Unraveling the Impact of Cumin-Centric Cropping Sequences on Cumin Yield, Economic Viability, and Dynamics of Soil Enzymatic Activities in Hot Arid Climatic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Characterization
2.2. Field Experiment
2.3. Cumin Equivalent Yield (CEY)
2.4. Economics
2.5. Analysis of Soil Enzymatic Activities and Soil Microbial Biomass Carbon
2.5.1. Fluorescein Diacetate (FDA) Hydrolysis
2.5.2. Alkaline Phosphatase (ALP)
2.5.3. Dehydrogenase Activity
2.5.4. Microbial Biomass Carbon
2.6. Statistical Analysis
3. Results
3.1. Effect of Cumin-Based Cropping Sequences on Cumin-Seed Yield
3.2. Effect of Cumin-Based Cropping Sequences on CEY
3.3. Effect of Cumin-Based Cropping Sequences on Economics
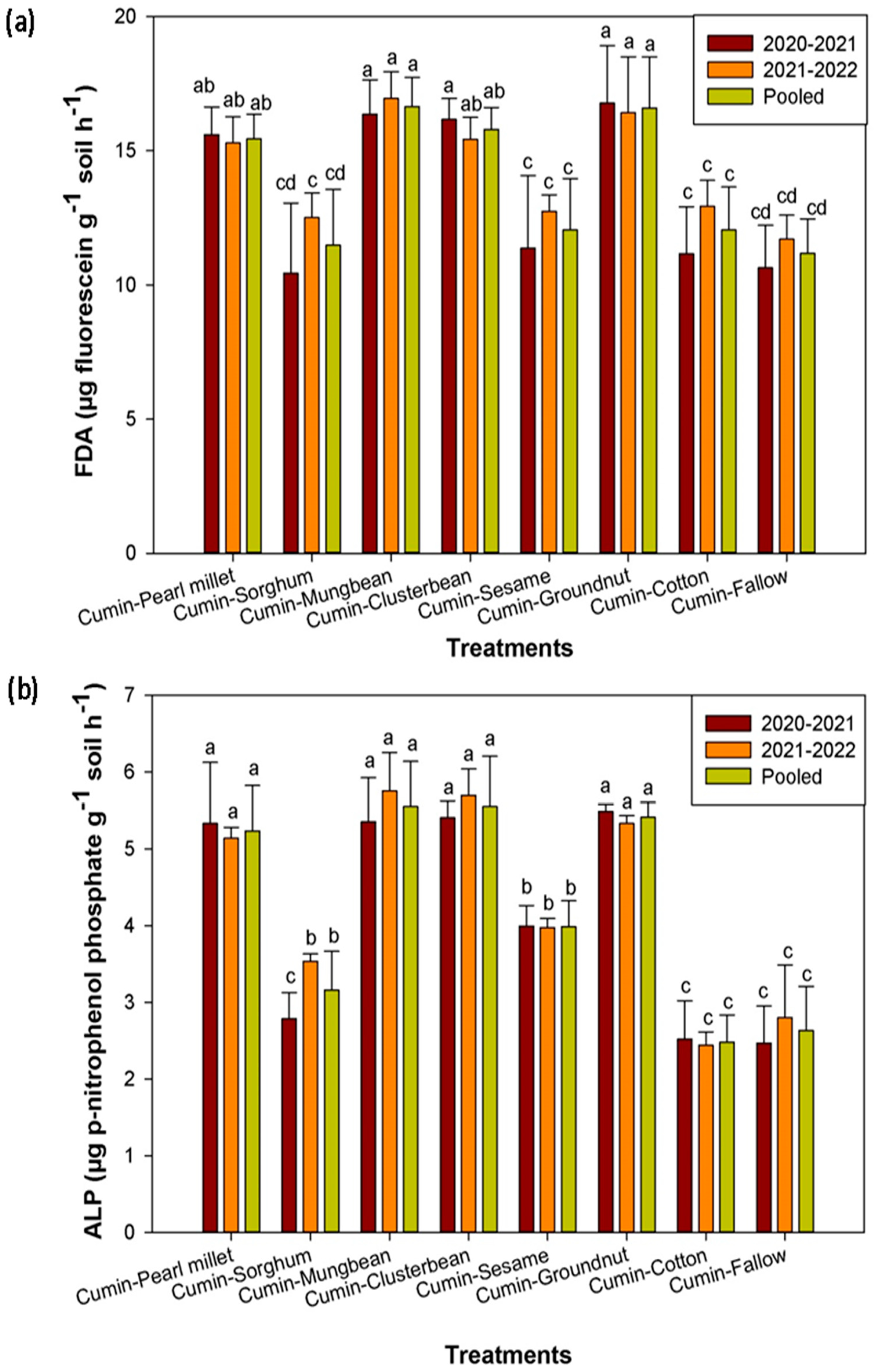
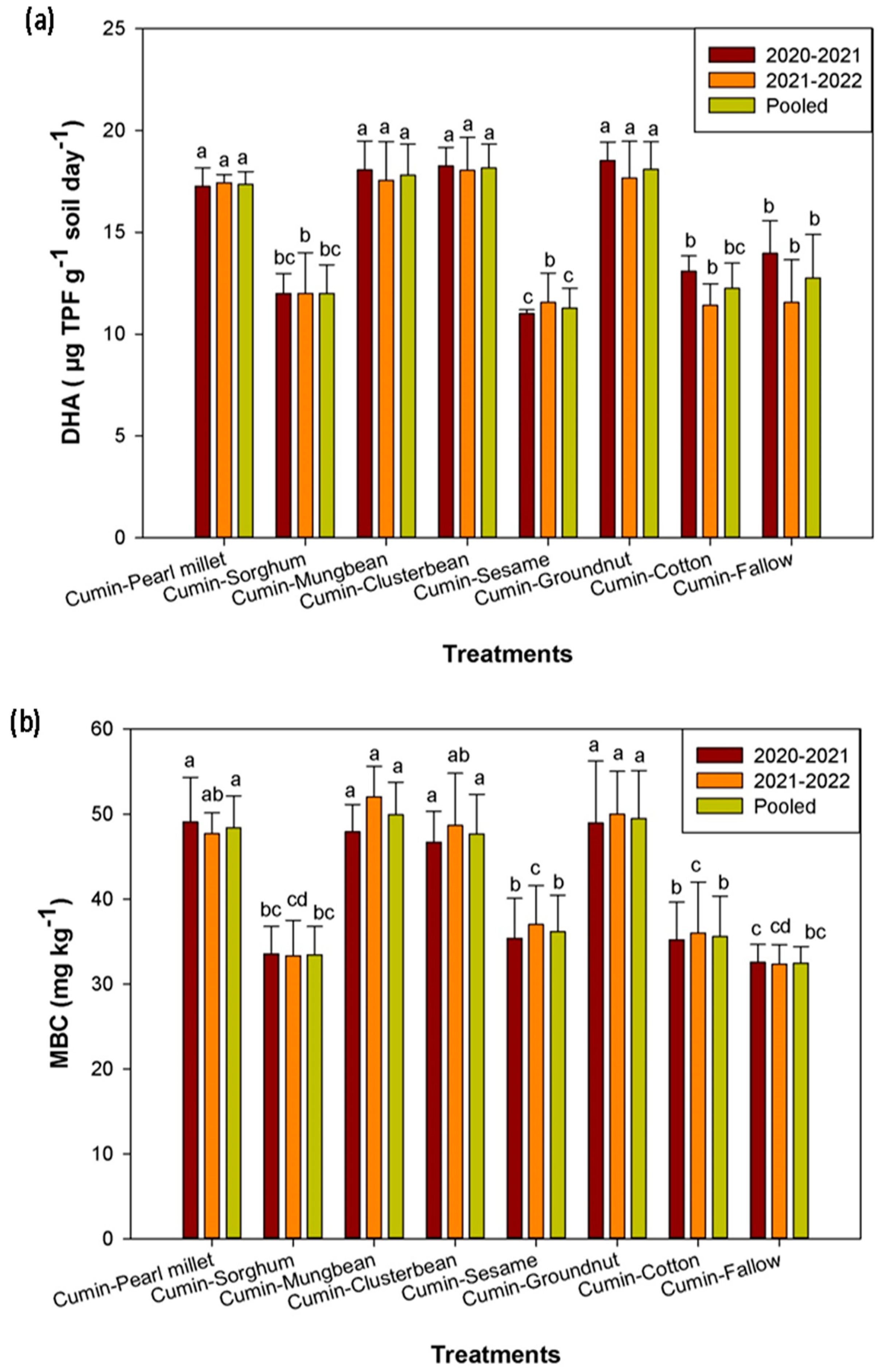
3.4. Effect of Cumin-Based Cropping Sequences on Soil Microbial Activities and Soil Microbial Biomass Carbon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naorem, A.; Jayaraman, S.; Dang, Y.P.; Dalal, R.C.; Sinha, N.K.; Rao, C.S.; Patra, A.K. Soil constraints in an arid environment-challenges, prospects, and implications. Agron. Res. 2023, 13, 220. [Google Scholar] [CrossRef]
- Manga, V.K.; Juktani, A.K.; Bhatt, R.K. Adaptation and selection of crop varieties for hot arid climate of Rajasthan. Indian J. Plant Sci. 2015, 4, 9. [Google Scholar]
- Brar, N.S.; Mahala, P.; Sharma, K.; Dhanda, P.S.; Yadav, A.; Sharma, M.; Kaushik, P. Cumin (Cuminium cyminium L.): A Seed Spice Crop with Adopted Production Technology in Cumin Cultivated Regions. In Ginger-Cultivation and Use; Intech Open: London, UK, 2022. [Google Scholar] [CrossRef]
- Mehriya, M.L.; Geat, N.; Sarita; Singh, H.; Mattar, M.A.; Elansary, H.O. Response of drip irrigation and fertigation on cumin yield, quality, and water-use efficiency grown under arid climatic conditions. Agronomy 2020, 10, 1711. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Bi, Y.; Miao, R.; Zhang, Z.; Su, H. Anti-inflammatory effects of cumin essential oil by blocking JNK, ERK, and NF-κB signaling pathways in LPS-stimulated RAW 264.7 cells. Evid. Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Davazdahemami, S.; Allahdadi, M. Essential oil yield and composition of four annual plants (ajowan, dill, Moldavian balm and black cumin) under saline irrigation. Food Ther. Health Care 2022, 4, 5. [Google Scholar] [CrossRef]
- Sumamad. Sustainable management of marginal drylands. In Proceedings of the 3rd Project Workshop, Djerba, Tunisia, 11–15 December 2004; pp. 1–184. [Google Scholar]
- Moswetsi, G.; Fanadzo, M.; Ncube, B. Cropping systems and agronomic management practices in smallholder farms in South Africa: Constraints, challenges and opportunities. J. Agron. 2017, 16, 51–64. [Google Scholar] [CrossRef]
- Kotir, J.H.; Bell, L.W.; Kirkegaard, J.A.; Whish, J.; Aikins, K.A. Labour demand–The forgotten input influencing the execution and adoptability of alternative cropping systems in Eastern Australia. Agric. Syst. 2022, 203, 103516. [Google Scholar] [CrossRef]
- Das, A.; Lyngdoh, D.; Ghosh, P.K.; Lal, R.; Layek, J.; Idapuganti, R.G. Tillage and cropping sequence effect on physico-chemical and biological properties of soil in Eastern Himalayas, India. Soil Tillage Res. 2018, 180, 182–193. [Google Scholar] [CrossRef]
- Negash, F.; Mulualem, T.; Fikirie, K. Effect of cropping sequence on agricultural crops: Implications for productivity and utilization of natural resources. Adv. Crop. Sci. Technol. 2018, 6, 326. [Google Scholar] [CrossRef]
- Singh, S.R.; Yadav, P.; Singh, D.; Tripathi, M.K.; Bahadur, L.; Singh, S.P.; Mishra, A.; Kumar, S. Cropping systems influence microbial diversity, soil quality and crop yields in Indo-Gangetic plains of India. Eur. J. Agron. 2020, 121, 126152. [Google Scholar] [CrossRef]
- Kumar, A.; Dubey, A. Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef]
- Sarrantonio, M.; Gallandt, E. The role of cover crops in North American cropping systems. J. Crop. Prod. 2003, 8, 53–74. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Wall, D.H.; Six, J. Soil Biodiversity and the Environment. Annu. Rev. Environ. Resour. 2015, 40, 63–90. [Google Scholar] [CrossRef]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Li, F. Soil Enzyme Activities and Soil Fertility Dynamics. In Advances in Citrus Nutrition; Srivastava, A., Ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2012; pp. 143–156. [Google Scholar] [CrossRef]
- Asirifi, I.; Werner, S.; Heinze, S.; Saba, C.K.; Lawson, I.Y.; Marschner, B. Short-term effect of biochar on microbial biomass, respiration and enzymatic activities in wastewater irrigated soils in urban agroecosystems of the West African savannah. Agronomy 2021, 11, 271. [Google Scholar] [CrossRef]
- Yu, P.; Fan, G.; Han, K.; Zhou, D. Soil quality assessment based on soil microbial biomass carbon and soil enzyme activities. Res. Agric. Modern. 2018, 39, 163–169. [Google Scholar]
- Malobane, M.E.; Nciizah, A.D.; Nyambo, P.; Mudau, F.N.; Wakindiki, I.I. Microbial biomass carbon and enzyme activities as influenced by tillage, crop rotation and residue management in a sweet sorghum cropping system in marginal soils of South Africa. Heliyon 2020, 6, e05513. [Google Scholar] [CrossRef]
- Jing, J.; Cong, W.F.; Bezemer, T.M. Legacies at work: Plant–soil–microbiome interactions underpinning agricultural sustainability. Trends Plant Sci. 2022, 27, 781–792. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to microbiome: A paradigm shift in the application of microorganisms for sustainable agriculture. Front. Microbiol. 2020, 11, 622926. [Google Scholar] [CrossRef]
- Subbiah, B.; Asija, G.A. Rapid procedure for the estimation of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Cole, V.C.; Watamable, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soil by Extraction with NaHCO3 Circular; United States Department of Agriculture: Washington, DC, USA, 1954; Volume 939, p. 21.
- Standfold, S.; English, L. Use of flame photometer in rapid soil test for K and Ca. Agron. J. 1949, 41, 446–447. [Google Scholar]
- Singh, D.; Chhonkar, P.K.; Pandey, R.N. Soil Reaction in Soil, Plant, Water Analysis Method: Manual; ICAR-IARI: New Delhi, India, 1949; p. 23. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Williams, C.H.; Steinberg, A. Soil sulphur fractions as chemical indices of available sulphur in some Australian soils. Aust. J. Agric. Res. 1969, 10, 340–352. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Klein, D.A.; Loh, T.C.; Goulding, R.L. A rapid procedure to evaluate the dehydrogenase activity of soils low in organic matter. Soil Biol. Biochem. 1971, 3, 385–387. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Patel, S.M.; Amin, A.U.; Patel, H.B. Effect of cropping sequence and nutrient management on cumin yield and fertility of soil. Int. J. Seed Spices 2017, 7, 66–71. [Google Scholar]
- SDAU. Annual Report, Seed Spices Research Station, SDAU, Jagudan Presented in AGRESCO Crop Production Committee; SDAU: Dantiwada, Gujarat, India, 2006; pp. 27–31. [Google Scholar]
- Ahmad, Z.F. Effects of crop rotation and residue management on bread Wheat. Afr. J. Plant Sci. 2013, 7, 176–184. [Google Scholar] [CrossRef]
- Dogan, R.; Goksoy, T.A.; Yagdi, K.; Turan, M.Z. Comparison of the effects of different crop rotation systems on winter wheat and sunflower under rain fed Conditions. Afr. J. Biotech. 2008, 1, 4076–4082. [Google Scholar]
- Alhameid, A.; Singh, J.; Sekaran, U.; Ozlu, E.; Kumar, S.; Singh, S. Crop rotational diversity impacts soil physical and hydrological properties under long-term no-and conventional-till soils. Soil Res. 2019, 58, 84–94. [Google Scholar] [CrossRef]
- Alhameid, A.; Singh, J.; Sekaran, U.; Kumar, S.; Singh, S. Soil biological health: Influence of crop rotational diversity and tillage on soil microbial properties. Soil Sci. Soc. Am. J. 2019, 83, 1431–1442. [Google Scholar] [CrossRef]
- Alhameid, A.; Ibrahim, M.; Kumar, S.; Sexton, P.; Schumacher, T.E. Soil organic carbon changes impacted by crop rotational diversity under no-till farming in South Dakota, USA. Soil Sci. Soc. Am. J. 2017, 81, 868–877. [Google Scholar] [CrossRef]
- Chary, G.R.; Sharma, K.L.; Reddy, K.S.; Hirpara, D.S.; Akbari, K.N.; Lal, M.; Gopinath, K.A.; Narsimlu, B.; Osman, M.; Srinivas, K.; et al. Effect of Cropping Sequences and Nutrient Management Practices on Soil Quality under Rainfed Semiarid (Hot dry) Vertisol Soils of Western India. J. Dryland Agric. Res. Dev. 2019, 34, 27–37. [Google Scholar] [CrossRef]
- Gikonyo, F.N.; Dong, X.; Mosongo, P.S.; Guo, K.; Liu, X. Long-term impacts of different cropping patterns on soil physico-chemical properties and enzyme activities in the low land plain of North China. Agronomy 2022, 12, 471. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, D.; Sharma, P. Effect of organics, biofertilizers and crop residue application on soil microbial activity in rice-wheat and rice-wheat mungbean cropping systems in the Indo-Gangetic plains. Cogent Geosci. 2015, 1, 1085296. [Google Scholar] [CrossRef]
- Patil, R.B.; Puranik, R.B. Microbial biomass C and N as influenced by cropping systems and nutrient management. PKV Res. J. 2001, 25, 73–77. [Google Scholar]
- Qin, S.; Yeboah, S.; Cao, L.; Zhang, J.; Shi, S.; Liu, Y. Breaking continuous potato cropping with legumes improves soil microbial communities, enzyme activities and tuber yield. PLoS ONE 2017, 12, 0175934. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Grandy, S. Soil microbial biomass and function are altered by 12 years of crop rotation. Soil 2016, 2, 583–599. [Google Scholar] [CrossRef]
- Dou, F.; Wright, A.L.; Mylavarapu, R.S.; Jiang, X.; Matocha, J.E. Soil enzyme activities and organic matter composition affected by 26 years of continuous cropping. Pedosphere 2016, 26, 618–625. [Google Scholar] [CrossRef]
- Wacal, C.; Ogata, N.; Basalirwa, D.; Sasagawa, D.; Ishigaki, T.; Handa, T.; Kato, M.; Tenywa, M.M.; Masunaga, T.; Yamamoto, S.; et al. Imbalanced soil chemical properties and mineral nutrition in relation to growth and yield decline of sesame on different continuously cropped upland fields converted paddy. Agronomy 2019, 9, 184. [Google Scholar] [CrossRef]
- Vallejo, V.E.; Roldan, F.; Dick, R.P. Soil enzymatic activities and microbial biomass in an integrated agroforestry chronosequence compared to monoculture and a native forest of Colombia. Biol. Fertil. Soils 2010, 46, 577–587. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Cruz, L.; Sotomayor-Ramírez, D.; Pérez-Alegría, L. Enzyme activities as affected by soil properties and land use in a tropical watershed. Appl. Soil Ecol. 2007, 35, 35–45. [Google Scholar] [CrossRef]
| Crop | Seed Rate (kg ha−1) | Date of Sowing | Spacing | Fertilizer | Irrigation |
|---|---|---|---|---|---|
| Cumin | 12 | 1st week of November | 30 × 7 cm | 30 kg N: 20 kg P: 15 kg K 10 kg ZnSO4 | Eight: 1st—just after sowing, 2nd—after 7 days of sowing, 3rd—7 days after 2nd irrigation; after that, 5 irrigations at 15–25 days interval |
| Pearl millet | 4 | 1st week of July | 45 × 15 cm | 60 kg N: 30 kg P | Three—at the time of plant growth, earhead formation, and grain formation |
| Sorghum | 10 | 1st week of July | 45 × 15 cm | 80 kg N: 40 kg P | Two—at the time of plant growth and earhead formation |
| Mung bean | 15 | 1st week of July | 30 × 10 cm | 15 kg N: 40 kg P | One |
| Cluster bean | 15 | 1st week of July | 30 × 10 cm | 10 kg N: 40 kg P | Two—20 days after sowing and after 20 days again if no rainfall |
| Sesame | 2.5 | 1st week of July | 30 × 10 cm | 40 kg N: 25 kg P 250 kg gypsum | One |
| Groundnut | 125 | 3rd week of June | 30 × 10 cm | 15 kg N: 60 kg P 250 kg gypsum | Two—at the time of flower formation and grain/pod formation |
| Cotton | 1.8 | 3rd week of May | 90 × 60 cm | 150 kg N: 40 kg P | Six (first irrigation 30–35 days after sowing; after that, 20–25 days interval) |
| Treatments | Seed Yield (kg ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2018–2019 | 2020–2021 | 2021–2022 | Pooled | |||||
| Cumin | Kharif Crops | Cumin | Kharif Crops | Cumin | Kharif Crops | Cumin | Kharif Crops | |
| Cumin–Pearl millet | 577 | 1932 | 628 | 1847 | 571 | 2114 | 592 | 1964 |
| Cumin–Sorghum | 532 | 1505 | 606 | 1504 | 550 | 1790 | 563 | 1600 |
| Cumin–Mung bean | 514 | 656 | 575 | 597 | 509 | 841 | 533 | 698 |
| Cumin–Cluster bean | 541 | 861 | 591 | 748 | 526 | 912 | 553 | 840 |
| Cumin–Sesame | 466 | 580 | 564 | 503 | 473 | 512 | 501 | 532 |
| Cumin–Groundnut | 510 | 1503 | 557 | 1457 | 446 | 1514 | 504 | 1491 |
| Cumin–Cotton | 483 | 1382 | 537 | 1424 | 422 | 1469 | 481 | 1425 |
| Cumin–Fallow | 485 | 0 | 539 | 0 | 529 | 0 | 518 | 0 |
| S.Em.± | 22.7 | 47.1 | 24.4 | 45.0 | 32 | 37 | 15.5 | 25.0 |
| C.D. (p = 0.05) | 68.9 | 142.7 | 74.0 | 136.4 | 98 | 113 | 44.2 | 71.2 |
| CV (%) | 7.7 | 7.7 | 7.4 | 7.7 | 11.1 | 5.6 | 8.8 | 7.0 |
| Treatments | CEY (kg ha−1) | |||
|---|---|---|---|---|
| 2018–2019 | 2020–2021 | 2021–2022 | Pooled | |
| Cumin–Pearl millet | 812 | 882 | 809 | 835 |
| Cumin–Sorghum | 760 | 870 | 796 | 809 |
| Cumin–Mung bean | 800 | 866 | 848 | 838 |
| Cumin–Cluster bean | 746 | 798 | 777 | 774 |
| Cumin–Sesame | 693 | 789 | 660 | 714 |
| Cumin–Groundnut | 969 | 1068 | 866 | 968 |
| Cumin–Cotton | 927 | 1054 | 973 | 985 |
| Cumin–Fallow | 485 | 539 | 596 | 540 |
| S.Em.± | 25.7 | 27.9 | 29 | 16 |
| C.D. (p = 0.05) | 78.0 | 84.6 | 88 | 45 |
| CV (%) | 5.8 | 5.6 | 6.3 | 5.9 |
| Treatments | Gross Returns (₹ ha−1) | Net Returns (₹ ha−1) | B:C Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018–2019 | 2020–2021 | 2021–2022 | Pooled | 2018–2019 | 2020–2021 | 2021–2022 | Pooled | 2018–2019 | 2020–2021 | 2021–2022 | Pooled | |
| Cumin–Pearl millet | 129,988 | 127,949 | 161,769 | 139,902 | 80,488 | 78,449 | 105,769 | 88,235 | 2.63 | 2.58 | 2.89 | 2.70 |
| Cumin–Sorghum | 121,666 | 126,192 | 159,148 | 135,669 | 69,436 | 73,962 | 100,148 | 81,182 | 2.33 | 2.42 | 2.70 | 2.48 |
| Cumin–Mung bean | 128,073 | 125,522 | 169,598 | 141,064 | 73,473 | 70,922 | 108,098 | 84,164 | 2.35 | 2.30 | 2.76 | 2.47 |
| Cumin–Cluster bean | 119,319 | 115,663 | 155,432 | 130,138 | 65,819 | 62,163 | 94,932 | 74,305 | 2.23 | 2.16 | 2.57 | 2.32 |
| Cumin–Sesame | 110,804 | 114,470 | 131,919 | 119,064 | 58,004 | 61,670 | 72,419 | 64,031 | 2.10 | 2.17 | 2.22 | 2.16 |
| Cumin–Groundnut | 155,080 | 154,909 | 173,179 | 161,056 | 64,080 | 63,909 | 73,179 | 67,056 | 1.70 | 1.70 | 1.73 | 1.71 |
| Cumin–Cotton | 148,382 | 152,773 | 194,595 | 165,250 | 64,382 | 68,773 | 99,595 | 77,583 | 1.77 | 1.82 | 2.05 | 1.88 |
| Cumin–Fallow | 77,600 | 78,203 | 119,174 | 91,659 | 41,600 | 42,203 | 77,174 | 53,659 | 2.16 | 2.17 | 2.84 | 2.39 |
| S.Em.± | 4115.8 | 4042.6 | 5785 | 2723 | 4115.8 | 4042.6 | 5785 | 2723 | - | - | - | - |
| C.D.(p = 0.05) | 12,484.0 | 12,262.0 | 17,548 | 7762 | 12,484.0 | 12,262.0 | 17,548 | 7762 | - | - | - | - |
| CV (%) | 5.8 | 5.6 | 6.3 | 6.0 | 11.0 | 10.7 | 11.0 | 11.1 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehriya, M.L.; Singh, D.; Verma, A.K.; Geat, N.; Alataway, A.; Al-Othman, A.A.; Dewidar, A.Z.; Mattar, M.A. Unraveling the Impact of Cumin-Centric Cropping Sequences on Cumin Yield, Economic Viability, and Dynamics of Soil Enzymatic Activities in Hot Arid Climatic Conditions. Agronomy 2023, 13, 3023. https://doi.org/10.3390/agronomy13123023
Mehriya ML, Singh D, Verma AK, Geat N, Alataway A, Al-Othman AA, Dewidar AZ, Mattar MA. Unraveling the Impact of Cumin-Centric Cropping Sequences on Cumin Yield, Economic Viability, and Dynamics of Soil Enzymatic Activities in Hot Arid Climatic Conditions. Agronomy. 2023; 13(12):3023. https://doi.org/10.3390/agronomy13123023
Chicago/Turabian StyleMehriya, Moti Lal, Devendra Singh, Anil Kumar Verma, Neelam Geat, Abed Alataway, Ahmed A. Al-Othman, Ahmed Z. Dewidar, and Mohamed A. Mattar. 2023. "Unraveling the Impact of Cumin-Centric Cropping Sequences on Cumin Yield, Economic Viability, and Dynamics of Soil Enzymatic Activities in Hot Arid Climatic Conditions" Agronomy 13, no. 12: 3023. https://doi.org/10.3390/agronomy13123023





