Molecular Dissection of the 5S Ribosomal RNA-Intergenic Transcribed Spacers in Saccharum spp. and Tripidium spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. DNA Extraction and Quantification
2.3. PCR Amplification, Agarose Gel, and CE Analyses
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniels, J.; Roach, B.T. Taxonomy and evolution. In Sugarcane Improvement through Breeding; Heinz, D.J., Ed.; Elsevier Press: Amsterdam, The Netherlands, 1987; pp. 7–84. [Google Scholar]
- Mukherjee, S.K. Origin and distribution of Saccharum. Bot. Gaz. 1957, 119, 55–61. [Google Scholar] [CrossRef]
- Valdés, B.; Scholz, H. The Euro+Med treatment of Gramineae—A generic synopsis and some new names. Willdenowia 2006, 36, 657–669. [Google Scholar] [CrossRef]
- Lloyd Evans, D.; Joshi, S.V.; Wang, J. Whole chloroplast genome and gene locus phylogenies reveal the taxonomic placement and relationship of Tripidium (Panicoideae: Andropogoneae) to sugarcane. BMC Evol. Biol. 2019, 19, 33. [Google Scholar] [CrossRef]
- Berding, N.; Roach, B.T. Germplasm collection, maintenance and use. In Sugarcane Improvement through Breeding; Heinz, D.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 143–210. [Google Scholar]
- Arro, J.A.; Veremis, J.C.; Kimbeng, C.A.; Botanga, C. Genetic diversity and relationships revealed by AFLP markers among Saccharum spontaneum and related species and genera. J. Am. Soc. Sugar Cane Technol. 2006, 26, 101–115. [Google Scholar]
- Hale, A.L.; Viator, R.P.; Veremis, J.C. Identification of freeze tolerant Saccharum spontaneum accessions through a pot-based study for use in sugarcane germplasm enhancement for adaptation to temperate climates. Biomass Bioenergy 2014, 61, 53–57. [Google Scholar] [CrossRef]
- Amalraj, V.A.; Balasundaram, N. On the taxonomy of the members of ‘Saccharum Complex’. Genet. Resour. Crop Evol. 2006, 53, 35–41. [Google Scholar] [CrossRef]
- Cloix, C.; Tutois, S.; Yukawa, Y.; Mathieu, O.; Cuvillier, C.; Espagnol, M.C.; Picard, G.; Tourmente, S. Analysis of the 5S RNA pool in Arabidopsis thaliana: RNAs are heterogeneous and only two of the genomic 5S loci produce mature 5S RNA. Genome Res. 2002, 12, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Grabiele, M.; Aguilera, P.M.; Ducasse, D.A.; Debat, H.J. Molecular characterization of the 5S rDNA non-transcribed spacer and reconstruction of phylogenetic relationships in Capsicum. Rodriguésia 2021, 72. [Google Scholar] [CrossRef]
- Schneeberger, R.G.; Creissen, G.P.; Cullis, C.A. Chromosomal and molecular analysis of 5S RNA gene organization in the flax, Linum usitatissinum. Gene 1989, 83, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Lee, D.; Thomas, C.M.; Simpson, P.R.; Cleary, W.G.; Newman, M.A.; Burcham, K.W.G. 5S rRNA genes in pisum: Sequence, long range and chromosomal organization. Mol. Gen. Genet. 1988, 214, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Campenhout, S.V.; Aert, R.; Volckaert, G. Orthologous sequence variation among 5S ribosomal RNA gene spacer sequences on homoeologous chromosomes 1B, 1D and 1R of wheat and rye. Genome 1998, 41, 244–255. [Google Scholar] [CrossRef]
- Baum, B.R.; Johnson, D.A. The 5S rRNA gene in sea barley (Hordeum marinum Hudson sensu labo): Sequence variation among repeat units and relationship to the X haplome in barley (hordeum). Genome 1998, 41, 652–661. [Google Scholar] [CrossRef]
- Schmidt, T.; Schwarzacher, T.; Heslop-Harrison, J.S. Physical mapping of rRNA genes by fluorescent in situ hybridization and structural analysis of 5S rRNA genes and intergenic spacer sequences in sugar beet (Beta vulgaris). Theor. Appl. Genet. 1994, 88, 629–636. [Google Scholar] [CrossRef] [PubMed]
- D’Hont, A.; Rao, P.S.; Feldmann, P.; Grivet, L.; Islam-Faridi, N.; Taylor, P.; Glaszmann, J.C. Identification and characterisation of sugarcane intergeneric hybrids, Saccharum officinarum x Erianthus arundinaceus with molecular markers and DNA in situ hybridisation. Theor. Appl. Genet. 1995, 91, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.B.; Burner, D.M.; Legendre, B.L. An assessment of the phylogenetic relationship among sugarcane and related taxa based on the nucleotide sequence of 5S rRNA intergenic spacers. Genetica 2000, 108, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhang, Z.; Yan, T.; Lin, Q.; Wang, Y.; Huang, W.; Huang, Y.; Li, Z.; Yu, Q.; Wang, J.; et al. Comprehensively characterizing the cytological features of Saccharum spontaneum by the development of a complete set of chromosome-specific oligo probes. Front. Plant Sci. 2018, 9, 1624. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.V.; Bennett, M.D.; Dyer, T.A. Use of the polymerase chain reaction to detect spacer size heterogeneity in plant 5S-rRNA gene clusters and to locate such clusters in wheat (Triticum aestivum L.). Theor. Appl. Genet. 1992, 83, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, M.; Vierna, J.; González-Tizón, A.M.; Martínez-Lage, A. The 5S rDNA gene family in mollusks: Characterization of transcriptional regulatory regions, prediction of secondary structures, and long-term evolution, with special attention to Mytilidae Mussels. J. Hered. 2011, 102, 433–444. [Google Scholar] [CrossRef]
- Fukuhara, S.; Terajima, Y.; Irei, S.; Sakaigaichi, T.; Ujihara, K.; Sugimoto, A.; Matsuoka, M. Identification and characterization of intergeneric hybrid of commercial sugarcane (Saccharum spp. hybrid) and Erianthus arundinaceus (Retz.) Jeswiet. Euphytica 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Nair, N.V.; Mohanraj, K.; Sunadaravelpandian, K.; Suganya, A.; Selvi, A.; Appunu, C. Characterization of an intergeneric hybrid of Erianthus procerus× Saccharum officinarum and its backcross progenies. Euphytica 2017, 213, 267. [Google Scholar] [CrossRef]
- Pachakkil, B.; Terajima, Y.; Ohmido, N.; Ebina, M.; Irei, S.; Hayashi, H.; Takagi, H. Cytogenetic and agronomic characterization of intergeneric hybrids between Saccharum spp. hybrid and Erianthus arundinaceus. Sci. Rep. 2019, 9, 1748. [Google Scholar] [CrossRef]
- Pan, Y.-B.; Burner, D.M.; Wei, Q. Developing species-specific DNA markers to assist in sugarcane breeding. Proc. Intl. Soc. Sugar Cane Technol. 2001, 24, 337–342. [Google Scholar]
- Yu, F.; Chai, J.; Li, X.; Yu, Z.; Yang, R.; Ding, X.; Wang, Q.; Wu, J.; Yang, X.; Deng, Z. Chromosomal characterization of Tripidium arundinaceum revealed by Oligo-FISH. Int. J. Mol. Sci. 2021, 22, 8539. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, M.; Liu, J.; Huang, Y.; Xu, L.; Deng, Z.; Zhao, X. Efficient anchoring of Erianthus arundinaceus chromatin introgressed into sugarcane by specific molecular markers. Int. J. Mol. Sci. 2022, 23, 9435. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Chen, P.H.; Pan, Y.B.; Chen, R.K. High-throughput procedure for single pollen grain collection and polymerase chain reaction in plants. J. Integr. Plant Biol. 2008, 50, 375–383. [Google Scholar] [CrossRef]
- Chae, W.B.; Hong, S.J.; Gifford, J.M.; Rayburn, A.L.; Sacks, E.J.; Juvik, J.A. Plant morphology, genome size, and SSR markers differentiate five distinct taxonomic groups among accessions in the genus Miscanthus. GCB Bioenergy 2014, 6, 646–660. [Google Scholar] [CrossRef]
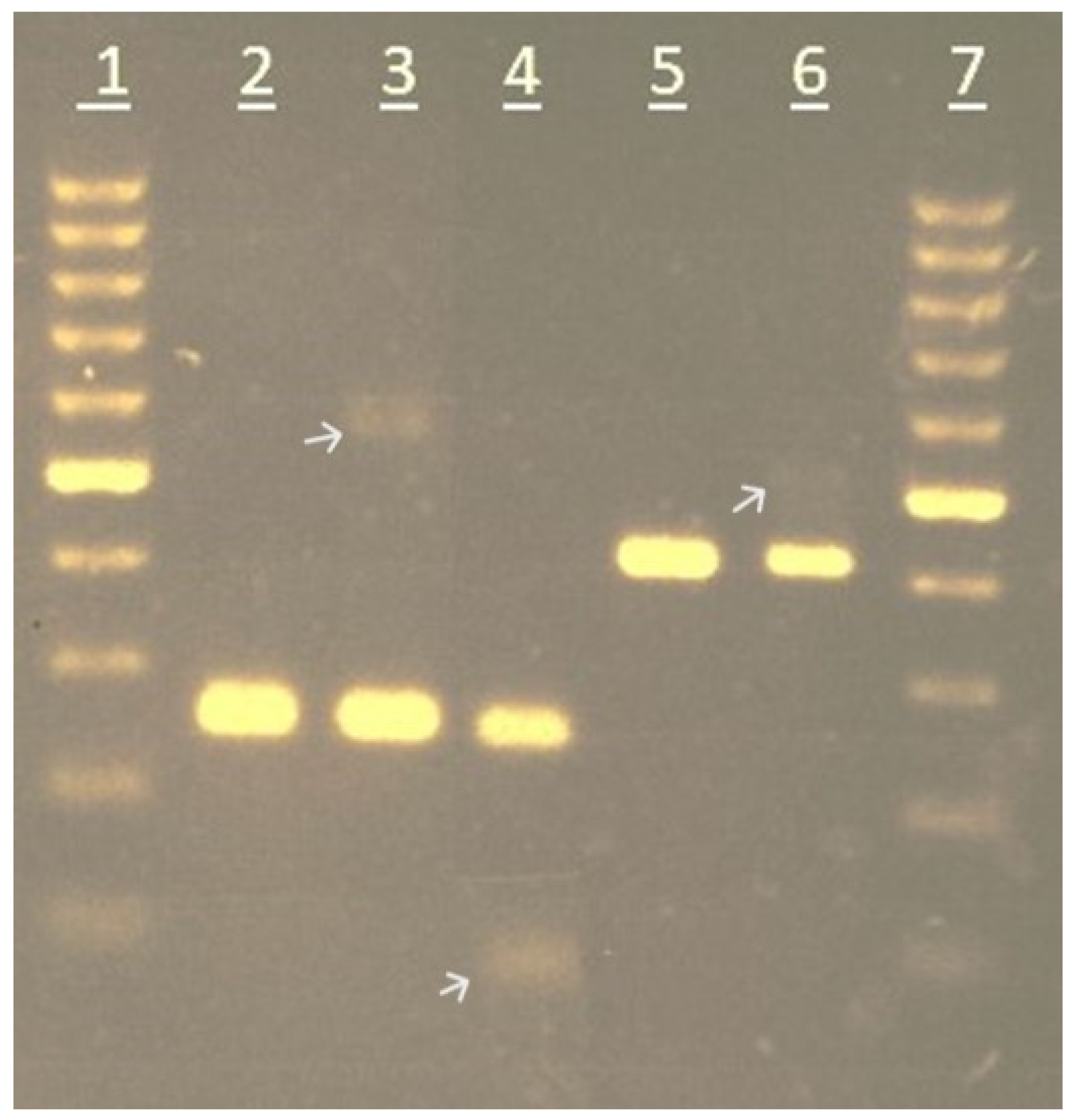
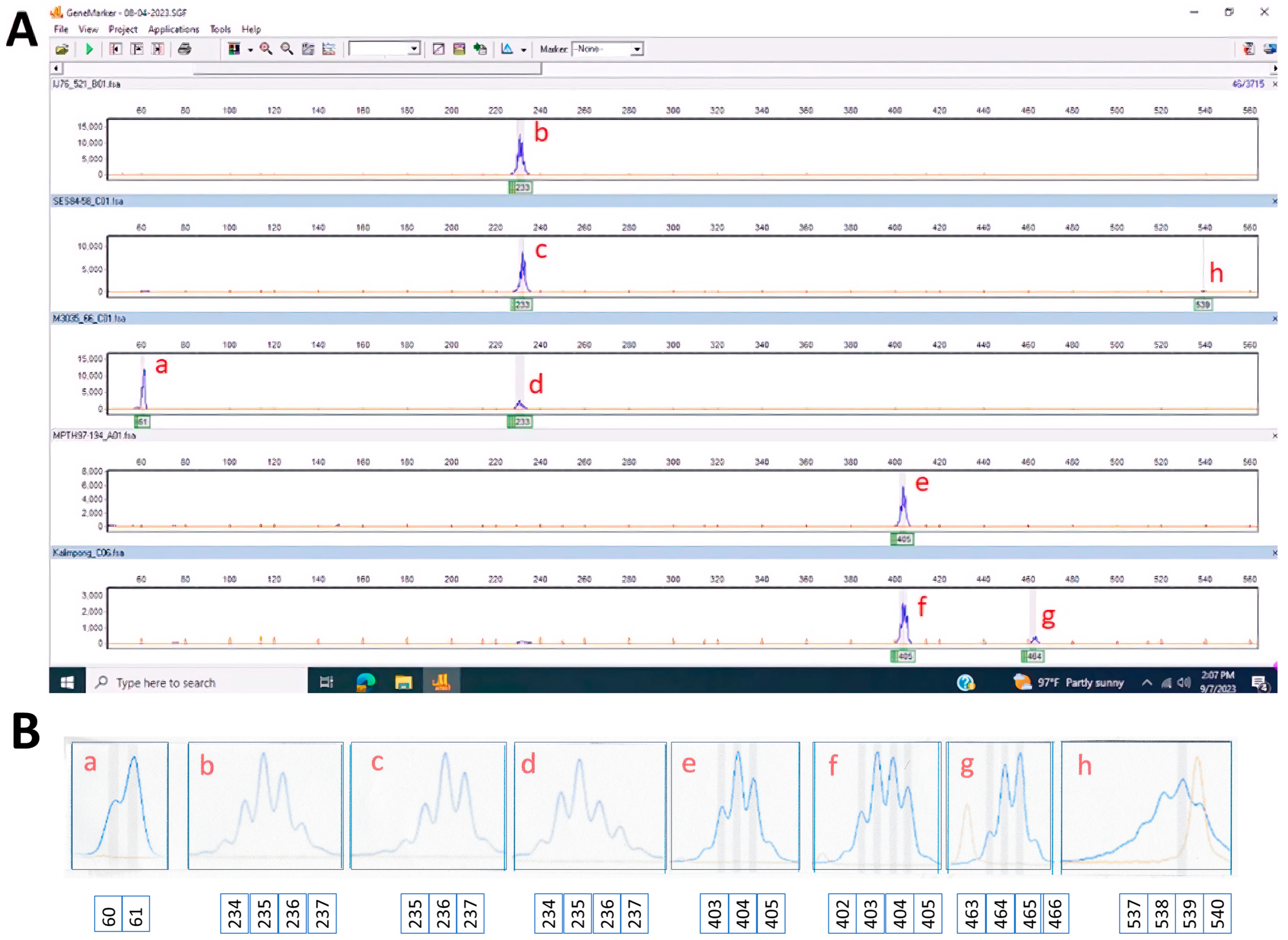
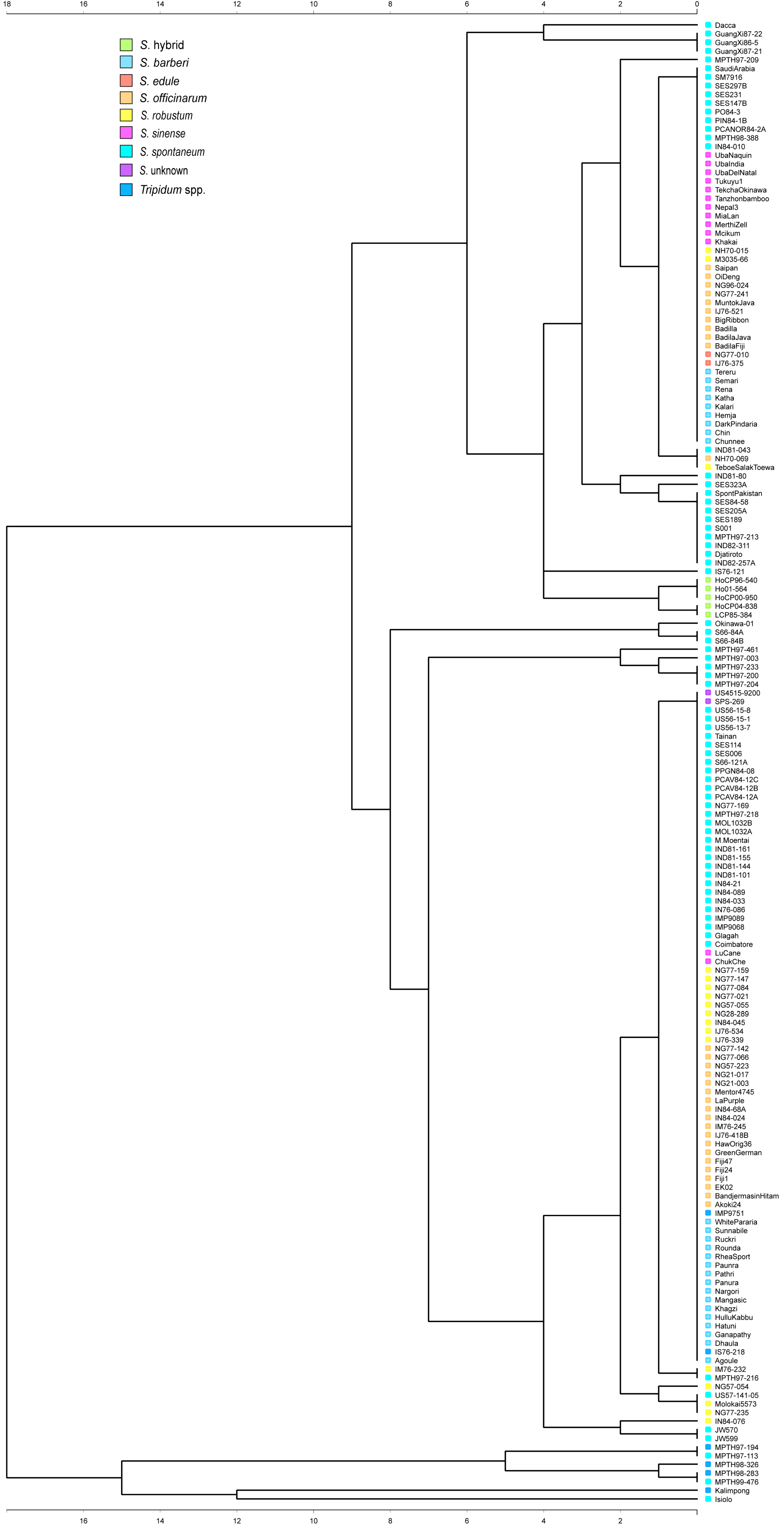
| Genus | Species | Accession | Total | Gel Banding Type |
|---|---|---|---|---|
| Tripidium | arundinaceum | IS76-218, MPTH97-194, MPTH98-283, MPTH98-326 | 4 | IV |
| bengalense | IMP9751 | 1 | IV | |
| procerum | Kalimpong | 1 | V | |
| Saccharum | officinarum | Akoki24 *, Badila Fiji *, Badila Java *, Badila, Bandjermasin Hitam *, Big Ribbon *, EK02 *, Fiji1, Fiji24 *, Fiji47, Green German*, Haw Orig 36 *, IJ76-418B *, IJ76-521 *, IM76-245 *, IN84-024 *, IN84-68A, Louisiana Purple*, Mentor4745, Muntok Java, NG21-003 *, NG21-017 *, NG57-223 *, NG77-066 *, NG77-142 *, NG77-241 *, NG96-024 *, NH70-069 *, Oi Deng, Saipan * | 30 | I, II |
| spontaneum | Coimbatore, Dacca *, Djatiroto, Glagah *, GuangXi86-5, GuangXi87-21, GuangXi87-22, IMP9068, IMP9089, IN76-086 *, IN84-010 *, IN84-033 *, IN84-089 *, IN84-21, IND81-043 *, IND81-101 *, IND81-144, IND81-155 *, IND81-161, IND81-80, IND82-257A, IND82-311, IS76-121 *, Isiolo *, JW570, JW599, M. Moentai *, MOL1032A, MOL1032B, MPTH97-003, MPTH97-113, MPTH97-200, MPTH97-204, MPTH97-209, MPTH97-213, MPTH97-216, MPTH97-218, MPTH97-233, MPTH97-461, MPTH98-388, MPTH99-476, NG77-169 *, Okinawa #01 *, PCANOR84-2A, PCAV84-12A, PCAV84-12B, PCAV84-12C, PIN84-1B, PO84-3, PPGN84-08 *, S001 *, S66-121A, S66-84A, S66-84B, SES006, SES114, SES147B, SES189, SES205A, SES231, SES297B *, SES323A, SES84-58, S spont Pakistan *, Saudi Arabia *, SM7916 *, Tainan, US56-013-07 *, US56-15-1, US56-15-8, US57-141-05 * | 71 | I, II | |
| robustum | IJ76-339 *, IJ76-534 *, IM76-232 *, IN84-045 *, IN84-076 *, M3035/66 *, Molokai 5573, NG28-289 *, NG57-054, NG57-055 *, NG77-021 *, NG77-084 *, NG77-147 *, NG77-159 *, NG77-235 *, NH70-015 *, Teboe SalakToewa * | 17 | I, II, III | |
| barberi | Agoule *, Chin, Chunnee *, Dark Pindaria *, Dhaula, Ganapathy, Hatuni, Hemja *, Hullu Kabbu *, Kalari, Katha, Khagzi, Manga sic *, Nargori *, Panura, Pathri *, Paunra *, Rena, Rhea Sport, Rounda *, Ruckri *, Semari *, Sunnabile *, Tereru *, White Pararia * | 25 | I, II | |
| sinense | Chuk Che *, Khakai *, Lu Cane *, Mcikum, Merthi Zell, Mia Lan *, Nepal 3 *, Tanzhon bamboo *, Tekcha Okinawa *, Tukuyu 1, Uba Del Natal *, Uba India *, Uba Naquin * | 13 | I, II | |
| edule | IJ76-375 *, NG77-010 * | 2 | I | |
| spp. hybrids | Ho01-564, HoCP00-950, HoCP04-838, HoCP96-540, LCP85-384 | 5 | III | |
| Unknown | US4515-9200, SPS-269 | 2 | II |
| Genus | Saccharum | Tripidium | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | barberi | edule | hybrid | officinarum | robustum | sinense | spontaneum | arundinaceum | bengalense | procerum | unknown |
| No. Accessions | 25 | 2 | 5 | 30 | 17 | 13 | 71 | 4 | 1 | 1 | 2 |
| Amplicons (bp) | Percent of each amplicon per species | ||||||||||
| 61 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 224–225 | 0 | 0 | 0 | 0 | 0 | 0 | 11.3 | 0 | 0 | 0 | 0 |
| 227 | 0 | 0 | 0 | 0 | 0 | 0 | 4.2 | 0 | 0 | 0 | 0 |
| 228–229 | 0 | 0 | 0 | 0 | 5.9 | 0 | 7 | 0 | 0 | 0 | 0 |
| 230–231 | 0 | 0 | 0 | 0 | 0 | 0 | 4.2 | 0 | 0 | 0 | 0 |
| 233 | 0 | 0 | 0 | 0 | 2.8 | 0 | 5.6 | 0 | 0 | 0 | 0 |
| 234 | 100 | 100 | 100 | 100 | 100 | 100 | 73.2 | 25 † | 100 | 0 | 100 |
| 235 | 100 | 100 | 100 | 100 | 100 | 100 | 94.4 | 25.0 † | 100 | 0 | 100 |
| 236 | 100 | 100 | 100 | 100 | 82.4 | 100 | 91.5 | 25.0 † | 100 | 0 | 100 |
| 237 | 36 | 100 | 60 | 36.7 | 17.6 | 84.6 | 42.3 | 0 | 0 | 0 | 0 |
| 238 | 0 | 0 | 0 | 3.3 | 8 | 0 | 2.8 | 0 | 0 | 0 | 0 |
| 247–248 | 0 | 0 | 0 | 0 | 0 | 0 | 2.8 | 0 | 0 | 0 | 0 |
| 382–385 | 0 | 0 | 0 | 0 | 0 | 0 | 2.8 † | 50 | 0 | 0 | 0 |
| 387, 388, 402 | 0 | 0 | 0 | 0 | 0 | 0 | 1.4 † | 0 | 0 | 0 | 0 |
| 403–405 | 0 | 0 | 0 | 0 | 0 | 0 | 1.4 † | 0 | 0 | 100 | 0 |
| 406 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| 407 | 0 | 0 | 0 | 0 | 0 | 0 | 2.8 † | 50 | 0 | 0 | 0 |
| 408–409 | 0 | 0 | 0 | 0 | 0 | 0 | 2.8 † | 0 | 0 | 0 | 0 |
| 463–466 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.-B.; Todd, J.R.; Lomax, L.; White, P.M., Jr.; Simpson, S.A.; Scheffler, B.E. Molecular Dissection of the 5S Ribosomal RNA-Intergenic Transcribed Spacers in Saccharum spp. and Tripidium spp. Agronomy 2023, 13, 2728. https://doi.org/10.3390/agronomy13112728
Pan Y-B, Todd JR, Lomax L, White PM Jr., Simpson SA, Scheffler BE. Molecular Dissection of the 5S Ribosomal RNA-Intergenic Transcribed Spacers in Saccharum spp. and Tripidium spp. Agronomy. 2023; 13(11):2728. https://doi.org/10.3390/agronomy13112728
Chicago/Turabian StylePan, Yong-Bao, James R. Todd, Lionel Lomax, Paul M. White, Jr., Sheron A. Simpson, and Brian E. Scheffler. 2023. "Molecular Dissection of the 5S Ribosomal RNA-Intergenic Transcribed Spacers in Saccharum spp. and Tripidium spp." Agronomy 13, no. 11: 2728. https://doi.org/10.3390/agronomy13112728









