Effects of Organic Liquid Waste Derived from Bioethanol Fermentation on Corn Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Treatments and Fertilizer Management
2.3. Corn Growth and Yield Surveys
2.4. Measurement of Soil Chemical Properties and Nitrogen Content in Corn
2.5. Analysis of Nitrogen Availability and Use Efficiency
2.6. Statistical Analysis
3. Results
3.1. Corn Growth and Yield
3.2. Nitrogen Content, Availability, and Use Efficiency of Corn
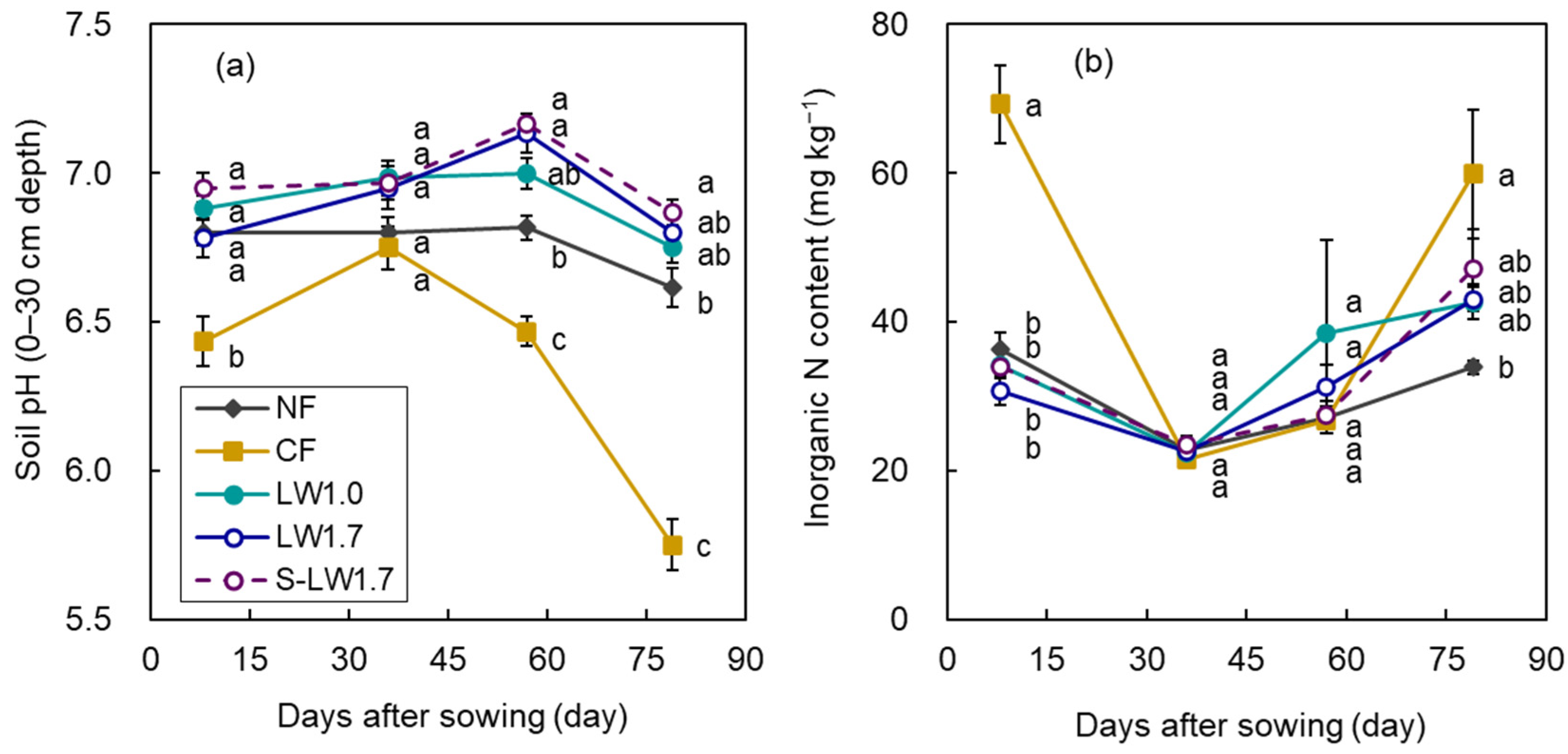
3.3. Soil Chemical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuess, L.T.; Rodrigues, I.J.; Garcia, M.L. Fertirrigation with sugarcane vinasse: Foreseeing potential impacts on soil and water resources through vinasse characterization. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2017, 52, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Christofoletti, C.A.; Escher, J.P.; Correia, J.E.; Marinho, J.F.U.; Fontanetti, C.S. Sugarcane vinasse: Environmental implications of its use. Waste Manag. 2013, 33, 2752–2761. [Google Scholar] [CrossRef]
- Jiang, Z.P.; Li, Y.R.; Wei, G.P.; Liao, Q.; Su, T.M.; Meng, Y.C.; Zhang, H.Y.; Lu, C.Y. Effect of long-term vinasse application on physico-chemical properties of sugarcane field soils. Sugar Tech. 2012, 14, 412–417. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane vinasse as organo-mineral fertilizers feedstock: Opportunities and environmental risks. Sci. Total Environ. 2022, 832, 154998. [Google Scholar] [CrossRef] [PubMed]
- Rachman, L.M.; Hartono, A.; Hazra, F.; Noorwicaksono, T.; Wasono, K.B.; Adityasari, A.D.; Prabowo, B.; Putri, N.; Davik. Essence, principle, and technique in utilization and converting vinasse waste to bio-organic fertilizer. IOP Conf. Ser. Earth Environ. Sci. 2023, 1133, 012023. [Google Scholar] [CrossRef]
- Kulichkova, G. Comparative characteristics of native (liquid) and concentrated up to 40% vinasse as a raw material for anaerobic fermentation. Eureka LS 2022, 6, 25–35. [Google Scholar] [CrossRef]
- Moraes, B.S.; Triolo, J.M.; Lecona, V.P.; Zaiat, M.; Sommer, S.G. Biogas production within the bioethanol production chain: Use of co-substrates for anaerobic digestion of sugar beet vinasse. Bioresour. Technol. 2015, 190, 227–234. [Google Scholar] [CrossRef]
- Nunes Ferraz Junior, A.D.N.F.; Etchebehere, C.; Perecin, D.; Teixeira, S.; Woods, J. Advancing anaerobic digestion of sugarcane vinasse: Current development, struggles and future trends on production and end-uses of biogas in Brazil. Renew. Sustain. Energy Rev. 2022, 157, 112045. [Google Scholar] [CrossRef]
- Ahmed, O.; Sulieman, A.M.E.; Elhardallou, S.B. Physicochemical, chemical and microbiological characteristics of vinasse, A by-product from ethanol industry. Am. J. Biochem. Biotechnol. 2013, 3, 80–83. [Google Scholar] [CrossRef]
- Karimi, S.; Mahboobi Soofiani, N.M.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of filamentous fungal biomass cultivated on vinasse as an alternative nutrient source of fish feed: Protein, lipid, and mineral composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef]
- dos Reis, K.C.; Coimbra, J.M.; Duarte, W.F.; Schwan, R.F.; Silva, C.F. Biological treatment of vinasse with yeast and simultaneous production of single-cell protein for feed supplementation. Int. J. Environ. Sci. Technol. 2019, 16, 763–774. [Google Scholar] [CrossRef]
- Peng, L.; Kong, X.; Wang, Z.; Ai-lati, A.; Ji, Z.; Mao, J. Baijiu vinasse as a new source of bioactive peptides with antioxidant and anti-inflammatory activity. Food Chem. 2021, 339, 128159. [Google Scholar] [CrossRef] [PubMed]
- Kaishev, A.S.; Kaisheva, N.S.; Larsky, M.V.; Karpenko, V.A. Processing of after-alcohol vinasse for environmentally friendly environment. IOP Conf. Ser. Earth Environ. Sci. 2022, 1052, 012118. [Google Scholar] [CrossRef]
- Xu, H.; Xu, X.; Tao, Y.; Yuan, F.; Gao, Y. Optimization by response surface methodology of supercritical carbon dioxide extraction of flavour compounds from Chinese liquor vinasse. Flavour Fragr. J. 2015, 30, 275–281. [Google Scholar] [CrossRef]
- Yang, S.D.; Liu, J.X.; Wu, J.; Tan, H.W.; Li, Y.R. Effects of vinasse and press mud application on the biological properties of soils and productivity of sugarcane. Sugar Tech. 2013, 15, 152–158. [Google Scholar] [CrossRef]
- Gallucci, A.D.; Natera, M.; Moreira, L.A.; Nardi, K.T.; Altarugio, L.M.; de Mira, A.B.; de Almeida, R.F.; Otto, R. Nitrogen-enriched vinasse as a means of supplying nitrogen to sugarcane fields: Testing the effectiveness of N source and application rate. Sugar Tech. 2019, 21, 20–28. [Google Scholar] [CrossRef]
- Otto, R.; de Freitas Júnior, J.C.M.; Zavaschi, E.; de Faria, I.K.P.; Paiva, L.A.; Bazani, J.H.; de Mira, A.B.; Kamogawa, M.Y. Combined application of concentrated vinasse and nitrogen fertilizers in sugarcane: Strategies to reduce ammonia volatilization losses. Sugar Tech. 2017, 19, 248–257. [Google Scholar] [CrossRef]
- Assimakopoulos, J.H. Effect of sodium-vinasse application on seed germination and growth of three species differing in salt tolerance. Commun. Soil Sci. Plant Anal. 2000, 31, 2803–2818. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L.; García-Martínez, A.M.; Parrado, J. Application of a green manure and green manure composted with beet vinasse on soil restoration: Effects on soil properties. Bioresour. Technol. 2008, 99, 4949–4957. [Google Scholar] [CrossRef]
- Madejón, E.; López, R.; Murillo, J.M.; Cabrera, F. Agricultural use of three (sugar-beet) vinasse composts: Effect on crops and chemical properties of a cambisol soil in the Guadalquivir River valley (SW Spain). Agric. Ecosyst. Environ. 2001, 84, 55–65. [Google Scholar] [CrossRef]
- Sahai, R.; Shukla, N.; Jabeen, S.; Saxena, P.K. Pollution effect of distillery waste on the growth behaviour of Phaseolus radiatus L. Environ. Pollut. Ser. A Ecol. Biol. 1985, 37, 245–253. [Google Scholar] [CrossRef]
- Srivastava, N.; Sahai, R. Effects of distillery waste on the performance of Cicer arietinum L. Environ. Pollut. 1987, 43, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kannan, A.; Upreti, R.K. Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J. Hazard. Mater. 2008, 153, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Algur, Ö.F.; Kadioǧlu, A. The effects of vinasse on the growth, biomass and primary productivity in pea (Pisum sativum) and sunflower (Helianthus annuus). Agric. Ecosyst. Environ. 1992, 39, 139–144. [Google Scholar] [CrossRef]
- Bastos, A.V.S.; Teixeira, M.B.; Soares, F.A.L.; da Silva, E.C.; dos Santos, L.N.S.; Gomes, F.H.F. Immediate and residual effects of mineral and organomineral nitrogen sources associated with concentrated vinasse on maize. J. Soil Sci. Plant Nutr. 2021, 21, 1382–1396. [Google Scholar] [CrossRef]
- Elmasry, H.M.M. Partial substitution of maize mineral fertilization with some organic and bio-fertilizers. IJAAS 2021, 2, 103–113. [Google Scholar] [CrossRef]
- de Resende, A.S.; Xavier, R.P.; de Oliveira, O.C.; Urquiaga, S.; Alves, B.J.R.; Boddey, R.M. Long-term effects of pre-harvest burning and nitrogen and vinasse applications on yield of sugar cane and soil carbon and nitrogen stocks on a plantation in Pernambuco, N.E. Brazil. Plant Soil 2006, 281, 339–351. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Oktafiani, O.; Hartanto, D.; Handayani, P.A. Effects of solid vinasse-based organic fertilizer on some growth indices of tomato plant. J. Bahan Alam Terbarukan 2017, 6, 190–197. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Hartanto, D.; Rohman, H.A.; Mitamaytawati; Qudus, N.; Daniyanto. Valorization of sugarcane-based bioethanol industry waste (vinasse) to organic fertilizer. Valoris. Agro-Ind. Resid. 2020, 2, 203–223. [Google Scholar] [CrossRef]
- Kheir, A.; Kamara, M. Effects of sugar beet factory lime, vinasse, and compost mixed with vinasse application on sandy soil properties and canola productivity. J. Soil Sci. Agric. Eng. 2019, 10, 69–77. [Google Scholar] [CrossRef]
- Tejada, M.; Moreno, J.L.; Hernandez, M.T.; Garcia, C. Application of two beet vinasse forms in soil restoration: Effects on soil properties in an arid environment in southern Spain. Agric. Ecosyst. Environ. 2007, 119, 289–298. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Beet vinasse applied to wheat under dryland conditions affects soil properties and yield. Eur. J. Agron. 2005, 23, 336–347. [Google Scholar] [CrossRef]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Organic amendment based on fresh and composted beet vinasse. Soil Sci. Soc. Am. J. 2006, 70, 900–908. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Application of different organic wastes on soil properties and wheat yield. Agron. J. 2007, 99, 1597–1606. [Google Scholar] [CrossRef]
- Toma, Y.; Higuchi, T.; Nagata, O.; Kato, Y.; Izumiya, T.; Oomori, S.; Ueno, H. Efflux of Soil nitrous oxide from Applied Fertilizer Containing Organic Materials in Citrus unshiu Field in Southwestern Japan. Agriculture 2017, 7, 10. [Google Scholar] [CrossRef]
- Charles, M.J.; Simmons, M.S. Methods for the determination of carbon in soils and sediments. A review. Analyst 1986, 111, 385–390. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Methodology for evaluation of lowland rice genotypes for nitrogen use efficiency. J. Plant Nutr. 2003, 26, 1315–1333. [Google Scholar] [CrossRef]
- Japan CROPs. Available online: https://japancrops.com/en/crops/sweet-corn (accessed on 20 October 2023).
- Pereira, N.C.M.; Galindo, F.S.; Gazola, R.P.D.; Dupas, E.; Rosa, P.A.L.; Mortinho, E.S.; Filho, M.C.M.T. Corn yield and phosphorus use efficiency response to phosphorus rates associated with plant growth promoting bacteria. Front. Environ. Sci. 2020, 8, 40. [Google Scholar] [CrossRef]
- Prado, R.M.; Caione, G.; Campos, C.N.S. Filter cake and vinasse as fertilizers contributing to conservation agriculture. Appl. Environ. Soil Sci. 2013, 2013, 581984. [Google Scholar] [CrossRef]
- Omori, W.P.; de Camargo, A.F.; Goulart, K.C.; Lemos, E.G.; de Souza, J.A. Influence of vinasse application in the structure and composition of the bacterial community of the soil under sugarcane cultivation. Int. J. Microbiol. 2016, 7, 2349514. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L. Effects of a microbial inoculant and organic fertilizers on the growth, photosynthesis and yield of sweet corn. J. Crop Prod. 2001, 3, 183–214. [Google Scholar] [CrossRef]
- Cerri, B.C.; Borelli, L.M.; Stelutti, I.M.; Soares, M.R.; da Silva, M.A. Evaluation of new environmental friendly particulate soil fertilizers based on agroindustry wastes biopolymers and sugarcane vinasse. Waste Manag. 2020, 108, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Carpanez, T.G.; Moreira, V.R.; Magalhães, N.C.; Assis, I.R.; Lange, L.C.; Amaral, M.C.S. Integrated membrane-based processes to obtain organo-mineral fertilizer, water, and energy from sugarcane vinasse. Sep. Purif. Technol. 2022, 302, 122180. [Google Scholar] [CrossRef]
- Andrade, L.C.L.; Putti, F.F.; Cremasco, C.P.; Gabriel Filho, L.R.A.G. New paradigm for vinasse use as fertilizer in hydroponics. Sugar Tech. 2022, 24, 1260–1271. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Effects of two beet vinasse forms on soil physical properties and soil loss. CATENA 2006, 68, 41–50. [Google Scholar] [CrossRef]
- Bolan, N.S.; Hedley, M.J.; White, R.E. Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil 1991, 134, 53–63. [Google Scholar] [CrossRef]
- Mattiazzo, M.E.; da Glória, N.A. Effect of vinasse on soil acidity. Water Sci. Technol. 1987, 19, 1293–1296. [Google Scholar] [CrossRef]
- Gutser, R.; Ebertseder, T.; Weber, A.; Schraml, M.; Schmidhalter, U. Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J. Plant Nutr. Soil Sci. 2005, 168, 439–446. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Yu, X.; Huang, Q. Effects of long-term fertilization on corn productivity and its sustainability in an ultisol of southern China. Agric. Ecosyst. Environ. 2010, 138, 44–50. [Google Scholar] [CrossRef]




| Depth (cm) | Bulk Density (g cm−3) | Soil Texture (IUSS) | Total C (g kg−1) | Total N (g kg−1) | C/N Ratio | CEC a (cmolc kg−1) | ||
|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | ||||||
| 0–24 | 1.17 | 76.3 | 10.1 | 13.6 | 19.4 | 2.21 | 9.04 | 11.1 |
| 24–35 | 1.61 | 82.7 | 7.04 | 10.3 | 3.21 | 1.56 | 2.09 | 7.97 |
| 35–40 | 1.73 | 78.6 | 7.70 | 13.7 | 3.31 | 0.94 | 3.56 | 7.40 |
| 40–75 | 1.54 | 78.5 | 8.37 | 13.1 | 3.74 | 1.52 | 2.48 | 7.50 |
| 75–93 | 1.47 | 68.7 | 6.78 | 24.6 | 3.17 | 1.15 | 2.76 | 9.19 |
| 93–102 | 1.61 | 76.4 | 9.75 | 13.8 | 4.28 | 0.97 | 4.50 | 6.24 |
| Treatment | Abbreviation | Nutrients | Amount of Fertilizer (kg 10a−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days after Sowing (Day) | Total | |||||||||
| −2 | 20 | 29 | 36 | 47 | 58 | 65 | ||||
| No fertilizer | NF | N | 0 | |||||||
| P2O5 | 0 | |||||||||
| K2O | 0 | |||||||||
| Chemical fertilizer | CF | N | 10.0 | 10.0 | 10.0 | 30.0 | ||||
| P2O5 | 10.0 | 10.0 | 10.0 | 30.0 | ||||||
| K2O | 10.0 | 10.0 | 10.0 | 30.0 | ||||||
| Liquid waste 1.0 | LW1.0 | N | 10.0 | 10.0 | 10.0 | 30.0 | ||||
| P2O5 | 1.07 | 1.07 | 1.07 | 3.21 | ||||||
| K2O | 12.9 | 12.9 | 12.9 | 38.7 | ||||||
| Liquid waste 1.7 | LW1.7 | N | 17.0 | 17.0 | 17.0 | 51.0 | ||||
| P2O5 | 1.82 | 1.82 | 1.82 | 5.46 | ||||||
| K2O | 22.0 | 22.0 | 22.0 | 66.0 | ||||||
| Split-application liquid waste 1.7 | S-LW1.7 | N | 8.50 | 8.50 | 8.50 | 8.50 | 8.50 | 8.50 | 51.0 | |
| P2O5 | 0.911 | 0.911 | 0.911 | 0.911 | 0.911 | 0.911 | 5.46 | |||
| K2O | 11.0 | 11.0 | 11.0 | 11.0 | 11.0 | 11.0 | 66.0 | |||
| Measurement Items | Treatments | Days after Sowing (Day) | ||||
|---|---|---|---|---|---|---|
| 16 | 29 | 44 | 58 | 79 | ||
| Plant height (cm) | N | 19.2 ± 0.24 a | 67.1 ± 0.94 a | 122 ± 1.3 b | 168 ± 1.8 c | N/A |
| CF | 19.6 ± 0.30 a | 63.0 ± 0.84 a | 133 ± 1.4 a | 184 ± 1.4 a | N/A | |
| LW1.0 | 20.1 ± 0.26 a | 62.7 ± 0.90 a | 128 ± 1.5 a | 176 ± 1.4 b | N/A | |
| LW1.7 | 19.5 ± 0.31 a | 62.4 ± 1.02 a | 132 ± 1.0 a | 180 ± 1.0 ab | N/A | |
| S-LW1.7 | 19.7 ± 0.22 a | 62.9 ± 0.88 a | 129 ± 1.2 a | 178 ± 1.0 b | N/A | |
| Number of leaves (number plant−1) | N | 3.8 ± 0.06 a | 7.8 ± 0.13 a | 8.8 ± 0.18 b | 8.8 ± 0.12 a | N/A |
| CF | 3.9 ± 0.05 a | 7.6 ± 0.10 a | 9.0 ± 0.12 ab | 9.1 ± 0.09 a | N/A | |
| LW1.0 | 4.0 ± 0.05 a | 7.8 ± 0.13 a | 8.8 ± 0.16 b | 9.0 ± 0.09 a | N/A | |
| LW1.7 | 3.9 ± 0.04 a | 7.7 ± 0.12 a | 9.4 ± 0.11 a | 9.1 ± 0.09 a | N/A | |
| S-LW1.7 | 3.7 ± 0.06 a | 8.0 ± 0.12 a | 9.4 ± 0.13 a | 9.0 ± 0.08 a | N/A | |
| SPAD value (SPAD) | N | 32.2 ± 0.41 a | 41.2 ± 0.46 ab | 39.4 ± 0.58 d | 37.5 ± 0.78 d | 30.1 ± 1.10 c |
| CF | 32.8 ± 0.43 a | 42.3 ± 0.43 a | 48.4 ± 0.54 a | 48.9 ± 0.69 a | 31.7 ± 1.21 bc | |
| LW1.0 | 32.4 ± 0.40 a | 40.3 ± 0.44 b | 43.6 ± 0.47 c | 41.8 ± 0.72 c | 34.6 ± 1.16 bc | |
| LW1.7 | 32.4 ± 0.46 a | 40.0 ± 0.45 b | 45.7 ± 0.43 b | 43.9 ± 0.70 bc | 34.9 ± 1.19 ab | |
| S-LW1.7 | 32.3 ± 0.38 a | 40.8 ± 0.43 ab | 44.7 ± 0.55 bc | 44.8 ± 0.77 b | 37.0 ± 1.18 a | |
| Treatment | N Input (kg 10a−1) | N Content (kg 10a−1) | N Availability (%) | NUE (kg DW kg N−1) | ||
|---|---|---|---|---|---|---|
| Stems, Leaves and Tassels | Ears | Total | ||||
| NF | 0 | 11.9 | N/A | N/A | N/A | N/A |
| CF | 30 | 16.6 | 100.0 | 29.4 | 10.9 | 40.3 |
| LW1.0 | 30 | 14.1 a | 45.1 a | 21.4 | 10.8 | 32.2 |
| LW1.7 | 51 | 15.2 | 40.5 | 16.6 | 6.9 | 23.5 |
| S-LW1.7 | 51 | 16.5 | 56.9 | 18.2 | 6.9 | 25.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, N.; Toma, Y.; Kato, Y.; Izumiya, T.; Ueno, H. Effects of Organic Liquid Waste Derived from Bioethanol Fermentation on Corn Production. Agronomy 2023, 13, 2904. https://doi.org/10.3390/agronomy13122904
Morita N, Toma Y, Kato Y, Izumiya T, Ueno H. Effects of Organic Liquid Waste Derived from Bioethanol Fermentation on Corn Production. Agronomy. 2023; 13(12):2904. https://doi.org/10.3390/agronomy13122904
Chicago/Turabian StyleMorita, Nobuki, Yo Toma, Yasuhiko Kato, Tooru Izumiya, and Hideto Ueno. 2023. "Effects of Organic Liquid Waste Derived from Bioethanol Fermentation on Corn Production" Agronomy 13, no. 12: 2904. https://doi.org/10.3390/agronomy13122904






