The Changes in Various Physio-Biochemical Parameters and Yield Traits of Faba Bean Due to Humic Acid Plus 6-Benzylaminopurine Application under Deficit Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Agronomic Management and Treatments
2.3. Irrigation Water Applied
2.4. Measurements
2.4.1. Water Status and Photosynthetic Capacity
2.4.2. Free Proline Content, Total Soluble Sugars and Enzyme
2.4.3. Growth Traits
2.4.4. Leaf Mineral Contents
2.4.5. Yield and Yield Components
2.4.6. Water Use Efficiency
2.5. Statistical Analysis
3. Results
3.1. Growth Response
3.2. Physiological Response
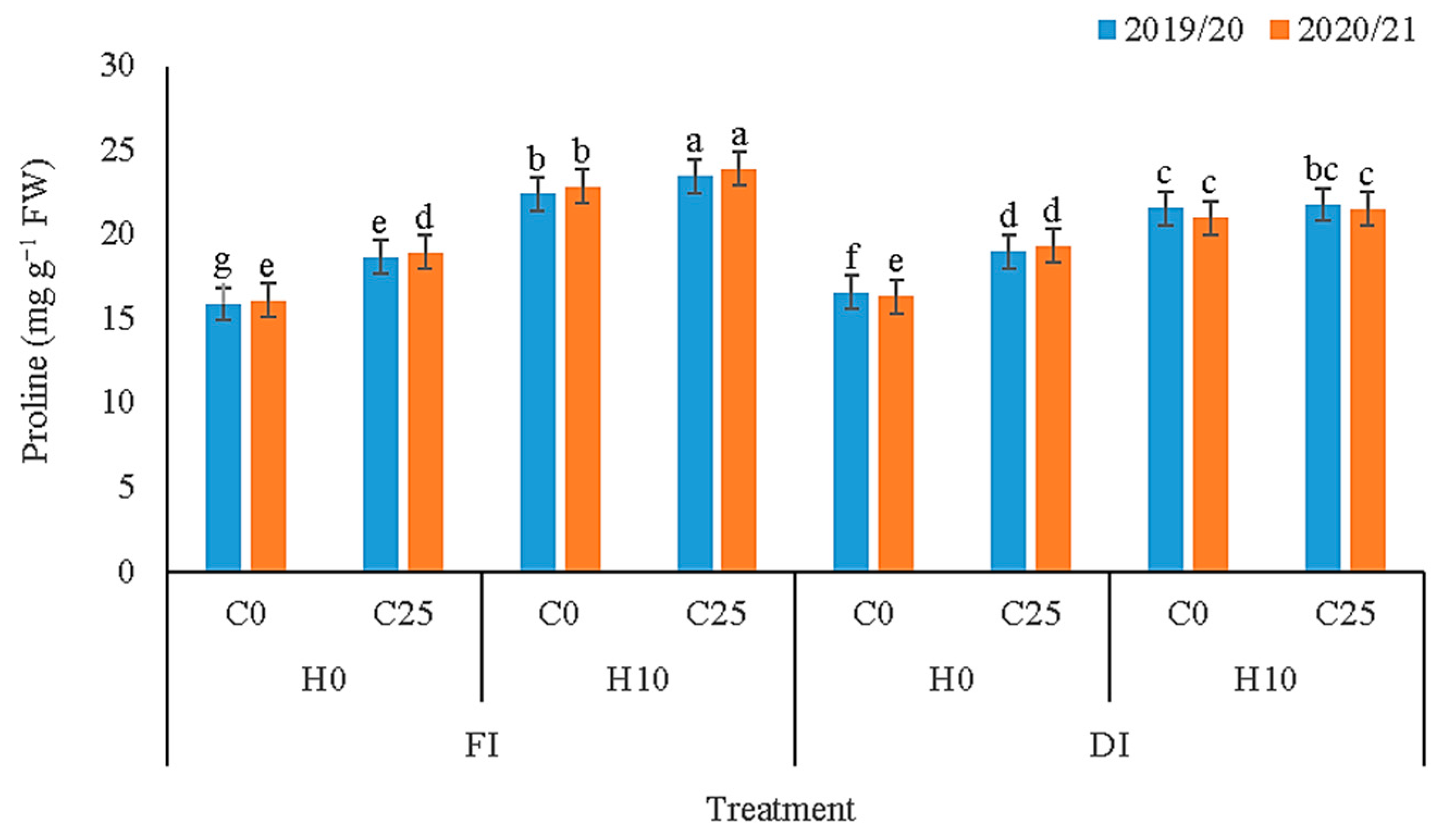
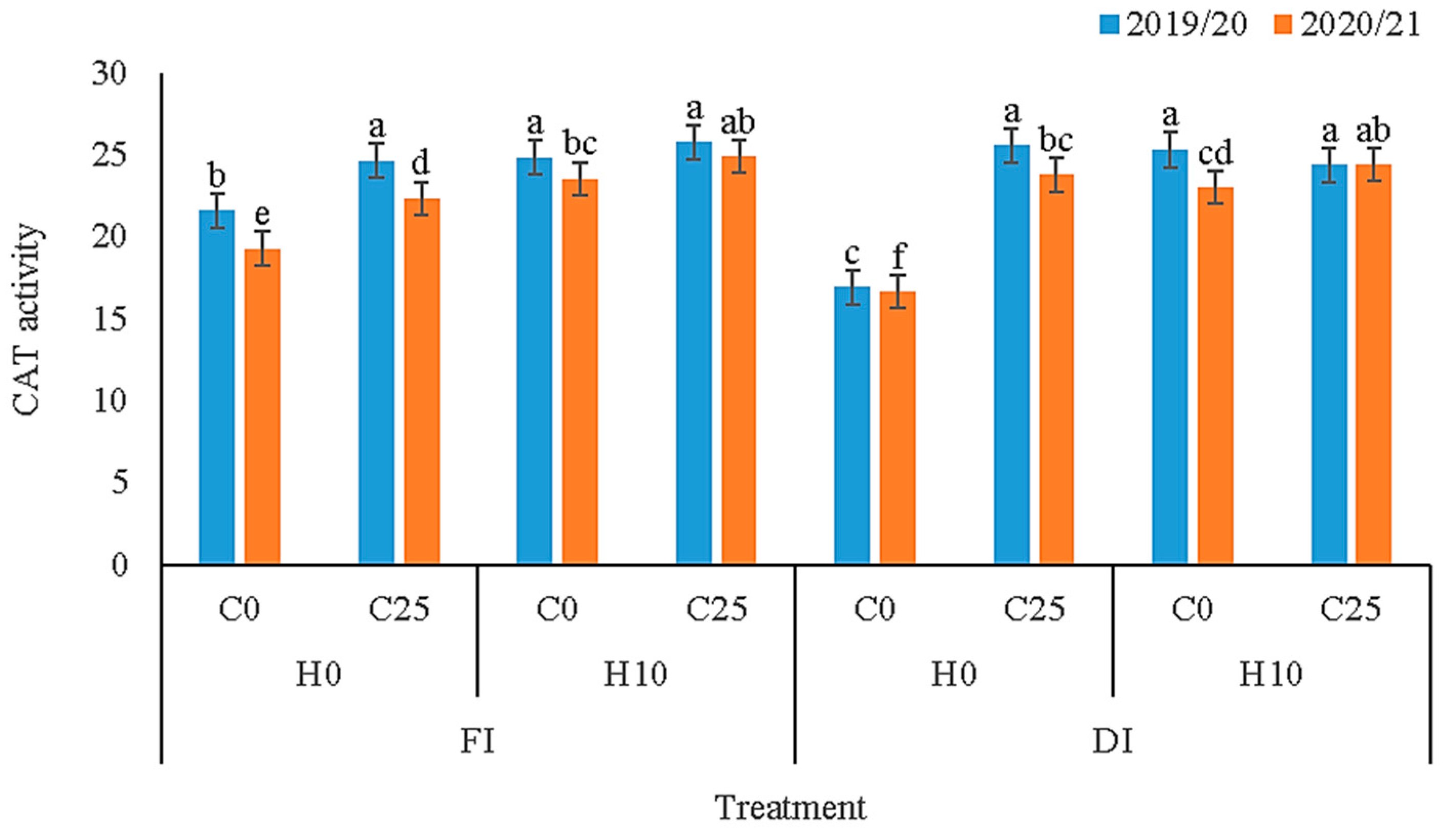
3.3. Biochemical Compounds
3.4. Nutrient Contents
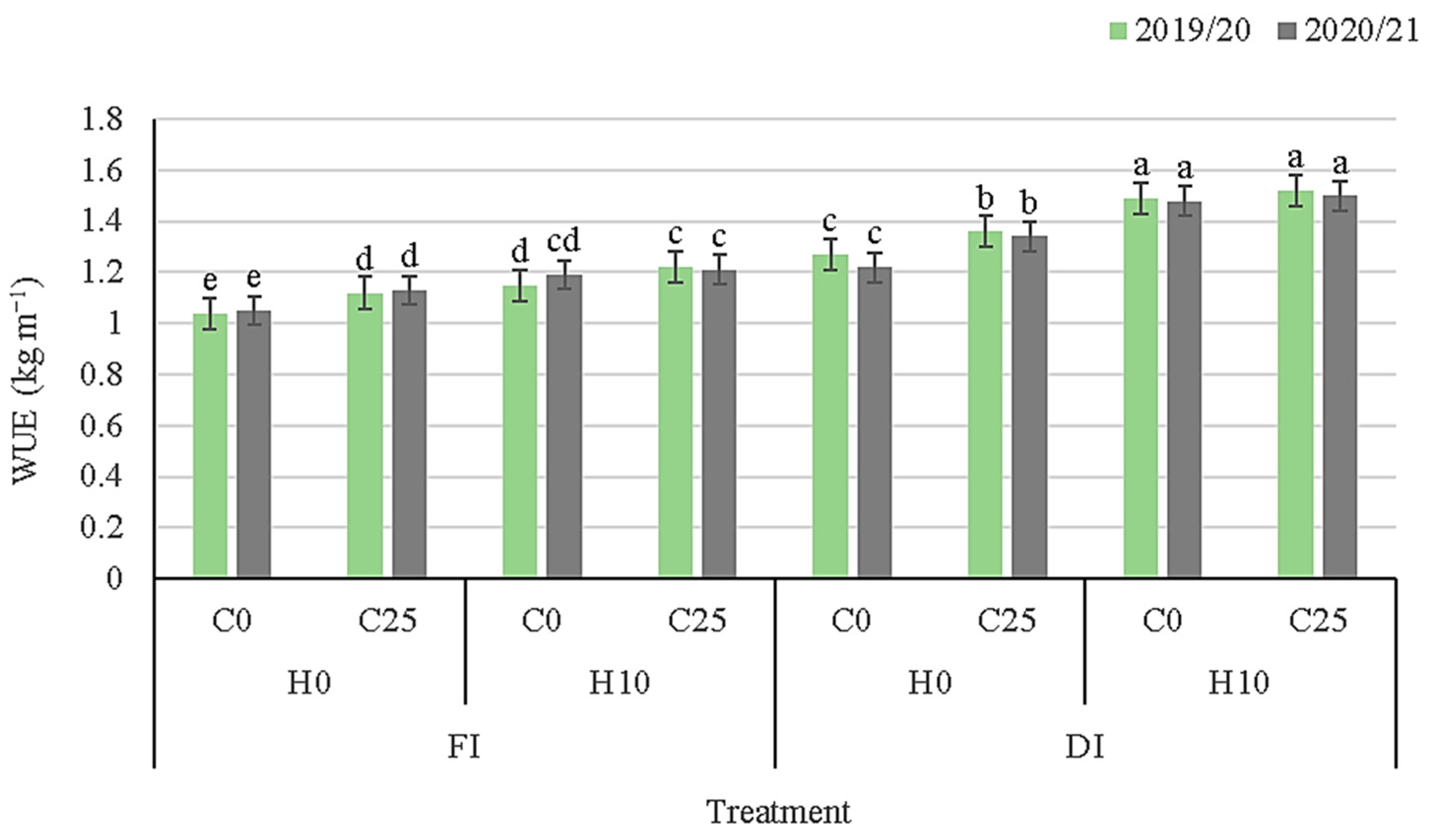
3.5. Yield Traits and Water Use Efficiency
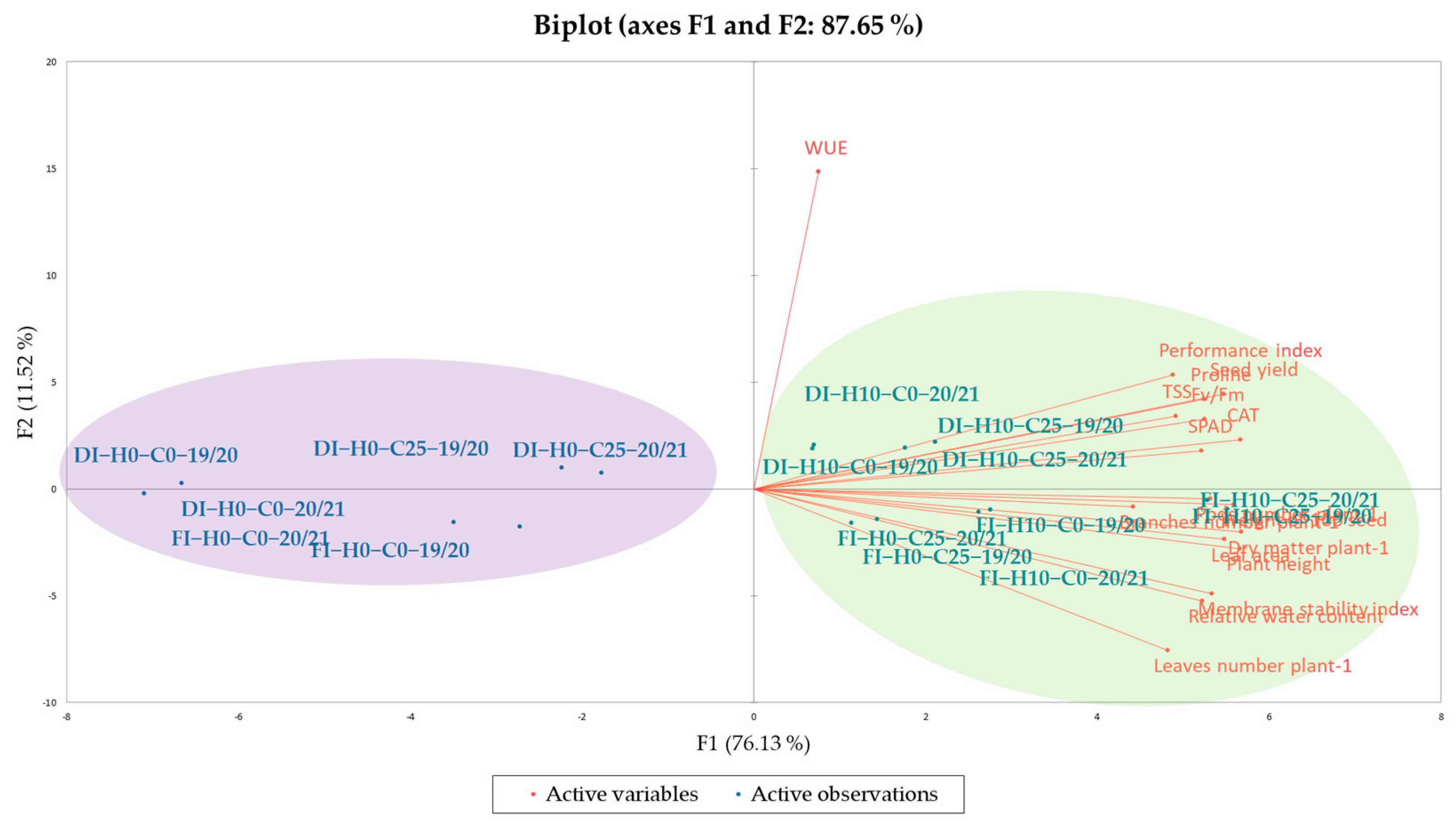
3.6. Chemometric Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neme, K.; Bultosa, G.; Bussa, N. Nutrient and Functional Properties of Composite Flours Processed from Pregelatinised Barley, Sprouted Faba Bean and Carrot Flours. Int. J. Food Sci. Technol. 2015, 50, 2375–2382. [Google Scholar] [CrossRef]
- Aisa, K.; Hamed, M.F.; Saudy, H.; El–Metwally, I.M.; Ramadan, K.M.A. Preliminary Study for Testing the Significance of Butyl–Iso–Butyl Phthalate in controlling Faba Bean Broomrape. Arab Univ. J. Agric. Sci. 2019, 27, 1399–1404. [Google Scholar] [CrossRef]
- Saudy, H.S.; Salem, E.M.M.; Abd El-Momen, W.R. Effect of Potassium Silicate and Irrigation on Grain Nutrient Uptake and Water Use Efficiency of Wheat Under Calcareous Soils. Gesunde Pflanz. 2022. [Google Scholar] [CrossRef]
- Saudy, H.S.; Hamed, M.F.; El–Metwally, I.M.; Ramadan, K.A.; Aisa, K.H. Assessing the Effect of Biochar or Compost Application as a Spot Placement on Broomrape Control in Two Cultivars of Faba Bean. J. Soil Sci. Plant Nutr. 2021, 21, 1856–1866. [Google Scholar] [CrossRef]
- Saudy, H.; El-Bially, M.; El-Metwally, I.; Shahin, M. Physio-Biochemical and Agronomic Response of Ascorbic Acid Treated Sunflower (Helianthus Annuus) Grown at Different Sowing Dates and Under Various Irrigation Regimes. Gesunde Pflanz. 2021, 73, 169–179. [Google Scholar] [CrossRef]
- El–Metwally, I.; Geries, L.; Saudy, H. Interactive Effect of Soil Mulching and Irrigation Regime on Yield, Irrigation Water Use Efficiency and weeds of Trickle–Irrigated Onion. Arch. Agron. Soil Sci. 2022, 68, 1103–1116. [Google Scholar] [CrossRef]
- Mubarak, M.; Salem, E.M.M.; Kenawey, M.K.M.; Saudy, H.S. Changes in Calcareous Soil Activity, Nutrient Availability, and Corn Productivity Due to the Integrated Effect of Straw Mulch and Irrigation Regimes. J. Soil Sci. Plant Nutr. 2021, 21, 2020–2031. [Google Scholar] [CrossRef]
- Salem, E.M.M.; Kenawey, M.K.M.; Saudy, H.S.; Mubarak, M. Influence of Silicon Forms on Nutrients Accumulation and Grain Yield of Wheat under Water Deficit Conditions. Gesunde Pflanz. 2022, 74, 539–548. [Google Scholar] [CrossRef]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.-H.; Siddique, K.H.M.; Zhuang, W.; et al. Developing drought-smart, ready-to-grow future crops. Plant Gen. 2023, 16, e20279. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Yang, F.; Miao, L.F. Adaptive responses to progressive drought stress in two poplar species originating from different altitudes. Silva Fennica 2010, 44, 23–37. [Google Scholar] [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Chen, X. Metabolic and growth responses of maize to successive drought and re-watering cycles. Agric. Water Manag. 2016, 172, 62–73. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A. Changes in non protein thiols, some antioxidant enzymes activity and ultrastructural alteration in radish plant (Raphanus sativus L.) grown under lead toxicity. Not. Bot. Horti. Agrobot. Cluj-Napoca 2010, 38, 76–85. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- de Jesus Souza, B.; do Carmo, D.L.; Santos, R.H.S.; de Oliveira, T.S.; Fernandes, R.B.A. Residual Contribution of Green Manure to Humic Fractions and Soil Fertility. J. Soil Sci. Plant Nutr. 2019, 19, 878–886. [Google Scholar] [CrossRef]
- Dinçsoy, M.; Sönmez, F. The Effect of Potassium and Humic Acid Applications on Yield and Nutrient Contents of Wheat (Triticum aestivum L. Var. Delfii) with Same Soil Properties. J. Plant Nutr. 2019, 42, 2757–2772. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Eissa, M.A. Effect of Biochar on Yield and Quality of Tomato Grown on a Metal-Contaminated Soil. Sci. Hortic. 2020, 265, 109210. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, Z.; Lin, X.; Zhao, F.; Wang, B.; Lin, F.; Ge, Y.; Eissa, M.A. Biochar Impacts on NH3-Volatilization Kinetics and Growth of Sweet Basil (Ocimum Basilicum L.) under Saline Conditions. Ind. Crops Prod. 2020, 157, 112903. [Google Scholar] [CrossRef]
- Rekaby, S.A.; Awad, M.Y.M.; Hegab, S.A.; Eissa, M.A. Effect of Some Organic Amendments on Barley Plants under Saline Condition. J. Plant Nutr. 2020, 43, 1840–1851. [Google Scholar] [CrossRef]
- Torun, H.; Toprak, B. Arbuscular Mycorrhizal Fungi and K-Humate Combined as Biostimulants: Changes in Antioxidant Defense System and Radical Scavenging Capacity in Elaeagnus Angustifolia. J. Soil Sci. Plant Nutr. 2020, 20, 2379–2393. [Google Scholar] [CrossRef]
- Aalipour, H.; Nikbakht, A.; Ghasemi, M.; Amiri, R. Morpho-Physiological and Biochemical Responses of Two Turfgrass Species to Arbuscular Mycorrhizal Fungi and Humic Acid Under Water Stress Condition. J. Soil Sci. Plant Nutr. 2020, 20, 566–576. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought Stress in Sunflower: Physiological Effects and Its Management through Breeding and Agronomic Alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising Toxicity of Cadmium in Plants—Role of Plant Growth Regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; Sadak, M.S.; Saudy, H.S. Stimulation Effects of Glutamic and 5-Aminolevulinic Acids On Photosynthetic Pigments, Physio-Biochemical Constituents, Antioxidant Activity, and Yield of Peanut. Gesunde Pflanz. 2022, 74, 915–924. [Google Scholar] [CrossRef]
- Al Mahmud, J.; Biswas, P.K.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Exogenous Application of Gibberellic Acid Mitigates Drought-Induced Damage in Spring Wheat. Acta Agrobot. 2019, 72, 2. [Google Scholar]
- Khalid, A.; Aftab, F. Effect of Exogenous Application of IAA and GA 3 on Growth, Protein Content, and Antioxidant Enzymes of Solanum Tuberosum L. Grown in Vitro under Salt Stress. Vitr. Cell. Dev. Biol. 2020, 56, 377–389. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin Signaling in Plant Development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Li, W.; Herrera-Estrella, L.; Tran, L.-S.P. The Yin–Yang of Cytokinin Homeostasis and Drought Acclimation/Adaptation. Trends Plant Sci. 2016, 21, 548–550. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Gaudinova, A.; Knirsch, V.; Körber, N.; Pieruschka, R.; Fiorani, F.; Brzobohatý, B.; Černý, M.; Spichal, L. Cytokinins: Their Impact on Molecular and Growth Responses to Drought Stress and Recovery in Arabidopsis. Front. Plant Sci. 2018, 9, 655. [Google Scholar] [CrossRef]
- Wu, X.; He, J.; Chen, J.; Yang, S.; Zha, D. Alleviation of Exogenous 6-Benzyladenine on Two Genotypes of Eggplant (Solanum Melongena Mill.) Growth under Salt Stress. Protoplasma 2014, 251, 169–176. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Huang, B. Cytokinin-Mitigation of Salt-Induced Leaf Senescence in Perennial Ryegrass Involving the Activation of Antioxidant Systems and Ionic Balance. Environ. Exp. Bot. 2016, 125, 1–11. [Google Scholar] [CrossRef]
- Rubio-Wilhelmi, M.M.; Sanchez-Rodriguez, E.; Rosales, M.A.; Begona, B.; Rios, J.J.; Romero, L.; Blumwald, E.; Ruiz, J.M. Effect of Cytokinins on Oxidative Stress in Tobacco Plants under Nitrogen Deficiency. Environ. Exp. Bot. 2011, 72, 167–173. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Shi, Y.; Cui, Z.; Luo, Y.; Zheng, M.; Chen, J.; Li, Y.; Yin, Y.; Wang, Z. Exogenous Cytokinins Increase Grain Yield of Winter Wheat Cultivars by Improving Stay-Green Characteristics under Heat Stress. PLoS ONE 2016, 11, e0155437. [Google Scholar] [CrossRef]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an Inflorescence Meristem-specific Cytokinin Oxidase–OsCKX2 in Rice Reduces Yield Penalty under Salinity Stress Condition. Plant. Cell Environ. 2018, 41, 936–946. [Google Scholar] [CrossRef]
- Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 2nd ed.; Page, A.L. (Ed.) The American Society of Agronomy, Inc., Soil Science Society of America, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Klute, A.; Dirksen, C. Hydraulic Conductivity and Diffusivity: Laboratory Methods. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; Volume 5, pp. 687–734. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell Membrane Stability, an Indicator of Drought Tolerance, as Affected by Applied Nitrogen in Soyabean. J. Agric. Sci. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus Sylvatica) Response to Ozone Exposure Assessed with a Chlorophyll a Fluorescence Performance Index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water Stress Induced Changes in Concentrations of Proline and Total Soluble Sugars in Nodulated Alfalfa (Medicago sativa) Plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Jacobs, M.B. Micro-Kjeldahl Method for Biologicals. J. Am. Pharm. Assoc. 1951, 40, 151–153. [Google Scholar] [CrossRef]
- AOAC The Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International; Prentice Hall India Pvt. Ltd.: New Delhi, India, 2000; p. 498. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Pentice Hall of India Pvt. Ltd.: New Delhi, India, 1973; Volume 498, pp. 151–154. [Google Scholar]
- Fernández, J.E.; Alcon, F.; Diaz-Espejo, A.; Hernandez-Santana, V.; Cuevas, M.V. Water use indicators and economic analysis for on-farm irrigation decision: A case study of a super high density olive tree orchard. Agric. Water Manag. 2020, 237, 106074. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984; ISBN 0471870927. [Google Scholar]
- Mahmoud, M.A.A.; Magdy, M.; Tybussek, T.; Barth, J.; Buettner, A. Comparative Evaluation of Wild and Farmed Rainbow Trout Fish Based on Representative Chemosensory and Microbial Indicators of Their Habitats. J. Agric. Food Chem. 2023, 71, 2094–2104. [Google Scholar] [CrossRef]
- Mahmoud, M.A.A.; Magdy, M. Metabarcoding Profiling of Microbial Diversity Associated with Trout Fish Farming. Sci. Rep. 2021, 11, 421. [Google Scholar] [CrossRef]
- Saudy, H.S.; El-Bially, M.E.; Hashem, F.A.; Shahin, M.G.; El-Gabry, Y.A. The Changes in Yield Response Factor, Water Use Efficiency, and Physiology of Sunflower Owing to Ascorbic and Citric Acids Application Under Mild Deficit Irrigation. Gesunde Pflanz. 2022. [Google Scholar] [CrossRef]
- Saudy, H.S.; Mohamed El–Metwally, I. Effect of Irrigation, Nitrogen Sources, and Metribuzin on Performance of Maize and Its Weeds. Commun. Soil Sci. Plant Anal. 2023, 54, 22–35. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; Saudy, H.S. Interactional Impacts of Drought and Weed Stresses on Nutritional Status of Seeds and Water Use Efficiency of Peanut Plants Grown in Arid Conditions. Gesunde Pflanz. 2021, 73, 407–416. [Google Scholar] [CrossRef]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of Abscisic Acid-Mediated Control of Stomatal Aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management BT—Sustainable Agriculture. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 153–188. ISBN 978-90-481-2666-8. [Google Scholar]
- Saudy, H.; Noureldin, N.; Mubarak, M.; Fares, W.; Elsayed, M. Cultivar Selection as a Tool for Managing Soil Phosphorus and Faba Bean Yield sustainability. Arch. Agron. Soil Sci. 2020, 66, 414–425. [Google Scholar] [CrossRef]
- Ashraf, M.; Ahmad, A.; McNeilly, T. Growth and Photosynthetic Characteristics in Pearl Millet under Water Stress and Different Potassium Supply. Photosynthetica 2001, 39, 389–394. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Raza, A.; Tabassum, J.; Kudapa, H.; Varshney, R.K. Can omics deliver temperature resilient ready-to-grow crops? Crit. Rev. Biotechnol. 2021, 41, 1209–1232. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategiesto tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Chen, L.; Li, P.; Cao, C. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci. Rep. 2019, 9, 3742. [Google Scholar] [CrossRef]
- Al-Shareef, A.R.; El-Nakhlawy, F.S.; Ismail, S.M. Enhanced Mungbean and Water Productivity under Full Irrigation and Stress Using Humic Acid in Arid Regions. Legum. Res. Int. J. 2018, 41, 428–431. [Google Scholar] [CrossRef]
- Puglisi, E.; Fragoulis, G.; Ricciuti, P.; Cappa, F.; Spaccini, R.; Piccolo, A.; Trevisan, M.; Crecchio, C. Effects of a Humic Acid and Its Size-Fractions on the Bacterial Community of Soil Rhizosphere under Maize (Zea Mays L.). Chemosphere 2009, 77, 829–837. [Google Scholar] [CrossRef]
- Yousefi Rad, M.; Masomi Zavarian, A. Effects of Humic Acid and Mycorrhiza on Morphological Characteristics and Nutrients Concentration of Red Bean (Vigna Unguiculata L.). J. Plant Environ. Physiol. 2017, 12, 92–102. [Google Scholar]
- El-Bassiony, A.M.; Fawzy, Z.F.; Abd El-Baky, M.M.H.; Mahmoud, A.R. Response of Snap Bean Plants to Mineral Fertilizers and Humic Acid Application. Res. J. Agric. Biol. Sci 2010, 6, 169–175. [Google Scholar]
- Ghorbani, S.; Khazaie, H.; Kafi, M.; Bannayan Aval, M. Effects of Humic Acid Application with Irrigation Water on Yield and Yield Components of Corn (Zea Mays L.). J. Agroecol. 2010, 2, 11–118. [Google Scholar] [CrossRef]
- Çelik, H.; Katkat, A.V.; Aşık, B.B.; Turan, M.A. Effect of Foliar-Applied Humic Acid to Dry Weight and Mineral Nutrient Uptake of Maize under Calcareous Soil Conditions. Commun. Soil Sci. Plant Anal. 2010, 42, 29–38. [Google Scholar] [CrossRef]
- Roudgarnejad, S.; Samdeliri, M.; Mirkalaei, A.M.; Moghaddam, M.N. The Role of Humic Acid Application on Quantitative and Qualitative Traits of Faba Bean (Vicia Faba L.). Gesunde Pflanz. 2021, 73, 603–611. [Google Scholar] [CrossRef]
- Makhlouf, B.S.I.; Khalil, S.R.A.E.; Saudy, H.S. Efficacy of Humic Acids and Chitosan for Enhancing Yield and Sugar Quality of Sugar Beet under Moderate and Severe Drought. J. Soil Sci. Plant Nutr. 2022, 22, 1676–1691. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, S.; Prakash, P. Exogenous Application of Cytokinin (6-BAP) Ameliorates the Adverse Effect of Combined Drought and High Temperature Stress in Wheat Seedling. J. Pharmacogn. Phytochem. 2018, 7, 1176–1180. [Google Scholar]
- Samea-Andabjadid, S.; Ghassemi-Golezani, K.; Nasrollahzadeh, S.; Najafi, N. Exogenous Salicylic Acid and Cytokinin Alter Sugar Accumulation, Antioxidants and Membrane Stability of Faba Bean. Acta Biol. Hung. 2018, 69, 86–96. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinin Biosynthesis and Metabolism. In Plant Hormones: Biosynthesis, Signal Transduction, Action; Davies, P.J., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 95–114. ISBN 978-1-4020-2686-7. [Google Scholar]
- Sakakibara, H.; Takei, K.; Hirose, N. Interactions between Nitrogen and Cytokinin in the Regulation of Metabolism and Development. Trends Plant Sci. 2006, 11, 440–448. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Nakamura, Y.; Yamaya, T.; Takahashi, H. A Novel Regulatory Pathway of Sulfate Uptake in Arabidopsis Roots: Implication of CRE1/WOL/AHK4-mediated Cytokinin-dependent Regulation. Plant J. 2004, 38, 779–789. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal Control of Nitrogen Acquisition: Roles of Auxin, Abscisic Acid, and Cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Albacete, A.; Smigocki, A.C.; Frebort, I.; Pospíšilová, H.; Martínez-Andújar, C.; Acosta, M.; Sanchez-Bravo, J.; Lutts, S.; Dodd, I.C. Root-Synthesized Cytokinins Improve Shoot Growth and Fruit Yield in Salinized Tomato (Solanum Lycopersicum L.) Plants. J. Exp. Bot. 2011, 62, 125–140. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Al-Otaibi, H.H.; Parmar, A.; Ramadan, K.; Lobato, A.K.d.S.; El-Mogy, M.M. Application of Potassium Humate and Salicylic Acid to Mitigate Salinity Stress of Common Bean. Life 2023, 13, 448. [Google Scholar] [CrossRef]
- Farag, H.A.; Ibrahim, M.F.; El-Yazied, A.A.; El-Beltagi, H.S.; El-Gawad, H.G.A.; Alqurashi, M.; Shalaby, T.A.; Mansour, A.T.; Alkhateeb, A.A.; Farag, R. Applied Selenium as a Powerful Antioxidant to Mitigate the Harmful Effects of Salinity Stress in Snap Bean Seedlings. Agronomy 2022, 12, 3215. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Yazied, A.A.; El-Gawad, H.G.A.; Kandeel, M.; Shalaby, T.A.; Mansour, A.T.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Alkhateeb, A.A.; Ibrahim, M.F.M. Synergistic Impact of Melatonin and Putrescine Interaction in Mitigating Salinity Stress in Snap Bean Seedlings: Reduction of Oxidative Damage and Inhibition of Polyamine Catabolism. Horticulturae 2023, 9, 285. [Google Scholar] [CrossRef]
- Afify, A.M.M.R.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012, 2, S772–S780. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Zhao, S.; Liu, Z.; Feng, Y.-Q.; Wu, Y. Cytokinin Antagonizes ABA Suppression to Seed Germination of Arabidopsis by Downregulating ABI5 Expression. Plant J. 2011, 68, 249–261. [Google Scholar] [CrossRef]
- Xu, Y.; Burgess, P.; Zhang, X.; Huang, B. Enhancing Cytokinin Synthesis by Overexpressing Ipt Alleviated Drought Inhibition of Root Growth through Activating ROS-Scavenging Systems in Agrostis Stolonifera. J. Exp. Bot. 2016, 67, 1979–1992. [Google Scholar] [CrossRef]
- Gashaw, A.; Theerawitaya, C.; Samphumphuang, T.; Cha-um, S.; Supaibulwatana, K. CPPU Elevates Photosynthetic Abilities, Growth Performances and Yield Traits in Salt Stressed Rice (Oryza Sativa L. Spp. Indica) via Free Proline and Sugar Accumulation. Pestic. Biochem. Physiol. 2014, 108, 27–33. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Xiao, X.O.; Zeng, Y.M.; Cao, B.H.; Lei, J.J.; Chen, Q.H.; Meng, C.M.; Cheng, Y.J. PSAG12-IPT Overexpression in Eggplant Delays Leaf Senescence and Induces Abiotic Stress Tolerance. J. Hortic. Sci. Biotechnol. 2017, 92, 349–357. [Google Scholar] [CrossRef]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The Antagonistic Action of Abscisic Acid and Cytokinin Signaling Mediates Drought Stress Response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Gujjar, R.S.; Supaibulwatana, K. The Mode of Cytokinin Functions Assisting Plant Adaptations to Osmotic Stresses. Plants 2019, 8, 542. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Samea-Andabjadid, S. Exogenous Cytokinin and Salicylic Acid Improve Amino Acid Content and Composition of Faba Bean Seeds Under Salt Stress. Gesunde Pflanz. 2022, 74, 935–945. [Google Scholar] [CrossRef]
- Gujjar, R.S.; Banyen, P.; Chuekong, W.; Worakan, P.; Roytrakul, S.; Supaibulwatana, K. A Synthetic Cytokinin Improves Photosynthesis in Rice under Drought Stress by Modulating the Abundance of Proteins Related to Stomatal Conductance, Chlorophyll Contents, and Rubisco Activity. Plants 2020, 9, 1106. [Google Scholar] [CrossRef]



| Season | Irrigation Regime | Treatments | Plant Height (cm) | Number of Leaves Plant−1 | Number of Branches Plant−1 | Leaf Area (dm2) | Dry Matter Plant−1 (g) | |
|---|---|---|---|---|---|---|---|---|
| 2019/2020 | FI | H0 | C0 | 87.1 ± 0.88 d | 97.0 ± 1.16 d | 5.56 ± 0.11 b | 189.7 ± 0.94 g | 49.7 ± 0.81 f |
| C25 | 90.7 ± 0.33 c | 101.3 ± 0.88 c | 5.89 ± 0.22 ab | 233.3 ± 1.3 b | 56.9 ± 1.1 bc | |||
| H10 | C0 | 95.7 ± 0.33 b | 106.6 ± 0.37 b | 6.33 ± 0.33 a | 227.6 ± 0.79 c | 58.0 ± 0.20 b | ||
| C25 | 103.0 ± 0.58 a | 114.9 ± 0.64 a | 6.00 ± 0.11 ab | 245.0 ± 1.37 a | 62.5 ± 0.35 a | |||
| DI | H0 | C0 | 75.2 ± 0.67 e | 73.7 ± 2.6 g | 4.00 ± 0.00 c | 178.6 ± 1.58 h | 45.61 ± 0.40 g | |
| C25 | 86.6 ± 0.67 d | 83.3 ± 1.8 f | 6.00 ± 0.33 ab | 205.5 ±1.58 f | 52.3 ± 0.40 e | |||
| H10 | C0 | 90.3 ± 0.33 c | 87.6 ± 0.32 e | 5.67 ± 0.32 b | 214.4 ± 0.79 e | 54.8 ± 0.20 d | ||
| C25 | 92.0 ± 0.58 c | 89.3 ± 0.56 e | 6.00 ± 0.33 ab | 218.3 ± 1.37 d | 55.8± 0.35 cd | |||
| 2020/2021 | FI | H0 | C0 | 84.5 ± 2.02 de | 90.3 ± 3.38 d | 4.3 ± 0.33 c | 184.1 ± 2.65 ef | 48.2 ± 0.92 d |
| C25 | 94.7 ± 4.33 bc | 93.8 ± 0.87 c | 4.6 ± 0.32 bc | 243.3 ± 10.3 ab | 59.5 ± 3.5 ab | |||
| H10 | C0 | 96.7 ± 0.33 b | 108.8 ± 0.32 b | 5.6 ± 0.33 a | 230.0 ± 0.97 bc | 58.6 ± 0.20 abc | ||
| C25 | 104.0 ± 0.53 a | 116.9 ± 0.56 a | 6.0 ± 0.31 a | 247.4 ± 1.37 a | 63.1 ± 0.5 a | |||
| DI | H0 | C0 | 73.7 ± 0.88 f | 78.3 ± 0.33 e | 3.7 ± 0.34 d | 174.8 ± 2.1 f | 44.7 ± 0.53 d | |
| C25 | 80.3 ± 2.7 e | 83.5 ± 6.7 de | 4.3 ± 0.33 c | 190.7 ± 6.3 e | 48.7 ± 1.6 d | |||
| H10 | C0 | 89.3 ± 0.33 cd | 86.7 ± 0.37 cde | 4.6 ± 0.29 bc | 212.0 ± 0.97 d | 54.2 ± 0.20 c | ||
| C25 | 91.0 ± 0.58 bc | 88.3 ± 0.64 cd | 5.0 ± 0.33 b | 216.0 ± 1.4 cd | 55.2 ± 0.35 bc | |||
| Season | Irrigation Regime | Treatments | SPAD | Fv/Fm | Performance Index | Relative Water Content | Membrane Stability Index | |
|---|---|---|---|---|---|---|---|---|
| 2019/2020 | FI | H0 | C0 | 44.6 ± 0.80 b | 0.79 ± 0.01 c | 3.1 ± 0.02 f | 78.34 ± 1.7 c | 39.0 ± 1.3 d |
| C25 | 46.0 ± 1.4 b | 0.82 ± 0.00 b | 5.0 ± 0.12 de | 80.7 ± 0.66 bc | 55.1 ± 1.6 b | |||
| H10 | C0 | 50.7 ± 0.60 a | 0.83 ± 0.03 ab | 6.2 ± 0.87 d | 82.8 ± 0.34 ab | 54.7 ± 1.8 b | ||
| C25 | 51.5 ± 0.23 a | 0.84 ± 0.01 a | 12.2 ± 0.33 a | 84.1 ± 0.55 a | 61.9 ± 1.6 a | |||
| DI | H0 | C0 | 33.8 ± 1.1 d | 0.76 ± 0.01 d | 2.6 ± 0.06 f | 67.1 ± 0.43 e | 34.0 ± 1.1 f | |
| C25 | 40.0 ± 0.573 c | 0.78 ± 0.00 c | 4.8 ± 0.48 e | 74.8 ± 1.2 d | 37.7 ±0.54 e | |||
| H10 | C0 | 46.2 ± 0.53 b | 0.83 ± 0.01 ab | 8.2 ± 1.2 c | 75.5 ± 0.79 d | 41.3 ± 0.88 d | ||
| C25 | 50.8 ± 0.33 a | 0.84 ± 0.01 a | 9.8 ± 0.78 b | 79.3 ± 0.54 c | 45.0 ± 0.58 c | |||
| 2020/2021 | FI | H0 | C0 | 43.4 ± 0.40 d | 0.80 ± 0.01 b | 2.9 ± 0.06 f | 79.6 ± 1.6 c | 36.0 ± 0.89 f |
| C25 | 45.9 ± 1.3 cd | 0.82 ± 0.01 ab | 4.9 ± 0.50 e | 81.6 ± 1.3 bc | 57.1 ± 1.1 b | |||
| H10 | C0 | 50.5 ± 0.81 ab | 0.82 ± 0.01 ab | 5.9 ± 0.33 de | 83.7 ± 0.79 ab | 56.7 ± 0.88 b | ||
| C25 | 52.1 ± 0.57 a | 0.83 ± 0.01 a | 12.8 ± 0.58 a | 85.1± 1.5 a | 63.9 ± 0.58 a | |||
| DI | H0 | C0 | 33.3 ± 1.3 e | 0.76 ± 0.01 d | 2.8 ± 0.20 f | 68.2 ± 1.4 e | 29.3 ± 0.33 g | |
| C25 | 48.5 ± 1.2 bc | 0.79 ± 0.01 c | 6.3 ± 0.80 cd | 74.7 ± 1.2 d | 39.7 ± 0.33 e | |||
| H10 | C0 | 51.6 ± 1.1 a | 0.84 ± 0.01 a | 7.6 ± 0.25 c | 77.3 ± 1.7 d | 43.3 ± 0.86 d | ||
| C25 | 50.1 ± 0.55 ab | 0.84 ± 0.01 a | 9.3 ± 0.82 b | 80.0 ± 0.54 c | 47.0 ± 1.1 c | |||
| Season | Irrigation Regime | Treatments | Nitrogen mg/g DW | Phosphorus mg/g DW | Potassium mg/g DW | |
|---|---|---|---|---|---|---|
| 2019/2020 | FI | H0 | C0 | 16.59 ± 0.30 d | 3.87 ± 0.10 e | 14.47 ± 0.46 c |
| C25 | 19.52 ± 0.24 b | 5.19 ± 0.16 bc | 17.74 ± 0.50 b | |||
| H10 | C0 | 18.36 ± 0.25 c | 4.87 ± 0.06 cd | 15.79 ± 0.45 c | ||
| C25 | 21.49 ± 0.24 a | 5.87 ± 0.15 a | 20.15 ± 0.51 a | |||
| DI | H0 | C0 | 9.82 ± 0.59 g | 2.50 ± 0.29 g | 12.46 ± 0.92 d | |
| C25 | 15.15 ± 0.53 e | 4.60 ± 0.31 d | 15.67 ± 0.33 c | |||
| H10 | C0 | 11.92 ± 0.59 f | 3.28 ± 0.28 f | 14.12 ± 0.60 cd | ||
| C25 | 17.49 ± 0.52 cd | 5.60 ± 0.30 ab | 17.76 ± 0.33 b | |||
| 2020/2021 | FI | H0 | C0 | 17.69 ± 0.30 c | 4.11 ± 0.06 d | 15.56 ± 0.45 de |
| C25 | 20.51 ± 0.23 b | 5.51± 0.15 ab | 18.83 ± 0.51 bc | |||
| H10 | C0 | 19.66 ± 0.20 b | 5.01 ±0.10 bc | 17.09 ± 0.46 cd | ||
| C25 | 21.88 ± 0.20 a | 6.03 ± 0.16 a | 21.45 ± 0.50 a | |||
| DI | H0 | C0 | 10.81 ± 0.58 f | 2.67 ± 0.28 f | 13.96 ± 0.91 e | |
| C25 | 16.14 ± 0.52 d | 4.73 ± 0.30 c | 17.17 ± 0.30 cd | |||
| H10 | C0 | 12.81 ± 0.58 e | 3.43 ± 0.30 e | 15.77 ± 0.60 de | ||
| C25 | 18.38 ± 0.53 c | 5.80 ± 0.30 a | 19.41 ± 0.30 b | |||
| Season | Irrigation Regime | Treatments | Number of Pods Plant−1 | Weight of 100 Seeds (g) | Seed Yield (t ha−1) | |
|---|---|---|---|---|---|---|
| 2019/2020 | FI | H0 | C0 | 12.3 ± 0.67 de | 80.7 ± 0.47 c | 4.10 ± 0.08 ef |
| C25 | 17.6 ± 0.89 ab | 87.6 ± 2.50 b | 4.40 ± 0.03 cd | |||
| H10 | C0 | 15.7 ± 0.67 bc | 89.7 ± 0.82 b | 4.53 ± 0.04 bc | ||
| C25 | 18.3 ± 0.67 a | 94.6 ± 0.42 a | 4.78 ± 0.02 a | |||
| DI | H0 | C0 | 12.0 ± 0.51 e | 73.7 ± 0.64 d | 3.98 ± 0.02 f | |
| C25 | 14.9 ± 0.48 c | 79.0 ± 0.60 c | 4.27 ± 0.03 de | |||
| H10 | C0 | 14.3 ± 0.33 cd | 86.7 ± 0.74 b | 4.53 ± 0.04 bc | ||
| C25 | 15.7 ± 0.67 bc | 88.3 ± 2.33 b | 4.78 ± 0.13 a | |||
| 2020/2021 | FI | H0 | C0 | 13.7 ± 1.33 cd | 82.0 ± 0.50 d | 4.05 ± 0.13 d |
| C25 | 17.0 ± 0.67 ab | 90.6 ± 2.70 b | 4.33 ± 0.07 b | |||
| H10 | C0 | 17.3 ± 0.58 ab | 87.0 ± 0.50 bc | 4.57 ± 0.09 ab | ||
| C25 | 19.7 ± 0.88 a | 96.8 ± 0.77 a | 4.67 ± 0.07 a | |||
| DI | H0 | C0 | 12.0 ± 1.00 d | 76.2 ± 0.61 e | 3.76 ± 0.03 e | |
| C25 | 15.0 ± 1.00 bc | 84.3 ± 1.67 cd | 4.13 ± 0.03 cd | |||
| H10 | C0 | 16.6 ± 0.35 b | 87.0 ± 0.58 bc | 4.57 ± 0.07 ab | ||
| C25 | 17.6 ± 0.74 ab | 89.3 ± 0.33 b | 4.63 ± 0.09 a | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadan, K.M.A.; El-Beltagi, H.S.; El-Mageed, T.A.A.; Saudy, H.S.; Al-Otaibi, H.H.; Mahmoud, M.A.A. The Changes in Various Physio-Biochemical Parameters and Yield Traits of Faba Bean Due to Humic Acid Plus 6-Benzylaminopurine Application under Deficit Irrigation. Agronomy 2023, 13, 1227. https://doi.org/10.3390/agronomy13051227
Ramadan KMA, El-Beltagi HS, El-Mageed TAA, Saudy HS, Al-Otaibi HH, Mahmoud MAA. The Changes in Various Physio-Biochemical Parameters and Yield Traits of Faba Bean Due to Humic Acid Plus 6-Benzylaminopurine Application under Deficit Irrigation. Agronomy. 2023; 13(5):1227. https://doi.org/10.3390/agronomy13051227
Chicago/Turabian StyleRamadan, Khaled M. A., Hossam S. El-Beltagi, Taia A. Abd El-Mageed, Hani S. Saudy, Hala Hazam Al-Otaibi, and Mohamed A. A. Mahmoud. 2023. "The Changes in Various Physio-Biochemical Parameters and Yield Traits of Faba Bean Due to Humic Acid Plus 6-Benzylaminopurine Application under Deficit Irrigation" Agronomy 13, no. 5: 1227. https://doi.org/10.3390/agronomy13051227








