. Agronomy 2023, 13, 1364. [Google Scholar] [CrossRef]
- Wang, W.S.; Zhao, X.Q.; Li, M.; Huang, L.Y.; Xu, J.L.; Zhang, F.; Cui, Y.R.; Fu, B.Y.; Li, Z.K. Complex molecular mechanisms underlying seedling salt tolerance in rice revealed by comparative transcriptome and metabolomic profiling. J. Exp. Bot. 2016, 67, 405–419. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Huang, G.; Jiang, X.; Liang, Y.; Yang, C.; Huang, L. Comparison of yield prediction models and estimation of the relative importance of main agronomic traits affecting rice yield formation in saline-sodic paddy fields. Eur. J. Agron. 2023, 148, 126870. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Nafees, M.; Chen, J.; Darras, A.; Ferrante, A.; Hancock, J.T.; Muhammad, A.; Zaid, A.; Latif, N.; Corpas, F.J.; et al. Chemical priming enhances plant tolerance to salt stress. Front. Plant Sci. 2022, 13, 946922. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kumar, P.; Verma, V.; Sharma, R.; Bargava, B.; Irfan, M. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol. Biochem. 2022, 179, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-M.; Rengasamy, P.; Wang, Z.C.; Yang, F.; Ma, H.Y.; Huang, L.H.; Liu, M.; Yang, H.Y.; Li, J.P.; An, F.H.; et al. Identification of the most limiting factor for rice yield using soil data collected before planting and during the reproductive stage. Land. Degrad. Dev. 2018, 29, 2310–2320. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hong, Y.; Yao, J.; Ren, Z.; Shi, H.; Zhu, J.K. The flowering repressor SVP confers drought resistance in Arabidopsis by regulating abscisic acid catabolism. Mol. Plant 2018, 11, 1184–1197. [Google Scholar] [CrossRef]
- Marusig, D.; Tombesi, S. Abscisic acid mediates drought and salt stress responses in Vitis vinifera—A review. Int. J. Mol. Sci. 2020, 21, 8648. [Google Scholar] [CrossRef]
- Yang, J.; He, R.; Qu, Z.; Gu, J.; Jiang, L.; Zhan, X.; Gao, Y.; Adelson, D.L.; Li, S.; Wang, Z.; et al. Long noncoding RNA ARTA controls ABA response through MYB7 nuclear trafficking in Arabidopsis. Dev. Cell 2023, 58, 1206–1217. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.; Chu, H.D.; Watanabe, Y.; Osakabe, Y.; Sato, M.; Toyooka, K.; Seo, M.; Tian, L.; Tian, C.; et al. Comparative functional analyses of DWARF14 and KARRIKIN INSENSITIVE 2 in drought adaptation of Arabidopsis thaliana. Plant J. 2020, 103, 111–127. [Google Scholar] [CrossRef]
- Wu, M.; Cai, R.; Liu, H.; Li, F.; Zhao, Y.; Xiang, Y. A moso bamboo drought-induced 19 protein, PeDi19-4, enhanced drought and salt tolerance in plants via the ABA-dependent signaling pathway. Plant Cell Physiol. 2019, 60, e1–e14. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Rabara, R. Abscisic acid-An enigma in the abiotic stress tolerance of crop plants. Plant Gene 2017, 11, 90–98. [Google Scholar] [CrossRef]
- Miyake, Y.; Takahashi, E. Effect of silicon on the growth of solution-cultured cucumber plant. Soil. Sci. Plant Nutr. 1983, 29, 71–83. [Google Scholar] [CrossRef]
- Kumar, G.M.; Knowles, N.R. Changes in lipid peroxidation and lipolytic and free-radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Datta, S.K. Micropropagation of gerbera: Lipid peroxidation and antioxidant enzyme activities during acclimatization process. Acta Physiol. Plant 2007, 30, 325–331. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Klapheck, S.; Zimmer, I.; Cosse, H. Scavenging of hydrogen peroxide in the endosperm of Ricinus communis by ascorbate peroxidase. Plant Cell Physiol. 1990, 31, 1005–1013. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xu, B.; Cao, L.; Zhang, Z.; Li, X.; Zhao, X.; Wang, X.; Wang, Y.; Wu, B.; Zhou, W.; Lin, C.; et al. Physiological effects of combined NaCl and NaHCO3 stress on the seedlings of two maple species. Front. Plant Sci. 2023, 14, 1209999. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.-J.; Wang, M.M.; Jin, Y.Y.; Zhang, G.H.; Liu, M.; Yang, H.Y.; Jiang, C.J.; Liang, Z.W. Abscisic acid priming creates alkaline tolerance in alfalfa seedlings (Medicago sativa L.). Agriculture 2021, 11, 608. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Gholami, R.; Abdelrahman, M.; Tran, L.P. Exogenous melatonin mitigates salinity-induced damage in olive seedlings by modulating ion homeostasis, antioxidant defense, and phytohormone balance. Physiol. Plant 2021, 173, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Breusegem, F.; Gechev, T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.J.; Liang, Z.W.; Xie, X.Z. Priming for saline-alkaline tolerance in rice: Current knowledge and future challenges. Rice Sci. 2023, 30, 7. [Google Scholar]
- Jisha, K.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Dodd, I.C.; Puertolas, J.; Huber, K.; Perez-Perez, J.G.; Wright, H.R.; Blackwell, M.S.A. The importance of soil drying and re-wetting in crop phytohormonal and nutritional responses to deficit irrigation. J. Exp. Bot. 2015, 66, 2239–2252. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Sabbatini, P.; Squeri, C.; Rodas, N.L.; Palliotti, A.; Poni, S. Kaolin reduces ABA biosynthesis through the inhibition of neoxanthin synthesis in grapevines under water deficit. Int. J. Mol. Sci. 2020, 21, 4950. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Zhao, L.; Shen, X.; Rao, G.; Meng, F.; Huang, A. Physiological mechanisms of ABA-induced salinity tolerance in leaves and roots of rice. Sci. Rep. 2022, 12, 8228. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Zhang, M.X.; Bai, R.; Nan, M.; Ren, W.; Wang, C.M.; Sabala, S.; Zhang, J.L. Evaluation of salt tolerance of oat cultivars and the mechanism of adaptation to salinity. J. Plant Physiol. 2022, 273, 153708. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-cis-Epoxycarotenoid dioxygenase 3 regulates plant growth and enhances multi-abiotic stress tolerance in rice. Front. Plant Sci. 2018, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.J.; Liu, X.L.; Liu, X.Q.; Zhang, H.; Yu, Y.J.; Liang, Z.W. Stunted growth caused by blast disease in rice seedlings is associated with changes in phytohormone signaling pathways. Front. Plant Sci. 2017, 8, 1558. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.J.; Jiang, C.J.; Jin, Y.Y.; Zhang, G.H.; Wang, M.M.; Liang, Z.W. Ca2+/Na+ Ratio as a Critical Marker for Field Evaluation of Saline-Alkaline Tolerance in Alfalfa (Medicago sativa L.). Agronomy 2020, 10, 191. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nan, N.; Li, N.; Liu, Y.; Wang, T.J.; Hwang, I.; Liu, B.; Xu, Z.Y. A DNA methylation reader-chaperone regulator-transcription factor complex activates OsHKT1;5 expression during salinity stress. Plant Cell 2020, 32, 3535–3558. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Rengel, Z.; Shabala, S. WITHDRAWN: HKT1;5 transporter gene expression and NHX-type Na+/H+ exchanger activity regulate adaptation of Echinacea species to salt stress. Environ. Exp. Bot. 2023, 105365. [Google Scholar] [CrossRef]
- Brindha, C.; Vasantha, S.; Raja, A.K.; Tayade, A.S. Characterization of the salt overly sensitive pathway genes in sugarcane under salinity stress. Physiol. Plant 2021, 171, 677–687. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Liu, X.-L.; Zhang, H.; Jin, Y.Y.; Wang, M.M.; Yang, H.Y.; Ma, H.Y.; Jiang, C.J.; Liang, Z.W. Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance-related genes. Plant Soil 2019, 438, 39–55. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Lu, G.; Sun, M.; Jin, Y.; Xu, Y.; Liu, X.; Wang, M.; Liu, M.; Yang, H.; Guan, Y.; et al. Comparative Study of the Priming Effect of Abscisic Acid on Tolerance to Saline and Alkaline Stresses in Rice Seedlings. Agronomy 2023, 13, 2698. https://doi.org/10.3390/agronomy13112698
Feng Z, Lu G, Sun M, Jin Y, Xu Y, Liu X, Wang M, Liu M, Yang H, Guan Y, et al. Comparative Study of the Priming Effect of Abscisic Acid on Tolerance to Saline and Alkaline Stresses in Rice Seedlings. Agronomy. 2023; 13(11):2698. https://doi.org/10.3390/agronomy13112698
Chicago/Turabian StyleFeng, Zhonghui, Guanru Lu, Miao Sun, Yangyang Jin, Yang Xu, Xiaolong Liu, Mingming Wang, Miao Liu, Haoyu Yang, Yi Guan, and et al. 2023. "Comparative Study of the Priming Effect of Abscisic Acid on Tolerance to Saline and Alkaline Stresses in Rice Seedlings" Agronomy 13, no. 11: 2698. https://doi.org/10.3390/agronomy13112698





