The Impact of Marasmius tricolor 310b on the Degradation of Cellulose in Rapeseed Straw Composting
Abstract
:1. Introduction
2. Material and Methods
2.1. Inoculants
2.2. Inoculation, Composting, and Sampling
2.3. Determination of Cellulose, Hemicellulose, and Lignin
2.4. DNA Extraction, PCR Amplification, and Sequencing Analysis
2.5. Sequence Processing
2.6. Molecular Ecological Network (MEN) Construction
2.7. Data Interpretation and Analysis
3. Results
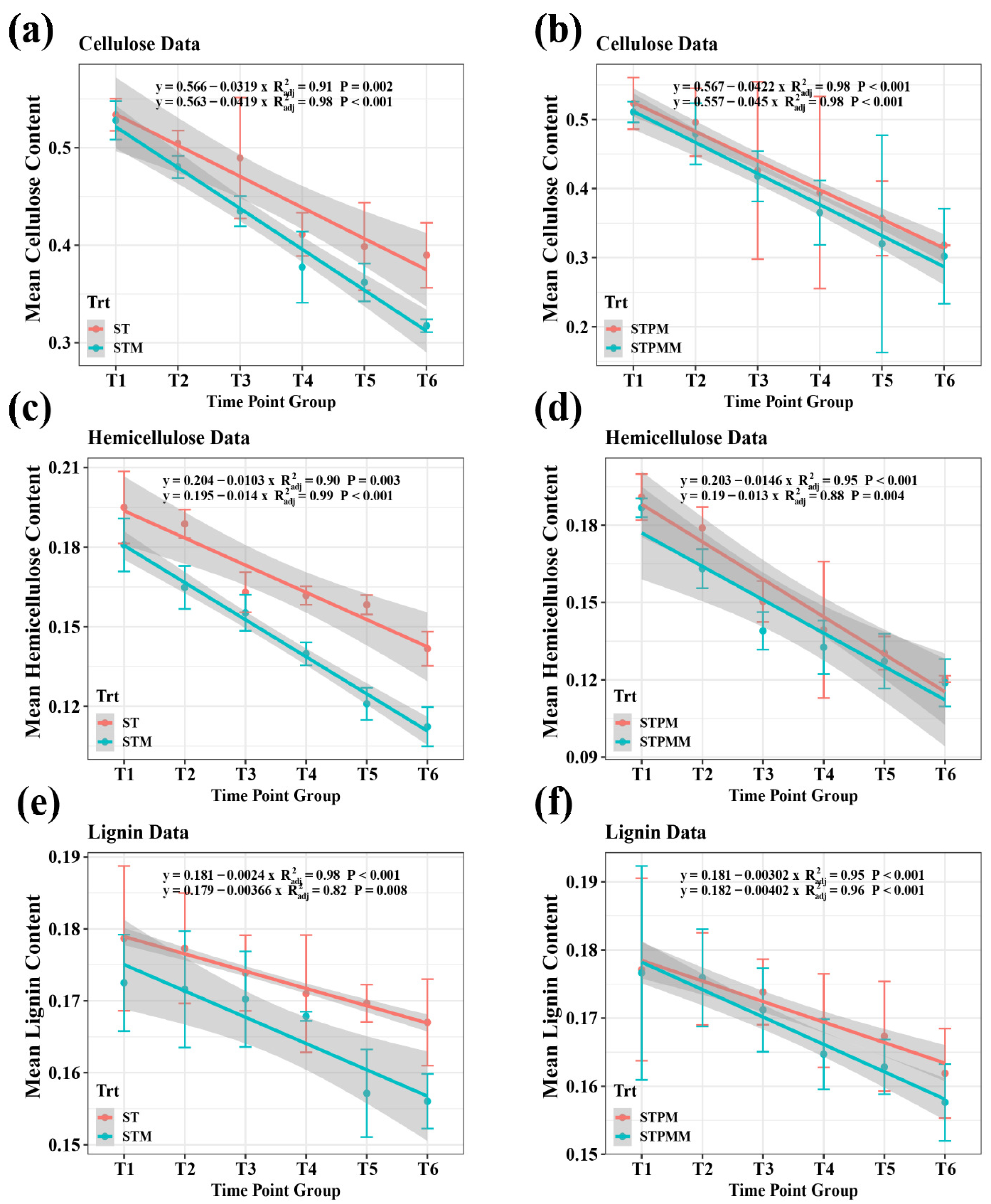
3.1. Changes in Temperature and Degradation of Lignocellulosic Components
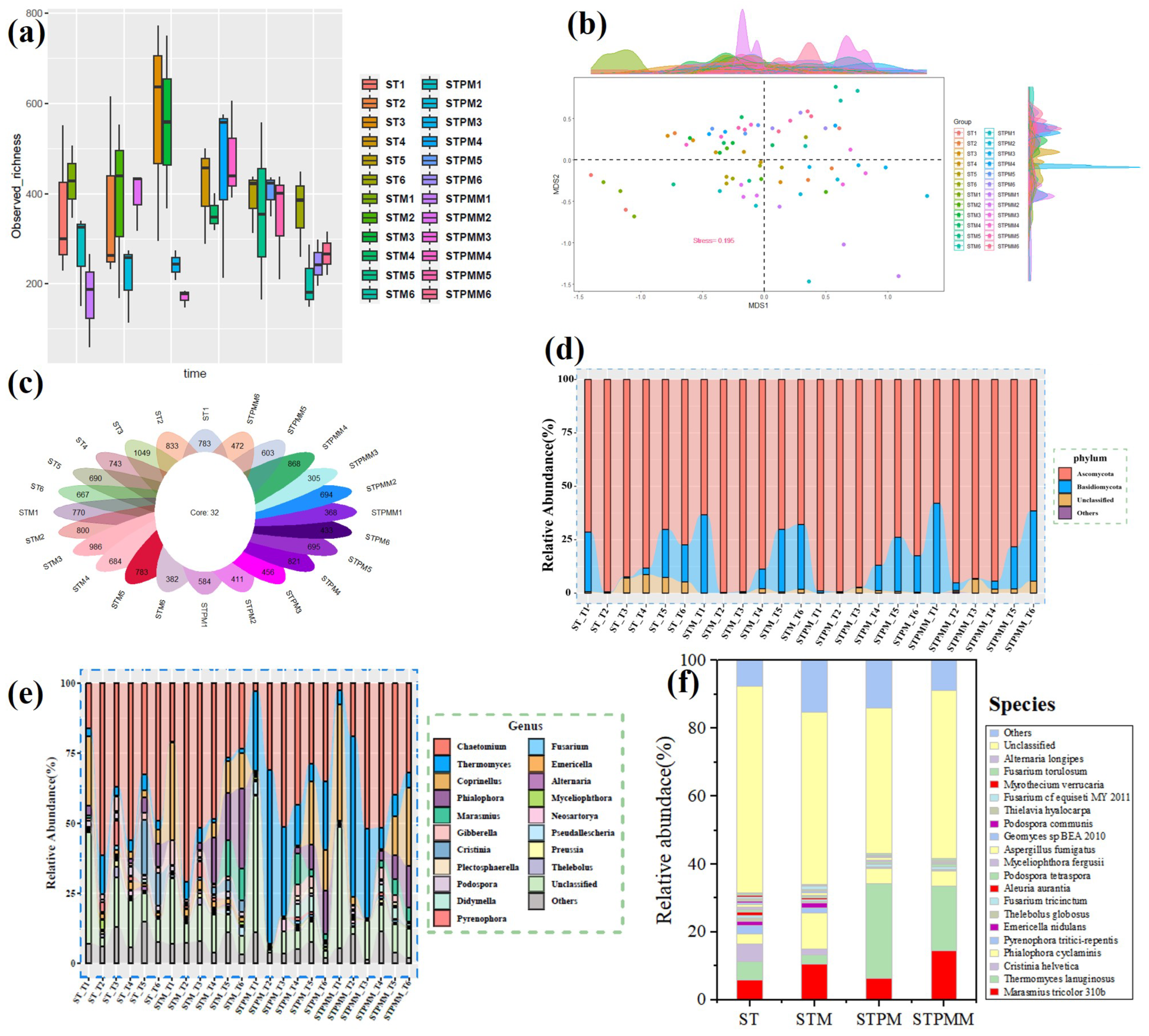
3.2. Influence of Marasmius Tricolor 310b on Fungal Community Dynamics
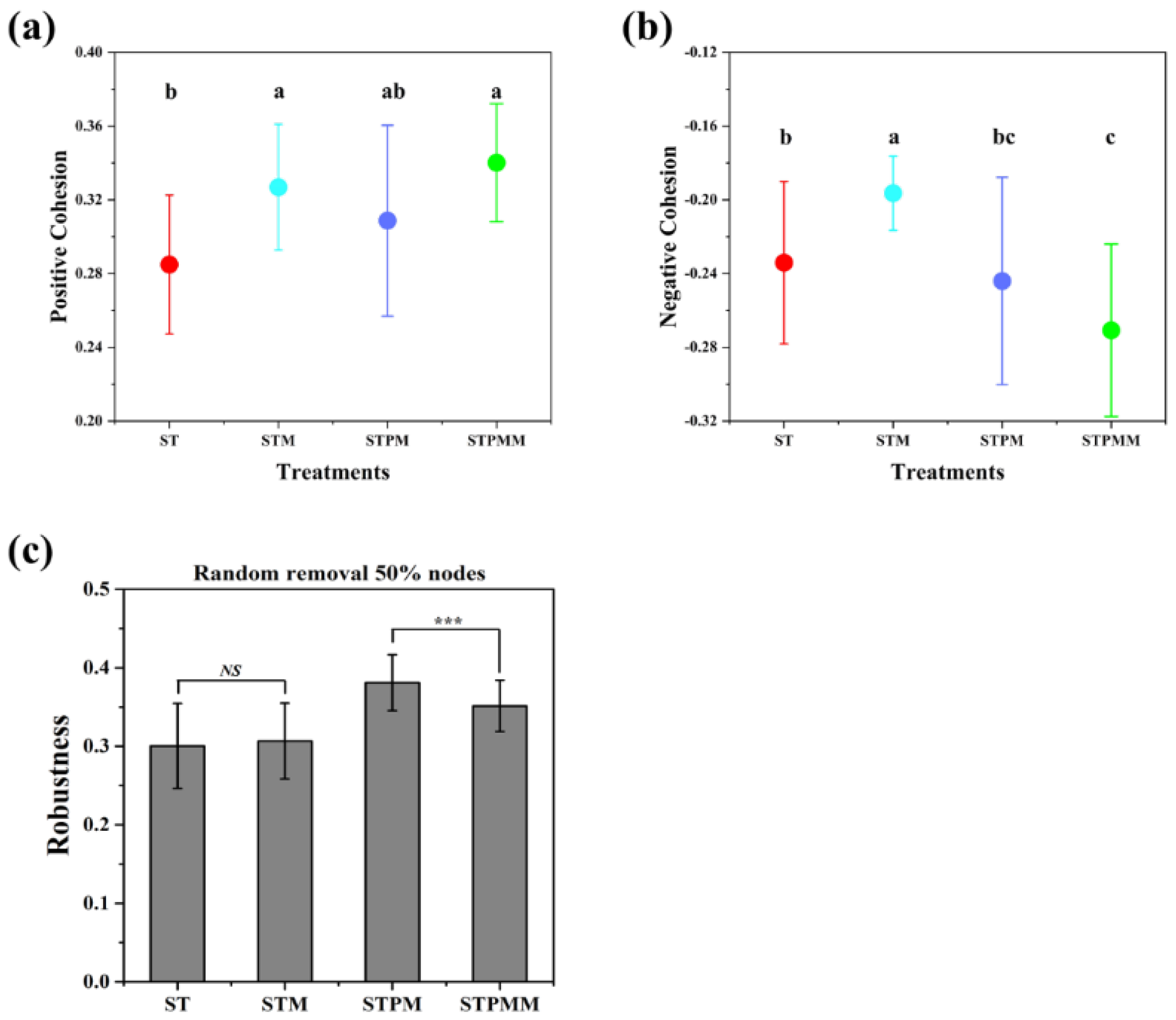
3.3. Inter-Domain Networks Analysis for Soil Microbial Communities under Composting Milieu
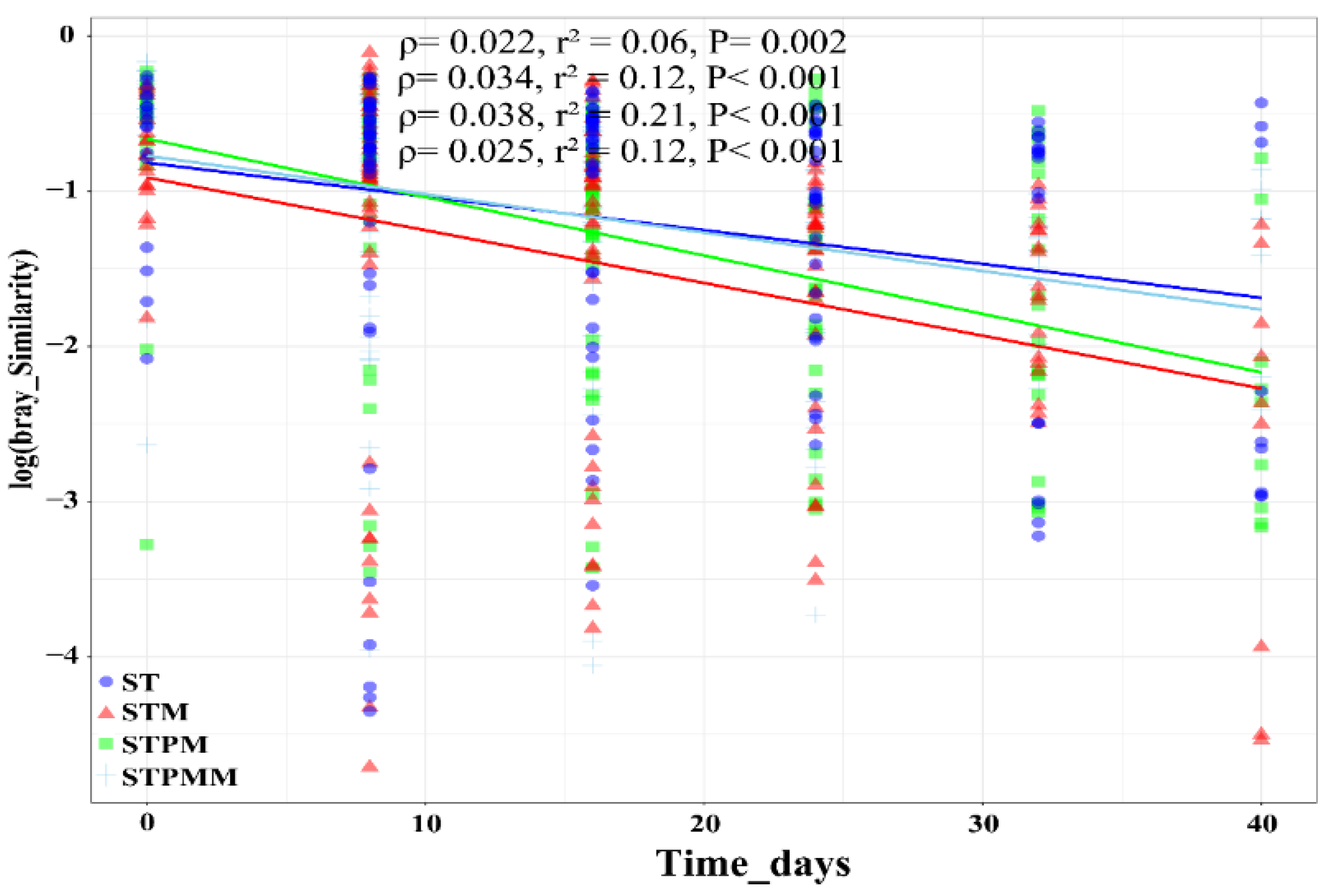
3.4. Impact of Inoculation on Temporal Stability of Fungal Communities in Compost
4. Discussion
4.1. Influence on Temperature and Lignocellulosic Degradation
4.2. Marasmius Tricolor 310b and Fungal Community Dynamics
4.3. Network Analysis and Community Interactions
4.4. Temporal Stability and Community Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pergola, M.; Persiani, A.; Palese, A.M.; Di Meo, V.; Pastore, V.; D’Adamo, C.; Celano, G. Composting: The way for a sustainable agriculture. Appl. Soil Ecol. 2018, 123, 744–750. [Google Scholar] [CrossRef]
- Ishola, T.M.; Ishola, E.T. Composting and Sustainable Development. In Encyclopedia of Sustainability in Higher Education; Filho, W.L., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 272–279. [Google Scholar]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste Management through Composting: Challenges and Potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, K.K. Fungal lignocellulolytic enzymes and lignocellulose: A critical review on their contribution to multiproduct biorefinery and global biofuel research. Int. J. Biol. Macromol. 2021, 193, 2304–2319. [Google Scholar] [CrossRef] [PubMed]
- Mazhari Mousavi, S.M.; Hosseini, S.Z.; Resalati, H.; Mahdavi, S.; Rasooly, E. Garmaroody Papermaking potential of rapeseed straw, a new agricultural-based fiber source. J. Clean. Prod. 2013, 52, 420–424. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.S.; Zhang, X.; Li, L.W.; Li, Y.Y.; Xu, H.M.; Li, X.X.; Yu, X.L.; Zhang, Z.S.; Liang, Y.Y.; et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 2017, 8, 14675. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, K. Worldwide rapeseed (Brassica napus L.) research: A bibliometric analysis during 2011–2021. Oil Crop Sci. 2022, 7, 157–165. [Google Scholar]
- Yu, Q.G.; Hu, X.; Ma, J.W.; Ye, J.; Sun, W.C.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy fields. Soil Tillage Res. 2020, 196, 104483. [Google Scholar] [CrossRef]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Grace, C.L. The genus Marasmius (Basidiomycota, Agaricales, Marasmiaceae) from Republic of São Tomé and Príncipe. West Afr. 2019, 414, 8. [Google Scholar] [CrossRef]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of Cellulose-Degrading Bacteria and Determination of Their Cellulolytic Potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, B.K.; Lee, B.H.; Jo, K.I.; Lee, N.K.; Chung, C.H.; Lee, Y.C.; Lee, J.W. Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresour. Technol. 2008, 99, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Yaseen, M. Global Agri-Food Sector: Challenges and Opportunities in COVID-19 Pandemic. Front. Sociol. 2021, 6, 647337. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.H.; Jiang, L.Y.; Mason, A.S.; Xiao, M.L.; Zhu, L.R.; Li, L.Z.; Zhou, Q.H.; Shen, C.J.; Huang, C.H. Research progress and strategies for multifunctional rapeseed: A case study of China. J. Integr. Agric. 2016, 15, 1673–1684. [Google Scholar] [CrossRef]
- Beal, T.; Massiot, E.; Arsenault, J.E.; Smith, M.R.; Hijmans, R.J. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS ONE 2017, 12, e0175554. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Li, W.; Abdalla, N.S.; Ai, P.; Zhang, Y.L.; Abomohra, A.E.F. Innovative approach for rapeseed straw recycling using black solider fly larvae: Towards enhanced energy recovery. Renew. Energy 2022, 188, 211–222. [Google Scholar] [CrossRef]
- Khalid, S.A.; Elsherif, W.M. Types of Microorganisms for Biodegradation. In Handbook of Biodegradable Materials; Ali, G.A.M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 195–220. [Google Scholar]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Garcia-Saravia Ortiz-de-Montellano, C.; Samani, P.; van der Meer, Y. How can the circular economy support the advancement of the Sustainable Development Goals (SDGs)? A comprehensive analysis. Sustain. Prod. Consum. 2023, 40, 352–362. [Google Scholar]
- Yao, S.T.; Lu, G.X.; Li, X.; Dang, N.; Wang, Y.C. Study on the degradation activity of oilseed rape straw by multi-enzymatic bacterial flora. J. Qinghai Univ. 2019, 37, 26–34. [Google Scholar]
- Zhang, X.P. Screening of Compound Bacteria Strains for Rape Straw Composting in Alpine Regions; Qinghai University: Xining, China, 2022. [Google Scholar]
- Wang, J.N. The Preparation and Evaluation of the Mixed Microbial Agents for Pig Manure Composting; Dalian University of Technology: Dalian, China, 2017. [Google Scholar]
- Sun, J.; Zhang, X.P.; Li, S.L.; Wang, Y.Y. Screening of fermentation strains of hog manure and rape straw in Qinghai Province. J. Qinghai Univ. 2022, 40, 55–61. [Google Scholar]
- GB/T 20806-2022; Determination of Neutral Detergent Fiber (NDF) in Feeds. National Administration for Market Regulation State Administration of Standardization Management: Shenzhen, China, 2022.
- NY/T 1459-2007; Determination of Acid Detergent Fiber (ADF) in Feeds. Industry Standard Agriculture: Beijing, China, 2007.
- GB/T 20805-2006; Determination of Acid Detergent Lignin (ADL) in Feeds. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Shenzhen, China, 2006.
- Feng, K.; Zhang, Z.J.; Cai, W.W.; Liu, W.Z.; Xu, M.Y.; Yin, H.Q.; Wang, A.J.; He, Z.L.; Deng, Y. Biodiversity and species competition regulate the resilience of microbial biofilm community. Mol. Ecol. 2017, 26, 6170–6182. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y. Btrim: A fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 2011, 98, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Robbertse, B.; Robert, V.; Vu, D.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for Fungi. Database 2014, 2014, bau061. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.E. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 2016, 081257. [Google Scholar]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.F.; He, Z.L.; Luo, F.; Zhou, J.Z. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Feng, K.; Peng, X.; Zhang, Z.; Gu, S.S.; He, Q.; Shen, W.L.; Wang, Z.J.; Wang, D.R.; Hu, Q.L.; Li, Y.; et al. iNAP: An integrated network analysis pipeline for microbiome studies. iMeta 2022, 1, e13. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P.; Melián, C.J.; Olesen, J.M. The nested assembly of plant–animal mutualistic networks. Proc. Natl. Acad. Sci. USA 2003, 100, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed]
- Herren, C.M.; McMahon, K.D. Cohesion: A method for quantifying the connectivity of microbial communities. ISME J. 2017, 11, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Dunne, J.A.; Williams, R.J.; Martinez, N.D. Food-web structure and network theory: The role of connectance and size. Proc. Natl. Acad. Sci. USA 2002, 99, 12917–12922. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Nandal, M.; Khosla, B. Microbes as vital additives for solid waste composting. Heliyon 2020, 6, e03343. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sun, H.; Chen, E.; Yang, M.; Wu, C.; Sun, X.; Wang, Q. From waste to wealth: Innovations in organic solid waste composting. Environ. Res. 2023, 229, 115977. [Google Scholar] [CrossRef]
- Bello, A.; Han, Y.; Zhu, H.F.; Deng, L.T.; Yang, W.; Meng, Q.X.; Sun, Y.; Egbeagu, U.U.; Sheng, S.Y.; Wu, X.T.; et al. Microbial community composition, co-occurrence network pattern and nitrogen transformation genera response to biochar addition in cattle manure-maize straw composting. Sci. Total Environ. 2020, 721, 137759. [Google Scholar] [CrossRef]
- Oviedo-Ocaña, E.R.; Soto-Paz, J.; Domínguez, I.; Sanchez-Torres, V.; Komilis, D. A Systematic Review on the Application of Bacterial Inoculants and Microbial Consortia During Green Waste Composting. Waste Biomass Valorization 2022, 13, 3423–3444. [Google Scholar] [CrossRef]
- Jack, C.N.; Petipas, R.H.; Cheeke, T.E.; Rowland, J.R.L.; Friesen, M.L. Microbial Inoculants: Silver Bullet or Microbial Jurassic Park? Trends Microbiol. 2021, 29, 299–308. [Google Scholar] [CrossRef]
- Finore, I.; Feola, A.; Russo, L.; Cattaneo, A.; Di Donato, P.; Nicolaus, B.; Poli, A.; Romano, I. Thermophilic bacteria and their thermozymes in composting processes: A review. Chem. Biol. Technol. Agric. 2023, 10, 7. [Google Scholar] [CrossRef]
- Wang, P.; Han, S.; Lin, Y. Role of microbes and microbial dynamics during composting. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 169–220. [Google Scholar]
- Wright, C.; Gryganskyi, A.P.; Bonito, G. Fungi in Composting. In Fungal Applications in Sustainable Environmental Biotechnology; Purchase, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–28. [Google Scholar]
- Karnaouri, A.; Topakas, E.; Antonopoulou, I.; Christakopoulos, P. Genomic insights into the fungal lignocellulolytic system of Myceliophthora thermophila. Front. Microbiol. 2014, 5, 281. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- De Gannes, V.; Eudoxie, G.; Hickey, W. Insights into fungal communities in composts revealed by 454-pyrosequencing: Implications for human health and safety. Front. Microbiol. 2013, 4, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Chang, Y.; Tao, Y.; Zhang, H.; Lin, Y.; Deng, J.; Ma, T.; Ding, G.; Wei, Y.; Li, J. Insight into the dynamic microbial community and core bacteria in composting from different sources by advanced bioinformatics methods. Environ. Sci. Pollut. Res. 2023, 30, 8956–8966. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, J.; Wang, X.; Xiao, H.; Yaser, A.Z.; Fu, J. Recent advances in research on microbial community in the composting process. Biomass Convers. Biorefinery 2023, 1–15. [Google Scholar] [CrossRef]
- Del Carmen Vargas-García, M.; Suárez-Estrella, F.F.; López, M.J.; Moreno, J. Influence of microbial inoculation and co-composting material on the evolution of humic-like substances during composting of horticultural wastes. Process Biochem. 2006, 41, 1438–1443. [Google Scholar] [CrossRef]
- Amelung, W.; Bossio, D.; de Vries, W.; Kögel-Knabner, I.; Lehmann, J.; Amundson, R.; Bol, R.; Collins, C.; Lal, R.; Leifeld, J.; et al. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020, 11, 5427. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Sun, L.; Men, M.; Wang, B.; Deng, L.; Zhao, L.; Han, Y.; Jong, C.; Bi, R.X.; et al. Microecological insight to fungal structure and key fungal communities regulating nitrogen transformation based on spatial heterogeneity during cow manure composting by multi-angle and multi-aspect analyses. Waste Manag. 2022, 142, 132–142. [Google Scholar] [CrossRef]
- Ji, Y.; Cao, Y.; Wang, Y.; Wang, C.; Qin, Z.; Cai, W.; Yang, Y.; Yan, S.; Guo, X. Effects of adding lignocellulose-degrading microbial agents and biochar on nitrogen metabolism and microbial community succession during pig manure composting. Environ. Res. 2023, 239, 117400. [Google Scholar] [CrossRef]
- Challacombe, J.F.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, L.V.; Porras-Alfaro, A.; Kuske, C.R. Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genom. 2019, 20, 976. [Google Scholar] [CrossRef]
- Taylor, T.N.; Krings, M.; Taylor, E.L. Chapter 8—Ascomycota. In Fossil Fungi; Elsevier: Amsterdam, The Netherlands, 2015; pp. 129–171. [Google Scholar]
- Wijayawardene, N.N.; Hyde, K.D.; Rajeshkumar, K.C.; Hawksworth, D.L.; Madrid, H.; Kirk, P.M.; Braun, U.; Singh, R.V.; Crous, P.W.; Kukwa, M.; et al. Notes for genera: Ascomycota. Fungal Divers. 2017, 86, 1–594. [Google Scholar] [CrossRef]
- Ryckeboer, J.; Mergaert, J.; Vaes, K.; Klammer, S.H.; Clercq, D.D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2003, 53, 349–410. [Google Scholar]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H.; Schreiber, F. Small-World Property. In Encyclopedia of Systems Biology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1957–1959. [Google Scholar]
- Barrat, A.; Weigt, M. On the properties of small-world network models. Eur. Phys. J. B Condens. Matter Complex Syst. 2000, 13, 547–560. [Google Scholar] [CrossRef]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling Interactions in the Microbiome: A Network Perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.B.; Martiny, A.C.; Martiny, J.B.H. Global biogeography of microbial nitrogen-cycling traits in soil. Proc. Natl. Acad. Sci. USA 2016, 113, 8033–8040. [Google Scholar] [CrossRef]
- Ronda, C.; Wang, H.H. Engineering temporal dynamics in microbial communities. Curr. Opin. Microbiol. 2022, 65, 47–55. [Google Scholar] [CrossRef]
- Hassell, N.; Tinker, K.A.; Moore, T.; Ottesen, E.A. Temporal and spatial dynamics in microbial community composition within a temperate stream network. Environ. Microbiol. 2018, 20, 3560–3572. [Google Scholar] [CrossRef]
- Liu, J.W.; Meng, Z.; Liu, X.Y.; Zhang, X.H. Microbial assembly, interaction, functioning, activity and diversification: A review derived from community compositional data. Mar. Life Sci. Technol. 2019, 1, 112–128. [Google Scholar] [CrossRef]
- Kalamdhad, A.S.; Kazmi, A.A. Effects of turning frequency on compost stability and some chemical characteristics in a rotary drum composter. Chemosphere 2009, 74, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Cheng, H.; Han, Z.; Wei, Z.; Song, C. Identification of driving factors of lignocellulose degrading enzyme genes in different microbial communities during rice straw composting. Bioresour. Technol. 2023, 381, 129109. [Google Scholar] [CrossRef] [PubMed]
- Eevers, N.; Hawthorne, J.R.; White, J.C.; Vangronsveld, J.; Weyens, N. Exposure of Cucurbita pepo to DDE-contamination alters the endophytic community: A cultivation dependent vs a cultivation independent approach. Environ. Pollut. 2016, 209, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.T.; Yang, X.D.; Zhang, J.J.; Lin, Z.A.; Zhao, B.Q. Microbial community structure and functional metabolic diversity are associated with organic carbon availability in an agricultural soil. J. Integr. Agric. 2015, 14, 2500–2511. [Google Scholar] [CrossRef]
- Whitman, T.; DeCiucies, S.; Hanley, K.; Enders, A.; Woolet, J.; Lehmann, J. Microbial Community Shifts Reflect Losses of Native Soil Carbon with Pyrogenic and Fresh Organic Matter Additions and Are Greatest in Low-Carbon Soils. Appl. Environ. Microbiol. 2021, 87, e02555-20. [Google Scholar] [CrossRef]
- Kingsley, M.K.; Bhat, B.V. Influence of Microbiome in Shaping the Newborn Immune System: An Overview. In Microbiome, Immunity, Digestive Health and Nutrition; Academic Press: Cambridge, MA, USA, 2022; pp. 11–24. [Google Scholar]


| Raw Material | Pig Manure | Rapeseed Straw |
|---|---|---|
| pH | 7.75 | — |
| Moisture content | 65.59 | 8.86 |
| Organic matter | 72.41 | 67.69 |
| Total N | 1.85 | 0.76 |
| Total P | 2.10 | 0.10 |
| Total K | 1.17 | 1.07 |
| C/N | 22.7:1 | 52.0:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shi, D.; Lu, G. The Impact of Marasmius tricolor 310b on the Degradation of Cellulose in Rapeseed Straw Composting. Agronomy 2023, 13, 3012. https://doi.org/10.3390/agronomy13123012
Wang Z, Shi D, Lu G. The Impact of Marasmius tricolor 310b on the Degradation of Cellulose in Rapeseed Straw Composting. Agronomy. 2023; 13(12):3012. https://doi.org/10.3390/agronomy13123012
Chicago/Turabian StyleWang, Zhihui, Dejun Shi, and Guangxin Lu. 2023. "The Impact of Marasmius tricolor 310b on the Degradation of Cellulose in Rapeseed Straw Composting" Agronomy 13, no. 12: 3012. https://doi.org/10.3390/agronomy13123012





