Soybean-Oil-Modified Petrochemical-Source Polyester Polyurethane Improves the Nutrient Release Performance of Coated Urea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Different CRFs
2.3. Wear Resistance Test
2.4. Statistical Analysis
2.5. FTIR Characterization
2.6. Characterization of Coating Materials
2.7. Nitrogen Release Characteristics of SMPCUs
3. Results and Discussion
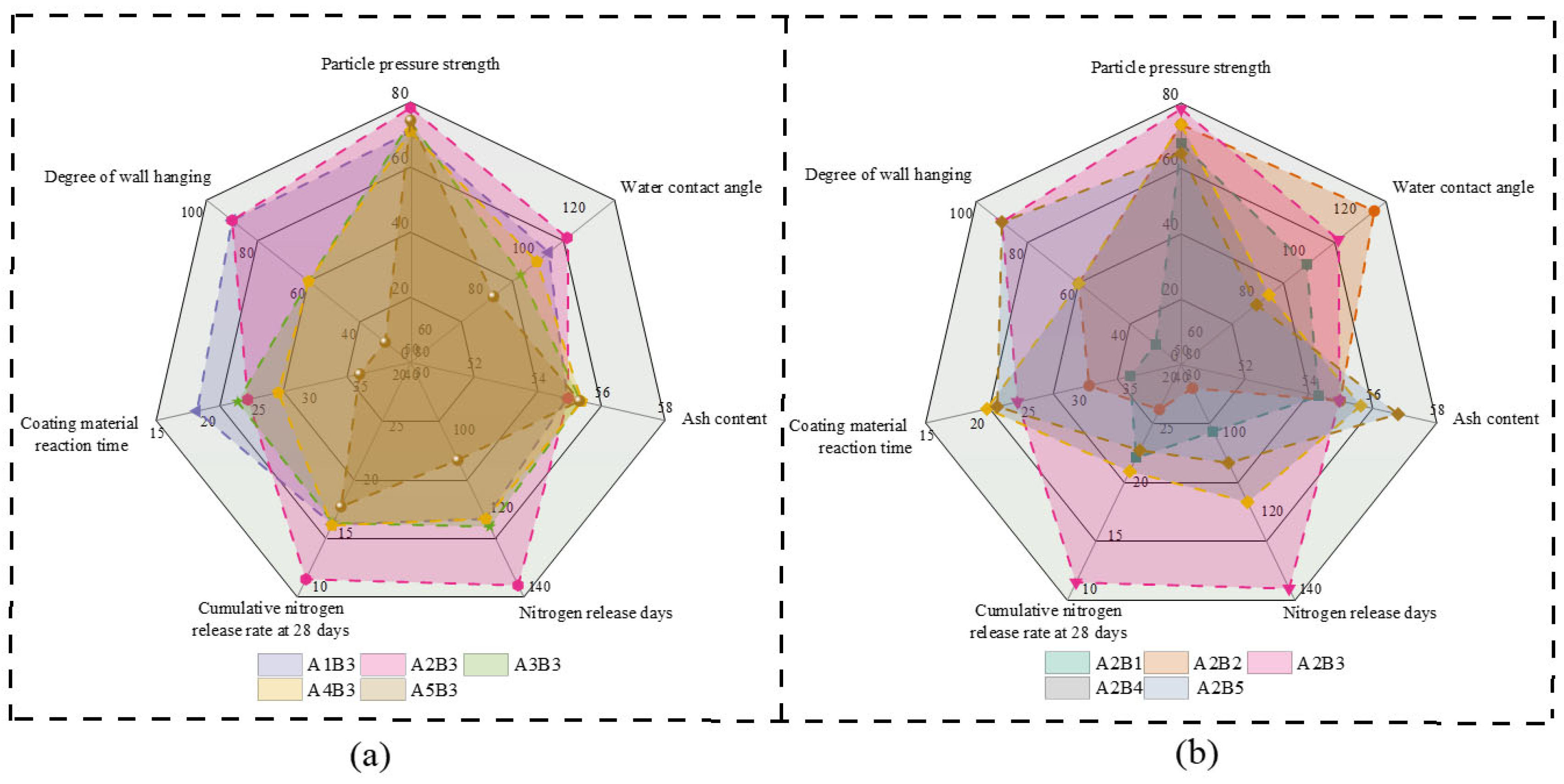
3.1. Characteristics of the Coating Materials
3.2. Nitrogen Release Behavior and Water Contact Angle of CRUs Coated with Different Materials
3.3. Microstructure of Different Coating Materials
3.4. Hardness and Wear Resistance of CRUs Coated with Different Materials
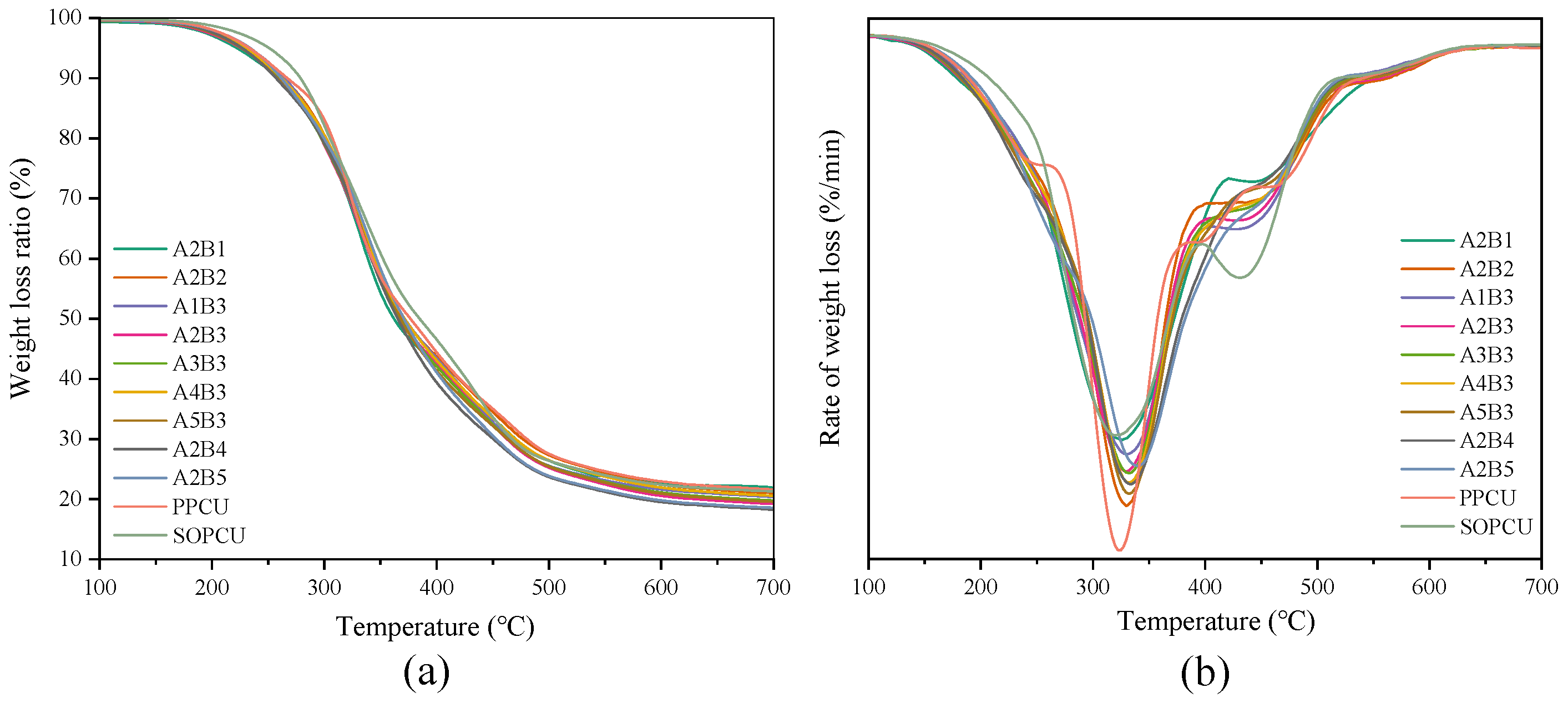
3.5. Thermal Stability Analysis of Coating Materials
3.6. Thermal Stability Analysis of Coating Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of Effectiveness of Enhanced-Efficiency Fertilizers as Mitigation Options for N2O and NO Emissions from Agricultural Soils: Meta-Analysis. Glob. Chang. Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Guilpart, N.; Grassini, P.; Sadras, V.O.; Timsina, J.; Cassman, K.G. Estimating Yield Gaps at the Cropping System Level. Field Crops Res. 2017, 206, 21–32. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Blaylock, A.D.; Chen, X. Mixture of Controlled Release and Normal Urea to Optimize Nitrogen Management for High-Yielding (>15 Mg ha−1) Maize. Field Crops Res. 2017, 204, 23–30. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Yang, X. The Diffusion Model of Nutrient Release from Membrane Pore of Controlled Release Fertilizer. Environ. Technol. Innov. 2022, 25, 102256. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on Materials & Methods to Produce Controlled Release Coated Urea Fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [PubMed]
- Zhang, M.; Chen, P.; Wang, G.; Dai, T.; Sun, P. Preparation of Bio-Inspired Liquid-Infused Surface on Epoxy Resin Coating for Reliable Hydrophobic and Anti-Corrosion.Properties. Prog. Org. Coat. 2023, 184, 107851. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled Release Fertilizer: A Review on Developments, Applications and Potential in Agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, M.; Li, Q.; Xu, Y. Structure and Properties of Controlled Release Fertilizers Coated with Thermosetting Resin. Polym. Plast. Technol. Eng. 2013, 52, 381–386. [Google Scholar] [CrossRef]
- Uzoh, C.F.; Onukwuli, O.D.; Ozofor, I.H.; Odera, R.S. Encapsulation of Urea with Alkyd Resin-Starch Membranes for Controlled N2 Release: Synthesis, Characterization, Morphology and Optimum N2 Release. Process Saf. Environ. Prot. 2019, 121, 133–142. [Google Scholar] [CrossRef]
- Park, W.J.; Hwangbo, M.; Chu, K.-H. Plastisphere and Microorganisms Involved in Polyurethane Biodegradation. Sci. Total Environ. 2023, 886, 163932. [Google Scholar] [CrossRef]
- Badr, E.C.; Ayoub, E.; Mohamed, Z.; Younes, E. Starch-Based Controlled Release Fertilizers: A Review. Int. J. Biol. Macromol. 2023, 238, 124075. [Google Scholar]
- Zhou, D.; Choi, P. Molecular Dynamics Study of Water Diffusivity at Low Concentrations in Non-Swollen and Swollen Polyurethanes. Polymer 2012, 53, 3253–3260. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Attributes of Natural and Synthetic Materials Pertaining to Slow-Release Urea Coating Industry. Rev. Chem. Eng. 2017, 33, 293–308. [Google Scholar] [CrossRef]
- Echeverria-Altuna, O.; Ollo, O.; Larraza, I.; Elizetxea, C.; Harismendy, I.; Eceiza, A. Development of a Novel Biobased Polyurethane Resin System for Structural Composites. Polymers 2022, 14, 4553. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, L.; Wang, M.; Li, L.X.; Cao, B.; Wang, H.; Huang, W.Q. Preparation and Properties of Starch-Based Polyurethane/Montmorillonite Composite Coatings for Controlled-Release Fertilizer. Polym. Compos. 2021, 42, 2293–2304. [Google Scholar] [CrossRef]
- Lu, H.; Dun, C.; Jariwala, H.; Wang, R.; Cui, P.; Zhang, H.; Dai, Q.; Yang, S.; Zhang, H. Improvement of Bio-Based Polyurethane and Its Optimal Application in Controlled Release Fertilizer. J. Control. Release 2022, 350, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhong, K.; Liu, W.; Cui, S.; Zhong, Y.; Jiang, S. Preparation and Oil Adsorption Properties of Hydrophobic Microcrystalline Cellulose Aerogel. Cellulose 2020, 27, 7663–7675. [Google Scholar] [CrossRef]
- Audrey, M.; Eric, P.; Vincent, P.; Avérous, L. Evaluation of Biological Degradation of Polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar]
- Tian, H.; Liu, Z.; Zhang, M.; Guo, Y.; Zheng, L.; Li, Y.C. Biobased Polyurethane, Epoxy Resin, and Polyolefin Wax Composite Coating for Controlled-Release Fertilizer. ACS Appl. Mater. 2019, 11, 5380–5392. [Google Scholar] [CrossRef]
- Chao, Y.; Yu, Z.; Yang, B. Improvement of Thermoregulating Performance for Outlast/Silk Fabric by the Incorporation of Polyurethane Microcapsule Containing Paraffin. Fibers Polym. 2013, 14, 1290–1294. [Google Scholar]
- Wang, S.; Huang, R.; Ren, K.; Shen, L.; Li, X.; Lei, G.; Shen, L.; Zhan, Y.; Zheng, Y.; Jiang, L. Structural Control of PEG-Intercalating Na-Bentonite and Its Influence on the Properties of Castor Oil-Based Polyurethane Coating. Prog. Org. Coat. 2023, 178, 107499. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Yang, Y.C.; Gao, B.; Li, Y.C. Organic silicone-modified transgenic soybean oil as bio-based coating material for controlled-release urea fertilizers. J. Appl. Polym. Sci. 2016, 133, 44097. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Ribeiro, C.; Polito, W.L. Controlled release of nitrogen-source fertilizers by natural-oil-based poly (urethane) coatings: The kinetic aspects of urea release. J. Appl. Polym. Sci. 2016, 133, 43790. [Google Scholar] [CrossRef]
- Yilgor, I.; Eynur, T.; Yilgor, E.; Wilkes, G.L. Contribution of Soft Segment Entanglement on the Tensile Properties of Silicone–Urea Copolymers with Low Hard Segment Contents. Polymer 2009, 50, 4432–4437. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Cavigelli, M.A.; Montes, S.E.; Schomberg, H.H.; Le, A.; Thompson, A.I.; Kramer, M.; Polito, W.l.; Ribeiro, C. Oil-based polyurethane-coated urea reduces nitrous oxide emissions in a corn field in a Maryland loamy sand soil. J. Clean. Prod. 2020, 249, 119329. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, P.; Zhang, Y.; Fan, B.; Zhu, M.; Wu, Z. Thermoplastic Starch Modified with Hydrophobic Polyurethane Microparticles. Starke 2011, 63, 373–380. [Google Scholar] [CrossRef]
- Li, S.; Bouzidi, L.; Narine, S.S. Polyols from Self-Metathesis-Generated Oligomers of Soybean Oil and Their Polyurethane Foams. Eur. Polym. J. 2017, 93, 232–245. [Google Scholar] [CrossRef]
- Lozada, Z.; Suppes, G.J.; Hsieh, F.-H.; Lubguban, A.; Tu, Y.-C. Preparation of Polymerized Soybean Oil and Soy-Based Polyols. J. Appl. Polym. Sci. 2009, 112, 2127–2135. [Google Scholar] [CrossRef]
- Li, R.; Loontjens, J.A.T.; Shan, Z. The varying mass ratios of soft and hard segments in waterborne polyurethane films: Performances of thermal conductivity and adhesive properties. Eur. Polym. J. 2019, 112, 423–432. [Google Scholar] [CrossRef]
- Fakhar, A.; Sadeghi, M.; Dinari, M.; Lammertink, R. Association of hard segments in gas separation through polyurethane membranes with aromatic bulky chain extenders. J. Membr. Sci. 2019, 574, 136–146. [Google Scholar] [CrossRef]
- Oprea, S. The Effect of Chain Extenders Structure on Properties of New Polyurethane Elastomers. Polym. Bull. 2010, 65, 753–766. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, V.O.; Oprea, V. Synthesis and Properties of New Crosslinked Polyurethane Elastomers Based on Isosorbide. Eur. Polym. J. 2016, 83, 161–172. [Google Scholar] [CrossRef]
- Pietrzak, K.; Kirpluks, M.; Cabulis, U.; Ryszkowska, J. Effect of the Addition of Tall Oil-Based Polyols on the Thermal and Mechanical Properties of Ureaurethane Elastomers. Polym. Degrad. Stab. 2014, 108, 201–211. [Google Scholar] [CrossRef]
- Mizera, K.; Ryszkowska, J. Polyurethane Elastomers from Polyols Based on Soybean Oil with a Different Molar Ratio. Polym. Degrad. Stab. 2016, 132, 21–31. [Google Scholar] [CrossRef]
- Huang, H.; Pang, H.; Huang, J.; Yu, P.; Li, J.; Lu, M.; Liao, B. Influence of Hard Segment Content and Soft Segment Length on the Microphase Structure and Mechanical Performance of Polyurethane-Based Polymer Concrete. Constr. Build Mater. 2021, 284, 122388. [Google Scholar] [CrossRef]
- Sun, Y.; Sheng, D.; Wu, H.; Tian, X.; Xie, H.; Shi, B.; Liu, X.; Yang, Y. Bio-Based Vitrimer-like Polyurethane Based on Dynamic Imine Bond with High-Strength, Reprocessability, Rapid-Degradability and Antibacterial Ability. Polymer 2021, 233, 124208. [Google Scholar] [CrossRef]
- Das, B.; Konwar, U.; Mandal, M.; Karak, N. Sunflower Oil Based Biodegradable Hyperbranched Polyurethane as a Thin Film Material. Ind. Crops Prod. 2013, 44, 396–404. [Google Scholar] [CrossRef]
- Rueda-Larraz, L.; d’Arlas, B.F.; Tercjak, A.; Ribes, A.; Mondragon, I.; Eceiza, A. Synthesis and microstructure–mechanical property relationships of segmented polyurethanes based on a PCL–PTHF–PCL block copolymer as soft segment. Eur. Polym. J. 2009, 45, 2096–432109. [Google Scholar] [CrossRef]
- Ye, L.; Meng, X.-Y.; Ji, X.; Li, Z.-M.; Tang, J.-H. Synthesis and Characterization of Expandable Graphite–Poly(Methyl Methacrylate) Composite Particles and Their Application to Flame Retardation of Rigid Polyurethane Foams. Polym. Degrad. Stab. 2009, 94, 971–979. [Google Scholar] [CrossRef]


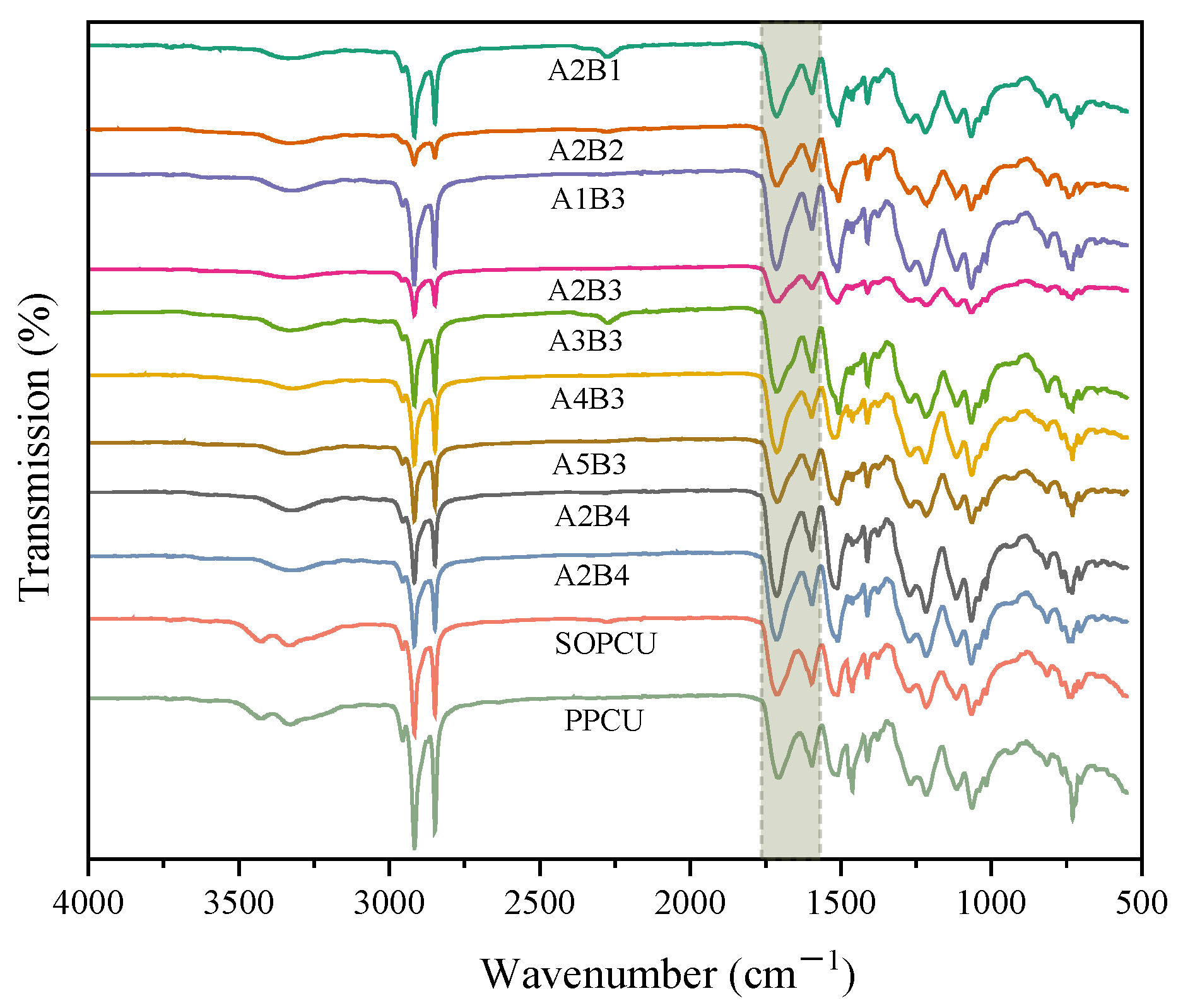

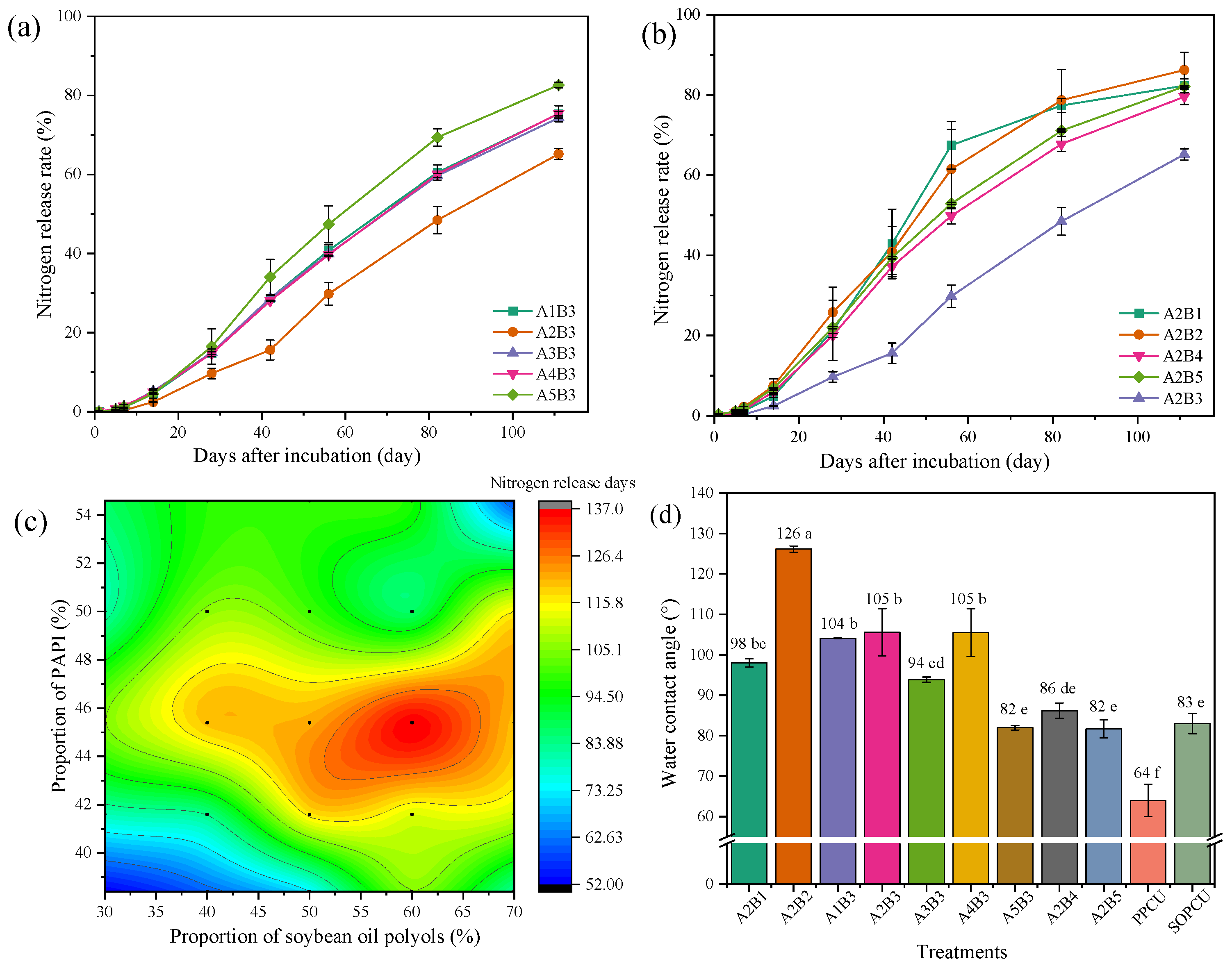
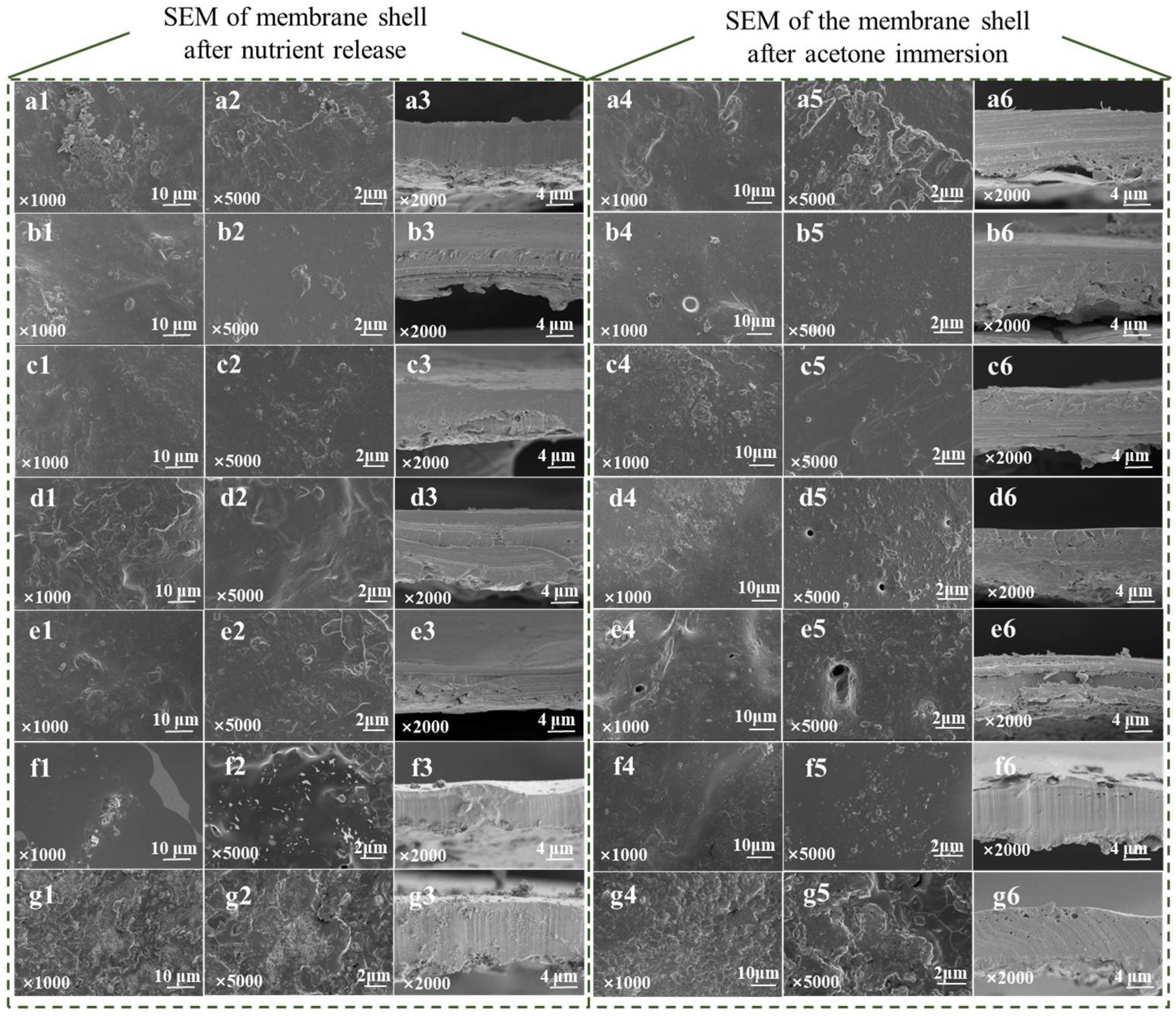
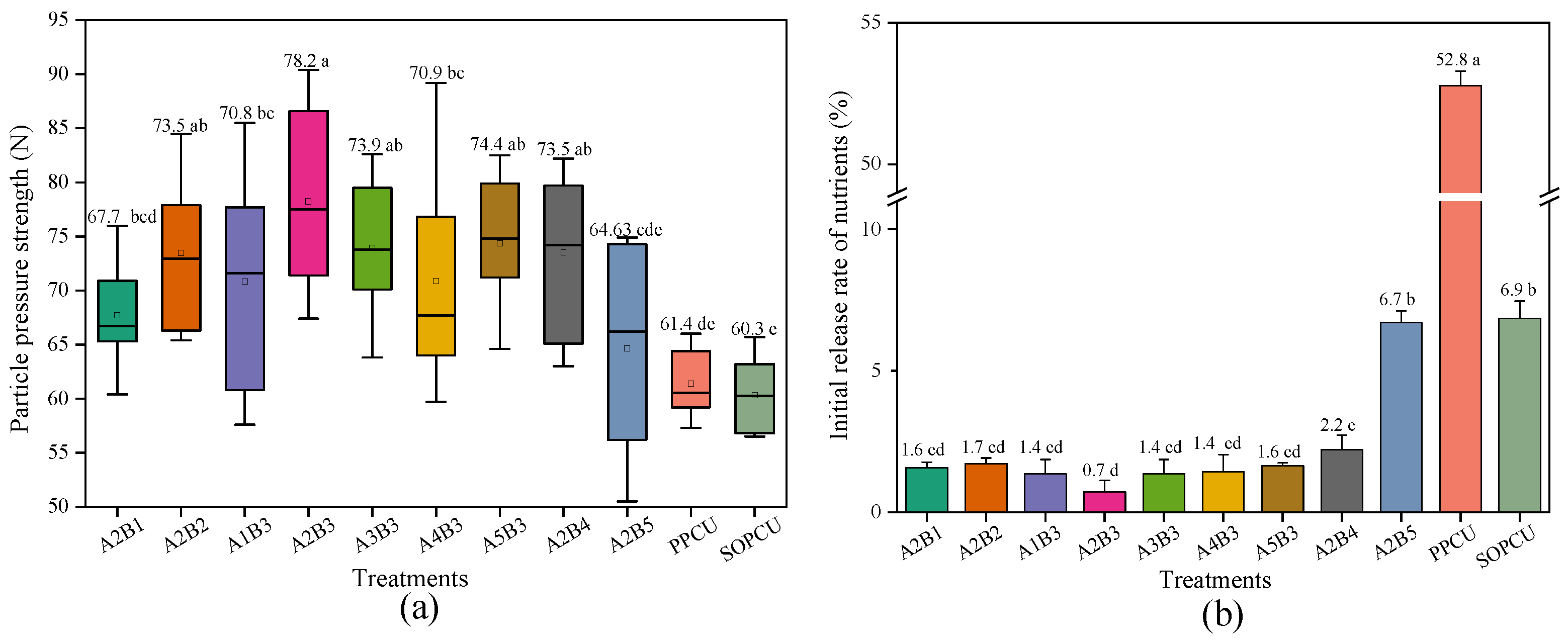
| Code | A | B |
|---|---|---|
| 1 | 0.7 | 1:1.2 |
| 2 | 0.6 | 1:1 |
| 3 | 0.5 | 1.2:1 |
| 4 | 0.4 | 1.4:1 |
| 5 | 0.3 | 1.6:1 |
| Sample Code | AH-bonded C=O | Afree C=O | HBI | DPS |
|---|---|---|---|---|
| A2B1 | 22,278.00 | 24,553.79 | 1.10 | 0.52 |
| A2B2 | 24,435.62 | 26,736.52 | 1.09 | 0.52 |
| A1B3 | 26,267.40 | 28,785.07 | 1.10 | 0.52 |
| A2B3 | 29,152.78 | 30,263.88 | 1.04 | 0.51 |
| A3B3 | 30,183.84 | 33,652.63 | 1.11 | 0.53 |
| A4B3 | 32,394.98 | 33,433.70 | 1.03 | 0.51 |
| A5B3 | 16,595.90 | 18,170.09 | 1.09 | 0.52 |
| A2B4 | 13,416.58 | 14,922.81 | 1.11 | 0.53 |
| A2B5 | 11,703.00 | 13,909.00 | 1.19 | 0.54 |
| SOPCU | 19,821.84 | 21,211.18 | 1.07 | 0.52 |
| PPCU | 19,101.54 | 20,354.39 | 1.07 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Zhang, Z.; Tian, H.; Chen, S.; Hu, X.; Li, M.; Wang, T.; Wan, J.; Yu, C.; Zhu, X.; et al. Soybean-Oil-Modified Petrochemical-Source Polyester Polyurethane Improves the Nutrient Release Performance of Coated Urea. Agronomy 2023, 13, 3008. https://doi.org/10.3390/agronomy13123008
Dong J, Zhang Z, Tian H, Chen S, Hu X, Li M, Wang T, Wan J, Yu C, Zhu X, et al. Soybean-Oil-Modified Petrochemical-Source Polyester Polyurethane Improves the Nutrient Release Performance of Coated Urea. Agronomy. 2023; 13(12):3008. https://doi.org/10.3390/agronomy13123008
Chicago/Turabian StyleDong, Jingjing, Zexi Zhang, Hongyu Tian, Siyu Chen, Xiaomin Hu, Mingyang Li, Ting Wang, Jinlan Wan, Changxin Yu, Xiaofan Zhu, and et al. 2023. "Soybean-Oil-Modified Petrochemical-Source Polyester Polyurethane Improves the Nutrient Release Performance of Coated Urea" Agronomy 13, no. 12: 3008. https://doi.org/10.3390/agronomy13123008






