Analysis of Trace Volatile Compounds Emitted from Flat Ground and Formed Bed Anaerobic Soil Disinfestation in Strawberry Field Trials on California’s Central Coast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trial Sites and Experimental Design
2.2. Flat ASD Field Trial
2.3. Formed Bed ASD Field Trials
2.4. Gas Collection and Analysis
2.5. FTIR Spectra Analysis and Data Limitations
2.6. Data Analysis of Gas Concentrations
3. Results
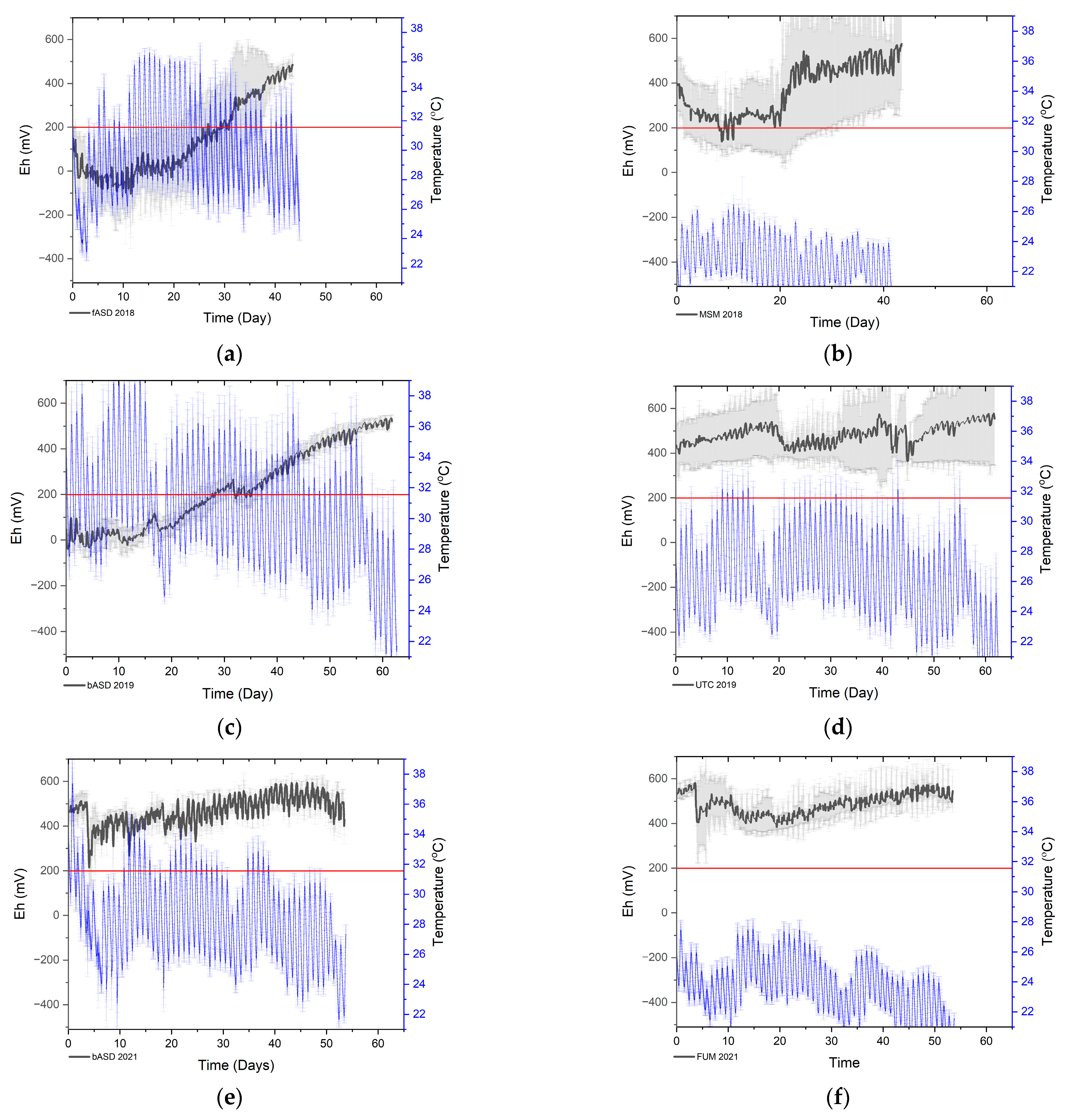
3.1. Monitoring of Anaerobic Conditions during ASD
3.2. Evaluating ASD Efficacy on Plant Health and Yield
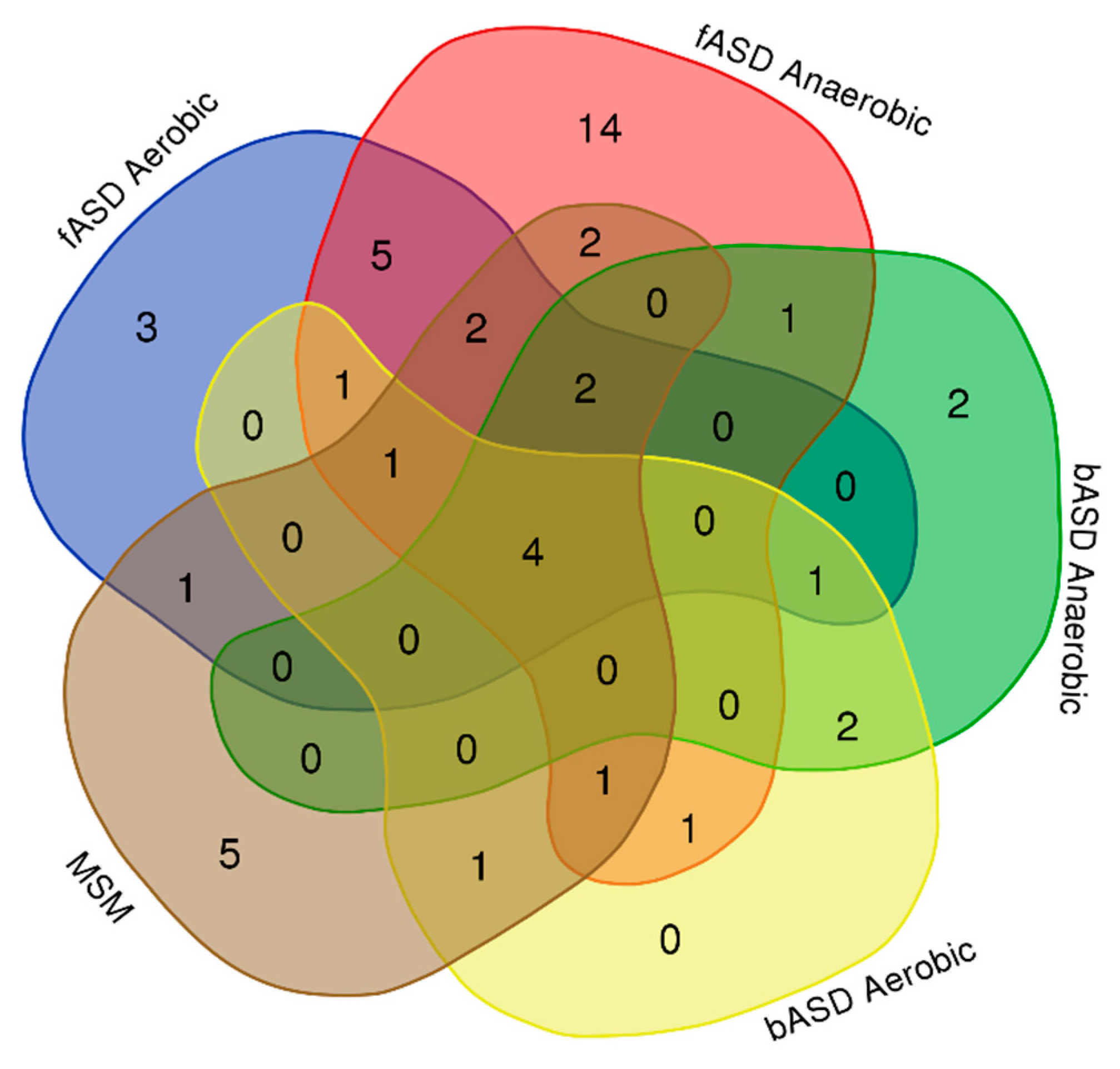
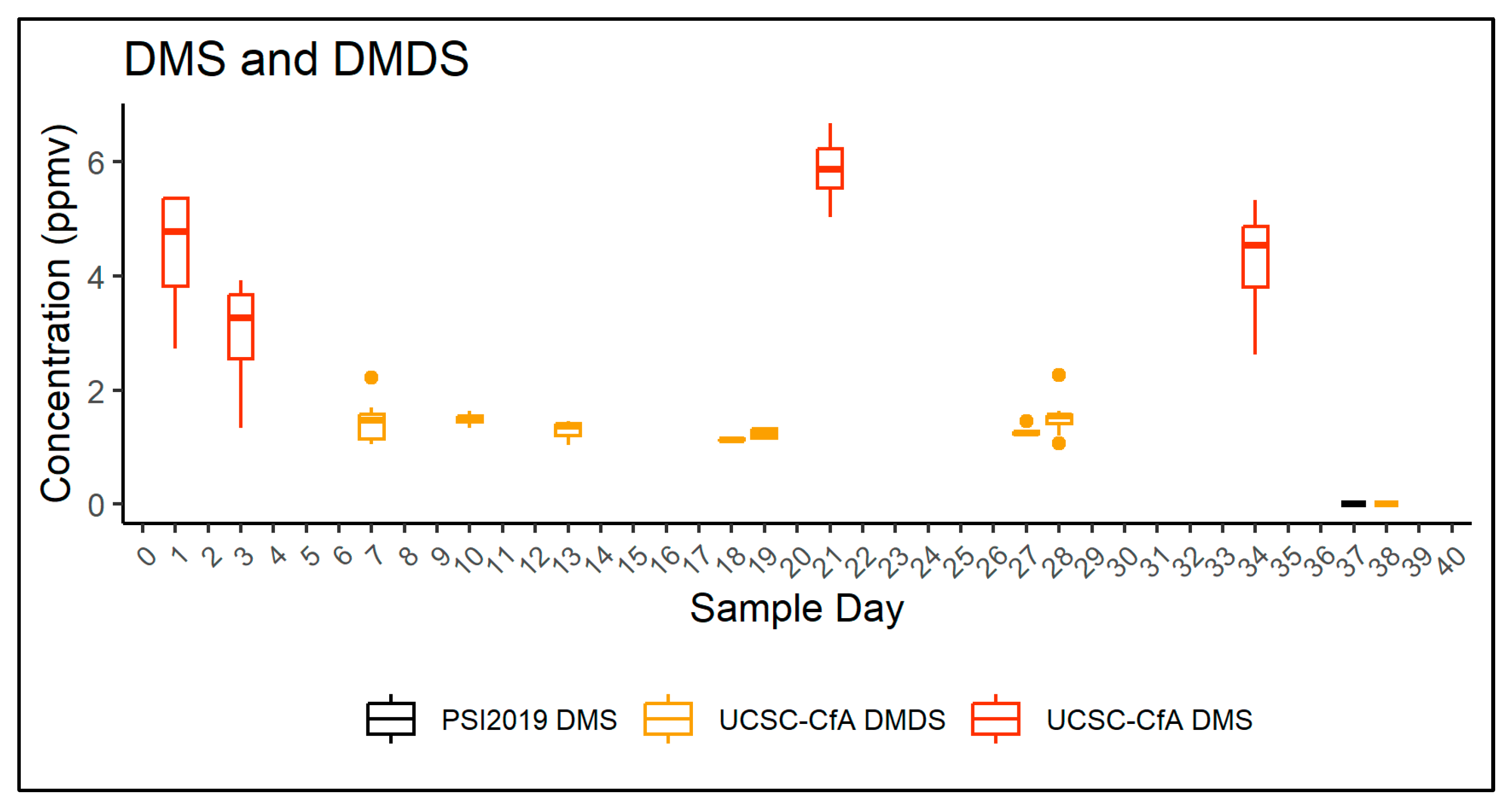
3.3. Gas Analysis
3.3.1. UCSC-CfA fASD Trial
3.3.2. 2019 PSI bASD Trial
4. Discussion
4.1. Challenges of FTIR
4.2. Gas Identification and Evidence of Biocontrol Organisms
4.3. Efficacy of fASD and bASD
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guthman, J.; Jiménez-Soto, E. Socioeconomic Challenges of California Strawberry Production and Disease Resistant Cultivars. Front. Sustain. Food Syst. 2021, 5, 446. [Google Scholar] [CrossRef]
- Guthman, J. Wilted: Pathogens, Chemicals, and the Fragile Future of the Strawberry Industry; University of California Press: Oakland, CA, USA, 2019. [Google Scholar]
- Carter, C.A.; Chalfant, J.A.; Goodhue, R.E.; Han, F.M.; De Santis, M. The methyl bromide ban: Economic impacts on the California strawberry industry. Rev. Agric. Econ. 2005, 27, 181–197. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Lamers, J.; Mazzola, M.; Rosskopf, E.N.; Kokalis-Burelle, N.; Momma, N.; Butler, D.M.; Kobara, Y.; Pugliese, M. Anaerobic soil disinfestation for soil borne disease control in strawberry and vegetable systems: Current knowledge and future directions. Acta Hortic. 2014, 1044, 165–175. [Google Scholar] [CrossRef]
- Rosskopf, E.N.; Serrano-Pérez, P.; Hong, J.; Shrestha, U.; del Carmen Rodríguez-Molina, M.; Martin, K.; Kokalis-Burelle, N.; Shennan, C.; Muramoto, J.; Butler, D. Anaerobic Soil Disinfestation and Soilborne Pest Management. In Organic Amendments and Soil Suppressiveness in Plant Disease Management; Meghvansi, M., Varma, A., Eds.; Soil Biology; Springer: Cham, Switzerland, 2015; Volume 46. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Koike, S.; Baird, G.; Fennimore, S.; Samtani, J.; Bolda, M.; Dara, S.; Daugovish, O.; Lazarovits, G.; et al. Anaerobic soil disinfestation is an alternative to soil fumigation for control of some soilborne pathogens in strawberry production. Plant Pathol. 2018, 67, 51–66. [Google Scholar] [CrossRef]
- Lopes, E.A.; Canedo, E.J.; Gomes, V.A.; Vieira, B.S.; Parreira, D.F.; Neves, W.S. Anaerobic soil disinfestation for the management of soilborne pathogens: A review. Appl. Soil Ecol. 2022, 174, 104408. [Google Scholar] [CrossRef]
- Goud, J.C.; Termorshuizen, A.J.; Blok, W.J.; van Bruggen, A.H.C. Long-Term Effect of Biological Soil Disinfestation on Verticillium Wilt. Plant Dis. 2004, 88, 688–694. [Google Scholar] [CrossRef]
- Messiha, N.A.S.; Van Diepeningen, A.D.; Wenneker, M.; Van Beuningen, A.R.; Janse, J.D.; Coenen, T.G.C.; Termorshuizen, A.J.; Van Bruggen, A.H.C.; Blok, W.J. Biological Soil Disinfestation (BSD), a new control method for potato brown rot, caused by Ralstonia solanacearum race 3 biovar 2. Eur. J. Plant Pathol. 2007, 117, 403–415. [Google Scholar] [CrossRef]
- Momma, N. Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. Jpn. Agric. Res. Q. 2008, 42, 7–12. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Ruddell, D.; Mazzola, M. Carbon source-dependent antifungal and nematicidal volatiles derived during anaerobic soil disinfestation. Eur. J. Plant Pathol. 2014, 140, 39–52. [Google Scholar] [CrossRef]
- Mazzola, M.; Muramoto, J.; Shennan, C. Anaerobic disinfestation induced changes to the soil microbiome, disease incidence and strawberry fruit yields in California field trials. Appl. Soil Ecol. 2018, 127, 74–86. [Google Scholar] [CrossRef]
- Ueki, A.; Kaku, N.; Ueki, K. Role of anaerobic bacteria in biological soil disinfestation for elimination of soil-borne plant pathogens in agriculture. Appl. Microbiol. Biotechnol. 2018, 102, 6309–6318. [Google Scholar] [CrossRef]
- Koike, S.T.; Gordon, T.R. Management of Fusarium wilt of strawberry. Crop Prot. 2015, 73, 67–72. [Google Scholar] [CrossRef]
- Wilhelm, S.; Koch, E.C. Verticillium Wilt Controlled chloropicrin achieves effective control of Verticillium wilt in strawberry plantings if properly applied as soil fumigant. Calif. Agric. 1956, 10, 3–14. [Google Scholar]
- Koike, S.T. Crown Rot of Strawberry Caused by Macrophomina phaseolina in California. Plant Dis. 2008, 92, 1253. [Google Scholar] [CrossRef]
- Insam, H.; Seewald, M.S.A. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils. 2010, 46, 199–213. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef] [PubMed]
- De Boer, W.; Li, X.; Meisner, A.; Garbeva, P. Pathogen suppression by microbial volatile organic compounds in soils. FEMS Microbiol. Ecol. 2019, 95, fiz105. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mo, M.; Zhang, L.; Zhang, K. Soil volatile fungistasis and volatile fungistatic compounds. Soil Biol. Biochem. 2004, 36, 1997–2004. [Google Scholar] [CrossRef]
- Momma, N.; Yamamoto, K.; Simandi, P.; Shishido, M. Role of organic acids in the mechanisms of biological soil disinfestation (BSD). J. Gen. Plant Pathol. 2006, 72, 247–252. [Google Scholar] [CrossRef]
- Zou, C.S.; Mo, M.H.; Gu, Y.Q.; Zhou, J.P.; Zhang, K.Q. Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol. Biochem. 2007, 39, 2371–2379. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Klarer, E.; Muramoto, J.; Shennan, C.; Mazzola, M. Analysis of Environmental Variables and Carbon Input on Soil Microbiome, Metabolome and Disease Control Efficacy in Strawberry Attributable to Anaerobic Soil Disinfestation. Microorganisms 2021, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Van Agtmaal, M.; van Os, G.J.; Gera Hol, W.H.; Hundscheid, M.P.J.; Runia, W.T.; Hordijk, C.A.; de Boer, W. Legacy effects of anaerobic soil disinfestation on soil bacterial community composition and production of pathogen-suppressing volatiles. Front. Microbiol. 2015, 6, 701. [Google Scholar] [CrossRef]
- Henry, P.M.; Haugland, M.; Lopez, L.; Munji, M.; Watson, D.C.; Gordon, T.R. The potential for Fusarium oxysporum f. sp. fragariae, cause of fusarium wilt of strawberry, to colonize organic matter in soil and persist through anaerobic soil disinfestation. Plant Pathol. 2020, 69, 1218–1226. [Google Scholar] [CrossRef]
- Mowlick, S.; Yasukawa, H.; Inoue, T.; Takehara, T.; Kaku, N.; Ueki, K.; Ueki, A. Suppression of spinach wilt disease by biological soil disinfestation incorporated with Brassica juncea plants in association with changes in soil bacterial communities. Crop Prot. 2013, 54, 185–193. [Google Scholar] [CrossRef]
- Cha, J.Y.; Han, S.; Hong, H.J.; Cho, H.; Kim, D.; Kwon, Y.; Kwon, S.K.; Crusemann, M.; Bok Lee, Y.; Kim, J.F.; et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef]
- Gray, C.M.; Monson, R.K.; Fierer, N. Biotic and abiotic controls on biogenic volatile organic compound fluxes from a subalpine forest floor. J. Geophys. Res. Biogeosci. 2014, 119, 487–507. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Mazzola, M. Carbon source-dependent effects of anaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology 2016, 106, 1015–1028. [Google Scholar] [CrossRef]
- Zavatta, M.; Muramoto, J.; Mazzola, M.; Shennan, C. Rotation length, crop rotation, anaerobic soil disinfestation and mustard seed meal affect organic strawberry yield and soil-borne disease incidence in California. Acta Hortic. 2021, 1309, 501–508. [Google Scholar] [CrossRef]
- Michuda, A.; Goodhue, R.; Klonsky, K.; Baird, G.; Toyama, L.; Zavatta, M.; Muramoto, J.; Shennan, C. The economic viability of suppressive crop rotations for the control of verticillium wilt in organic strawberry production. Agroecol. Sustain. Food Syst. 2019, 43, 984–1008. [Google Scholar] [CrossRef]
- Parkin, T.B.; Venterea, R.T. USDA-ARS GRACEnet Project Protocols Chapter 3. Chamber-Based Trace Gas Flux Measurements. Flux 2010, 1–39. Available online: https://www.ars.usda.gov/ARSUserFiles/np212/chapter%203.%20gracenet%20Trace%20Gas%20Sampling%20protocols.pdf (accessed on 5 February 2023).
- Rochette, P.; Eriksen-Hamel, N.S. Chamber Measurements of Soil Nitrous Oxide Flux: Are Absolute Values Reliable? Soil Sci. Soc. Am. J. 2008, 72, 331. [Google Scholar] [CrossRef]
- Momma, N.; Kobara, Y.; Uematsu, S.; Kita, N.; Shinmura, A. Development of biological soil disinfestations in Japan. Appl. Microbiol. Biotechnol. 2013, 97, 3801–3809. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. VOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.W.; Senter, S.D. Use of digital aroma technology and SPME GC-MS to compare volatile compounds produced by bacteria isolated from processed poultry. J. Sci. Food Agric. 1998, 78, 343–348. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Stotzky, G.; Schenck, S.; Papavizas, G.C. Volatile Organic Compounds and Microorganisms. CRC Crit. Rev. Microbiol. 2008, 4, 333–382. [Google Scholar] [CrossRef]
- Müller, A.; Faubert, P.; Hagen, M.; zu Castell, W.; Polle, A.; Schnitzler, J.P.; Rosenkranz, M. Volatile profiles of fungi—Chemotyping of species and ecological functions. Fungal Genet. Biol. 2013, 54, 25–33. [Google Scholar] [CrossRef]
- Weikl, F.; Tischer, C.; Probst, A.J.; Heinrich, J.; Markevych, I.; Jochner, S.; Pritsch, K. Fungal and Bacterial Communities in Indoor Dust Follow Different Environmental Determinants. PLoS ONE 2016, 11, e0154131. [Google Scholar] [CrossRef] [PubMed]
- Preti, G.; Thaler, E.; Hanson, C.W.; Troy, M.; Eades, J.; Gelperin, A. Volatile compounds characteristic of sinus-related bacteria and infected sinus mucus: Analysis by solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Weise, T.; Thürmer, A.; Brady, S.; Kai, M.; Daniel, R.; Gottschalk, G.; Piechulla, B. VOC emission of various Serratia species and isolates and genome analysis of Serratia plymuthica 4Rx13. FEMS Microbiol. Lett. 2014, 352, 45–53. [Google Scholar] [CrossRef]
- Ma, Q.; Fonseca, A.; Liu, W.; Fields, A.T.; Pimsler, M.L.; Spindola, A.F.; Tarone, A.M.; Crippen, T.L.; Tomberlin, J.K.; Wood, T.K. Proteus mirabilis interkingdom swarming signals attract blow flies. ISME J. 2012, 6, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Dandurishvili, N.; Toklikishvili, N.; Ovadis, M.; Eliashvili, P.; Giorgobiani, N.; Keshelava, R.; Tediashvili, M.; Vainstein, A.; Khmel, I.; Szegedi, E.; et al. Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumours on tomato plants. J. Appl. Microbiol. 2011, 110, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of Bioactive Volatiles by Different Burkholderia ambifaria Strains. J. Chem. Ecol. 2013, 39, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Zweers, H.; de Boer, W.; Garbeva, P. Volatiles in inter-specific bacterial interactions. Front. Microbiol. 2015, 6, 1412. [Google Scholar] [CrossRef]
- De Vrieze, M.; Pandey, P.; Bucheli, T.D.; Varadarajan, A.R.; Ahrens, C.H.; Weisskopf, L.; Bailly, A. Volatile organic compounds from native potato-associated Pseudomonas as potential anti-oomycete agents. Front. Microbiol. 2015, 6, 1295. [Google Scholar] [CrossRef]
- Biwer, P.; Neumann-Schaal, M.; Henke, P.; Jahn, D.; Schulz, S. Thiol Metabolism and Volatile Metabolome of Clostridioides difficile. Front. Microbiol. 2022, 13, 864587. [Google Scholar] [CrossRef]
- Conti, S.; Villari, G.; Faugno, S.; Melchionna, G.; Somma, S.; Caruso, G. Effects of organic vs. conventional farming system on yield and quality of strawberry grown as an annual or biennial crop in southern Italy. Sci. Hortic. 2014, 180, 63–71. [Google Scholar] [CrossRef]
- Han, D.; Yan, D.; Wang, Q.; Fang, W.; Wang, X.; Li, J.; Wang, D.; Li, Y.; Ouyang, C.; Cao, A. Effects of soil type, temperature, moisture, application dose, fertilizer, and organic amendments on chemical properties and biodegradation of dimethyl disulfide in soil. Land Degrad. Dev. 2018, 29, 4282–4290. [Google Scholar] [CrossRef]
- Muramoto, J.; Shennan, C.; Mazzola, M.; Wood, T.; Miethke, E.; Resultay, E.; Zavatta, M.; Koike, S.T. Use of a summer cover crop as a partial carbon source for anaerobic soil disinfestation in coastal California. Acta Hortic. 2020, 1270, 37–43. [Google Scholar] [CrossRef]


| Gas Species | fASD Aerobic | fASD Anaerobic | MSM (2018) | bASD Aerobic (2019) | bASD Anaerobic (2019) | FUM (2021) | UTC (2021) |
|---|---|---|---|---|---|---|---|
| 1,2,3-Trimethylbenzene | 0.842 | 1.475 | 0.666 | ||||
| 1,3,5-Trimethylbenzene | 1.320 | ||||||
| 1-Butanethiol | 5.203 | 6.042 | |||||
| 1-Butene | 1.730 | 5.706 | 1.158 | 1.102 | 0.654 | ||
| 1-Heptene | 1.055 | 1.080 | 0.319 | ||||
| 1-Hexanol | 1.439 | 2.303 | 1.174 | 0.103 | |||
| 1-Methylimidazol | 7.672 | ||||||
| 1-Pentanol | 1.966 | ||||||
| 1-Pentene | 1.475 | 1.306 | 0.538 | 0.938 | 0.175 | 0.178 | |
| 2,3-Dimethylpyrazine | 1.290 | 0.571 | 0.029 | ||||
| 2,3-Heptanedione | 0.417 | 0.163 | |||||
| 2,3-Hexanedione | 1.221 | ||||||
| 2-Ethylhexanol | 1.895 | ||||||
| 2-Methylpyrazine | 0.237 | 0.238 | 0.073 | ||||
| 3-Chloro-2-methyl-1-propene | 1.190 | ||||||
| 4-Ethyltoluene | 1.505 | ||||||
| 4-Methyl-3-penten-2-one | 1.154 | ||||||
| Allylcyanide-3-butenenitrile | 1.312 | ||||||
| Ammonia | 0.023 | 0.229 | |||||
| Butylamine-1-butanamine | 5.842 | 1.799 | 2.845 | ||||
| Carbon dioxide | 49,690 | 82,123 | 883 | 10,335 | 48,227 | 12,863 | 11,952 |
| Carbon monoxide | 0.129 | 0.733 | 0.001 | ||||
| Cis-2-Butene | 0.205 | ||||||
| Cis-2-Pentene | 1.020 | ||||||
| Cyclohexane | 2.830 | ||||||
| Dimethyl disulfide | 0.875 | 1.468 | |||||
| Dimethyl sulfide | 5.868 | 3.952 | |||||
| Dodecane | 7.082 | 1.090 | |||||
| Ethanol | 4.2 | 41.0 | |||||
| Ethanolamine | 0.793 | ||||||
| Ethyl chloride | 0.861 | 0.855 | |||||
| Ethyl fluoride | 15.957 | ||||||
| Heptane | 12.927 | 2.652 | |||||
| Hexane | 0.428 | ||||||
| Hexanoic acid | 5.646 | ||||||
| Hexene | 4.566 | 0.998 | 0.838 | 1.040 | 1.464 | 0.197 | |
| Hexylamine | 2.930 | 1.260 | 1.250 | ||||
| Isobutanol | 1.530 | ||||||
| Isohexane | 36.378 | ||||||
| Isopentane | 1.247 | ||||||
| Methane | 1545.48 | 679.86 | 1.82 | 1.78 | 15.87 | 1.68 | 1.71 |
| Methyl chloride | 20.347 | ||||||
| M-Xylene | 0.639 | ||||||
| Nitrogen dioxide | 13.308 | ||||||
| Nitrous oxide | 9.54 | 8.34 | 0.34 | 33.82 | 5.38 | 4.09 | 2.07 |
| Nonane | 5.937 | ||||||
| O-Cresol | 0.603 | 0.584 | |||||
| Pentane | 1.113 | 2.538 | |||||
| Propene | 1.399 | 1.153 | |||||
| Propylamine | 0.797 | 0.080 | |||||
| Propylene oxide | 3.192 | 4.148 | 0.444 | 0.036 | 0.963 | 2.038 | 0.374 |
| t-Butanol | 2.050 | ||||||
| Tetrahydrofuran | 0.233 | ||||||
| Tetrahydrothiopene | 1.910 | ||||||
| Undecane | 1.374 | 9.532 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prescott, K.; Kortman, S.; Duque, J.; Muramoto, J.; Shennan, C.; Greenstein, G.; Haffa, A.L.M. Analysis of Trace Volatile Compounds Emitted from Flat Ground and Formed Bed Anaerobic Soil Disinfestation in Strawberry Field Trials on California’s Central Coast. Agronomy 2023, 13, 1190. https://doi.org/10.3390/agronomy13051190
Prescott K, Kortman S, Duque J, Muramoto J, Shennan C, Greenstein G, Haffa ALM. Analysis of Trace Volatile Compounds Emitted from Flat Ground and Formed Bed Anaerobic Soil Disinfestation in Strawberry Field Trials on California’s Central Coast. Agronomy. 2023; 13(5):1190. https://doi.org/10.3390/agronomy13051190
Chicago/Turabian StylePrescott, Kali, Stefanie Kortman, Josue Duque, Joji Muramoto, Carol Shennan, Gloria Greenstein, and Arlene L. M. Haffa. 2023. "Analysis of Trace Volatile Compounds Emitted from Flat Ground and Formed Bed Anaerobic Soil Disinfestation in Strawberry Field Trials on California’s Central Coast" Agronomy 13, no. 5: 1190. https://doi.org/10.3390/agronomy13051190







