Response of Rice Grain Yield and Soil Fertility to Fertilization Management under Three Rice-Based Cropping Systems in Reclaimed Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Materials
2.3. Cropping System
2.4. Fertilization Management
2.5. Plant and Soil Analysis
2.6. Statistical Analysis
3. Results
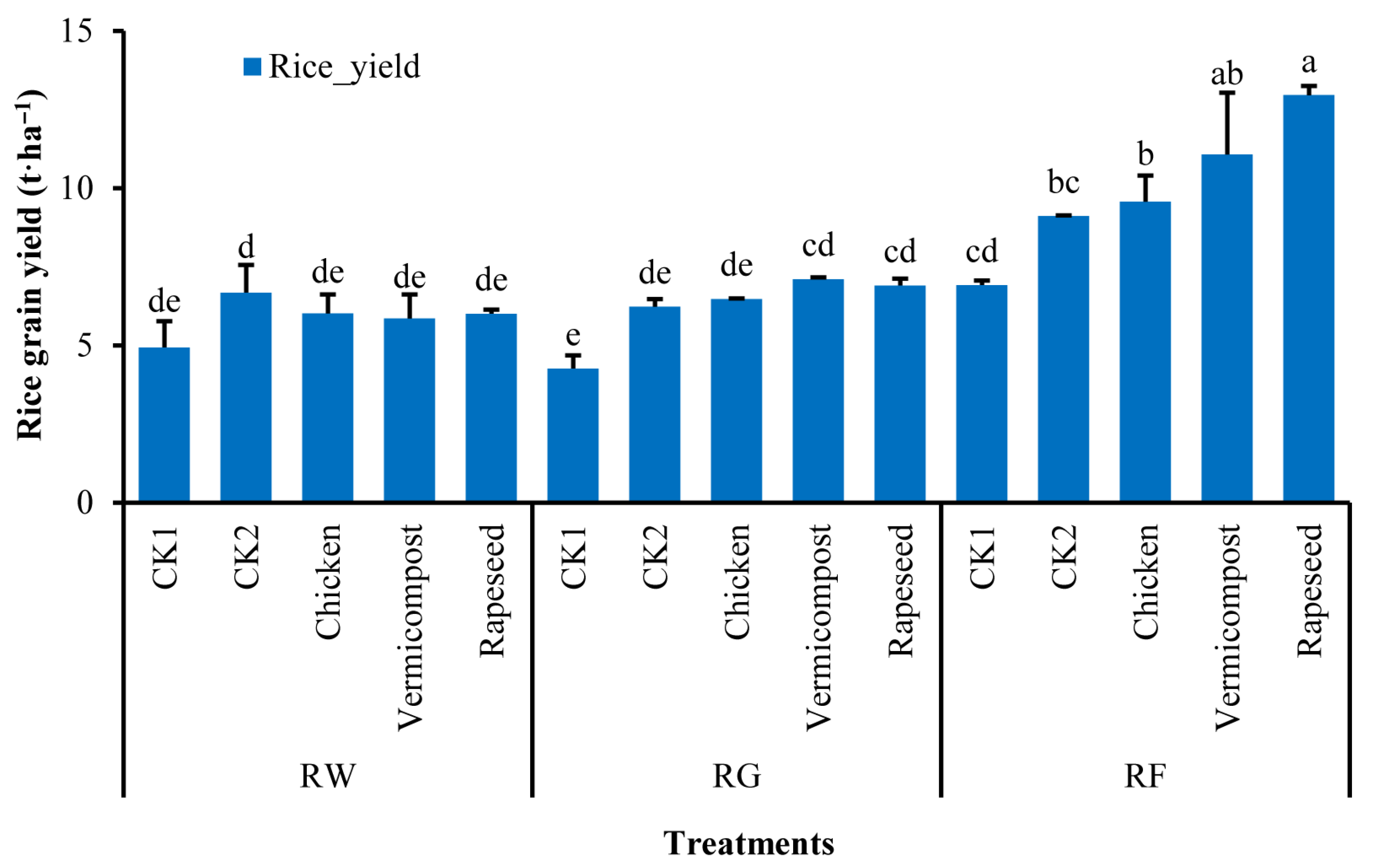
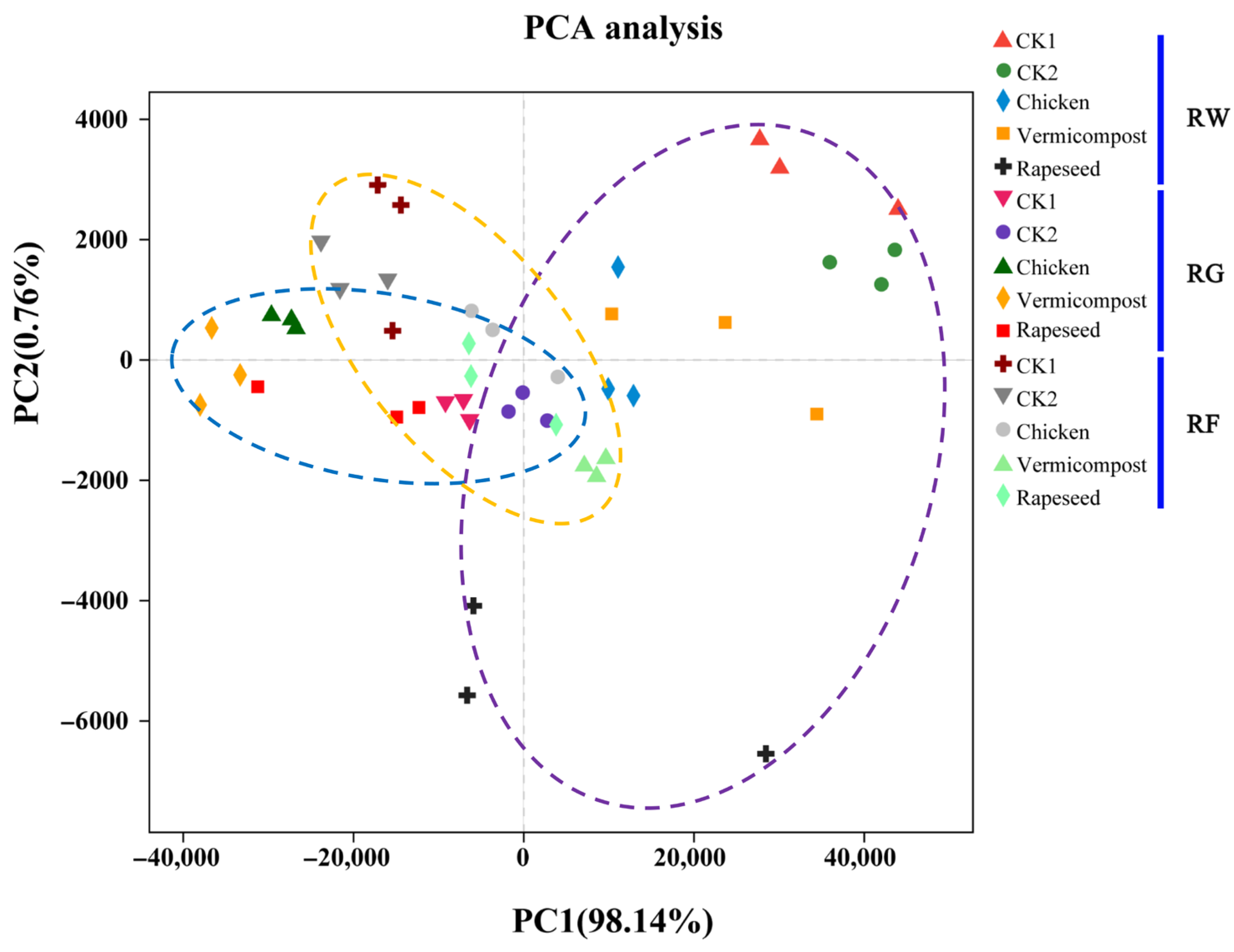
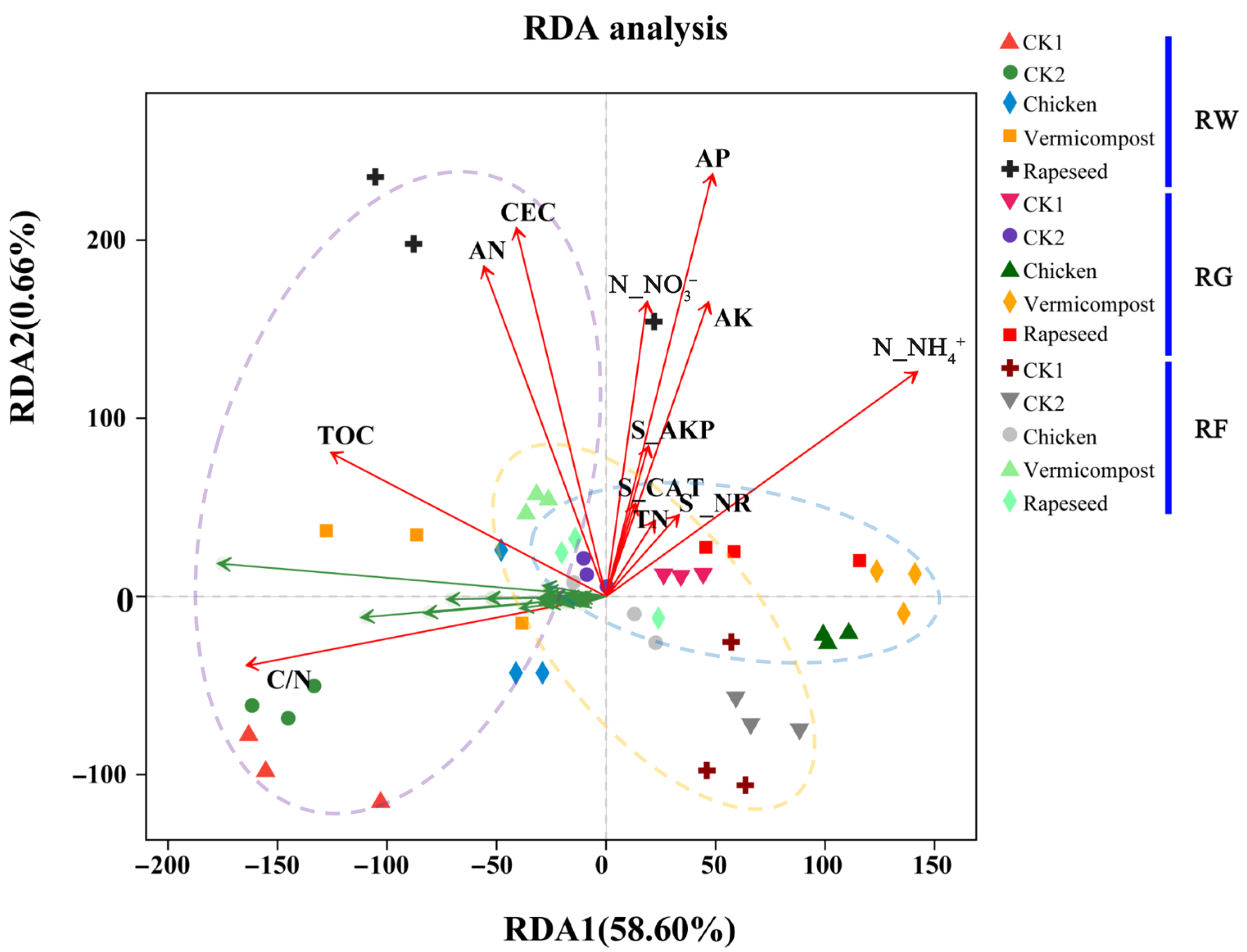
3.1. Rice Grain Field and Soil Physic-Chemical Properties
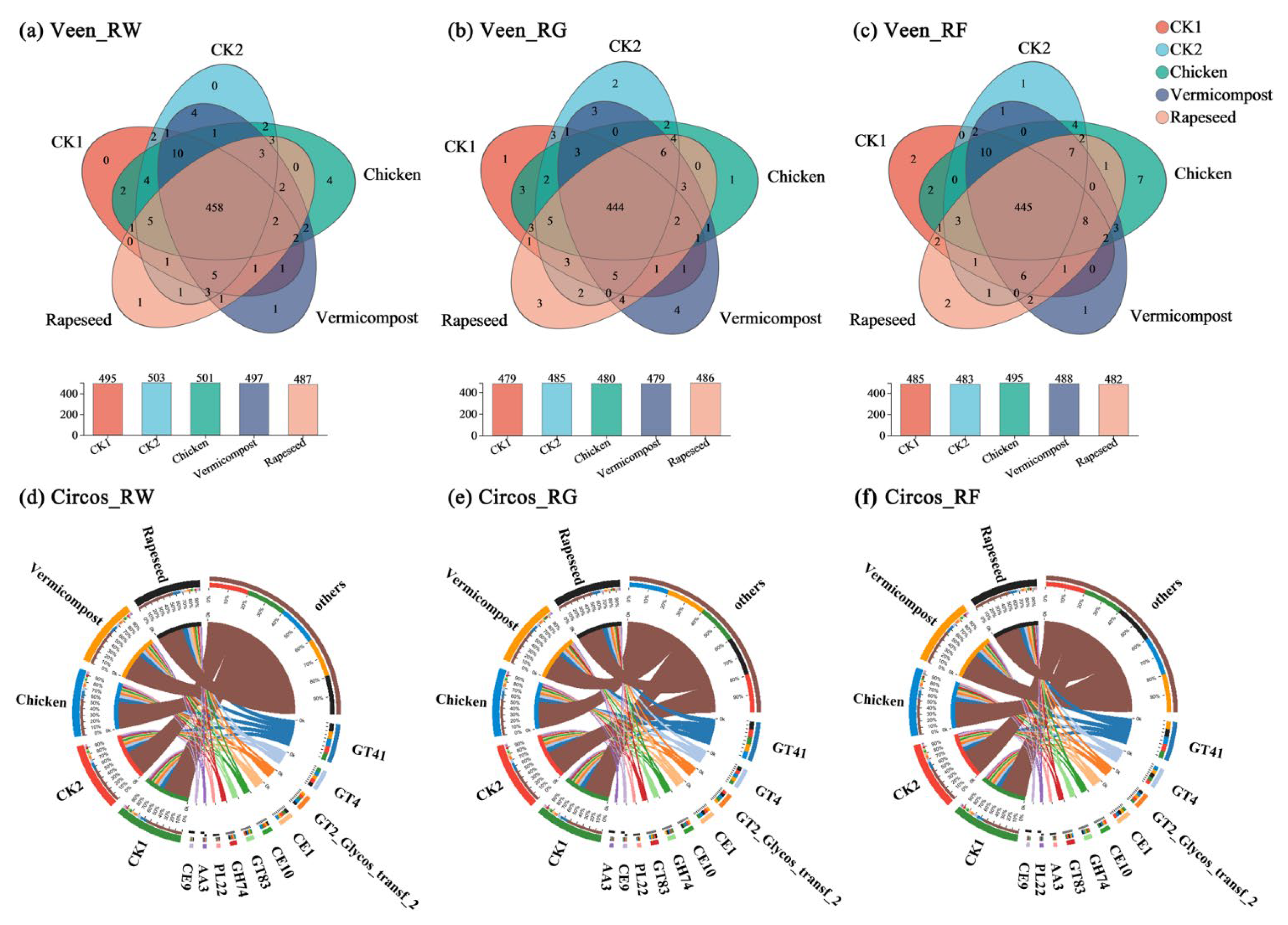
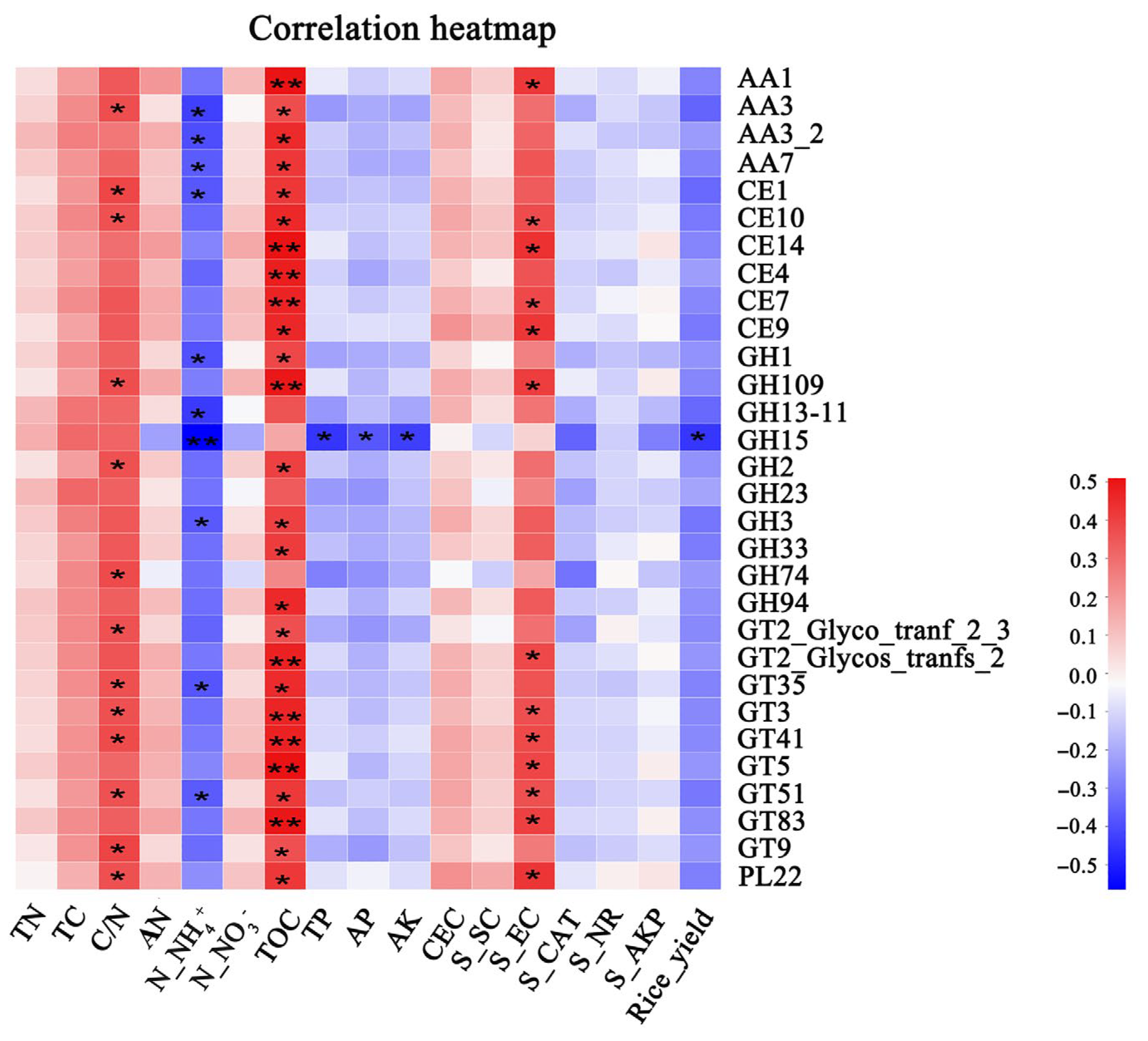
3.2. Functional Composition of Carbohydrate-Active Enzymes
4. Discussion
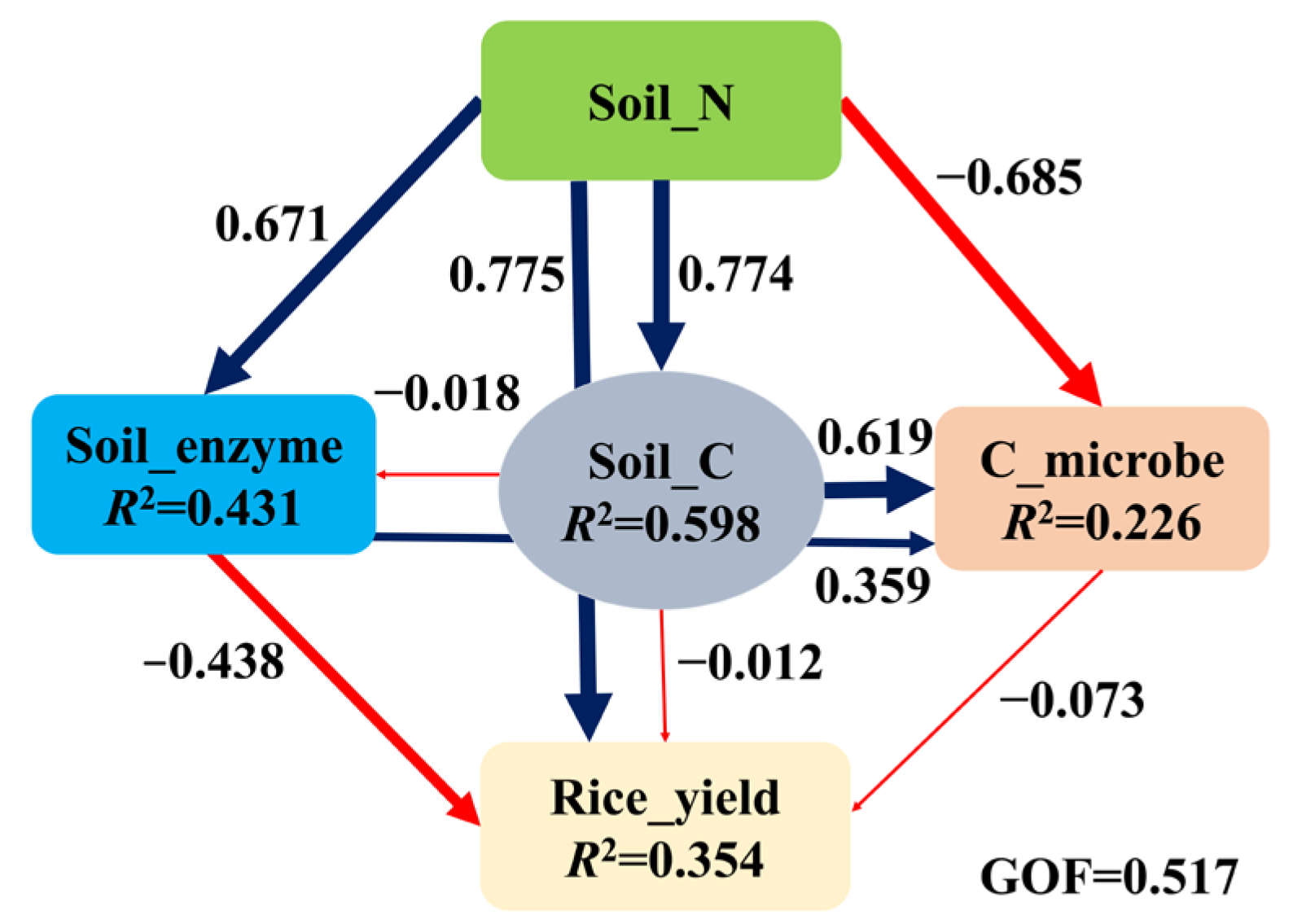
4.1. Effects of Cropping Systems and Organic Fertilizers on Soil Properties and Rice Grain Yield
4.2. Responses of Carbohydrate-Active Enzymes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Assefa, S.; Tadesse, S. The principle role of organic fertilizer on soil properties and agricultural productivity—A review. Agric. Res. Technol. 2019, 22, 556192. [Google Scholar] [CrossRef]
- Tang, Q.; Cotton, A.; Wei, Z.; Xia, Y.; Daniell, T.; Yan, X. How does partial substitution of chemical fertilizer with organic forms increase sustainability of agricultural production? Sci. Total Environ. 2022, 803, 149933. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, X.; Pan, H.; Zhang, X.; Cao, H.; Ulgiati, S.; Wu, J.; Zhang, Y.; Wang, G.; Xiao, Y. Impact of fertilization schemes with different ratios of urea to controlled release nitrogen fertilizer on environmental sustainability, nitrogen use efficiency and economic benefit of rice production: A study case from Southwest China. J. Clean. Prod. 2021, 293, 126198. [Google Scholar] [CrossRef]
- Shaji, H.; Chandran, V.; Mathew, L. Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 231–245. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Dutt, D.; Singh, W.K.; Upadhyay, P.K.; Meena, A.L.; Kumar, A.; Mishra, R.P.; Dwivedi, B.S.; Shukla, A.K.; Yadav, G.S.; Tewari, R.B.; et al. Long-term impact of organic and inorganic fertilizers on soil organic carbon dynamics in a rice-wheat system. Land Degrad. Dev. 2022, 33, 1862–1877. [Google Scholar] [CrossRef]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: A review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Dincǎ, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Correction: Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0216006. [Google Scholar] [CrossRef]
- Iabal, A.; He, L.; Khan, A.; Wei, S.; Akhtar, K.; Ali, I.; Ullah, S.; Munsif, F.; Zhao, Q.; Jiang, L. Organic manure coupled with inorganic fertilizer: An approach for the sustainable production of rice by improving soil properties and nitrogen use efficiency. Agronomy 2019, 9, 651. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fang, Q.; Zhang, T.; Ma, W.; Velthof, G.L.; Hou, Y.; Oenema, O.; Zhang, F. Benefits and trade-offs of replacing synthetic fertilizers by animal manures in crop production in China: A meta-analysis. Glob. Change Biol. 2020, 26, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Halin, J.; Heiniger, R. Soil fertility management for better crop production. Agronomy 2020, 10, 1349. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential—Global and Irish perspective. Renew. Sustain. Energ. Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Hussain, B.; Ashraf, M.N.; Rahman, S.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kastner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Global Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root effects on soil organic carbon: A double-edged sword. New Phytol. 2021, 230, 60–65. [Google Scholar] [CrossRef]
- Basile-Doelsch, I.; Balesdent, J.; Pellerin, S. Reviews and syntheses: The mechanisms underlying carbon storage in soil. Biogeosciences 2020, 17, 5223–5242. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Bellitürk, K.; Iqbal, N.; Naeem, S.; Idrees, M.; Kaleem, Z.; Nawaz, M.Y.; Nawaz, M.; Sajjad, M.; et al. Vermicomposting methods from different wastes: An environment friendly, economically viable and socially acceptable approach for crop nutrition: A review. Int. J. Food Agric. 2021, 5, 58–68. [Google Scholar] [CrossRef]
- Saba, S.; Zara, G.; Bianco, A.; Garau, M.; Bononi, M.; Deroma, M.; Pais, A.; Budroni, M. Comparative analysis of vermicompost quality produced from brewers’ spend grain and cow manure by the red earthworm Eisenia fetida (Sav.). Bioresour. Technol. 2019, 293, 122019. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Xu, W.; Liu, X.; Li, Y.; Wei, J.; Gao, M.; Bi, J.; Lu, X.; Wang, Z.; et al. Challenges for global sustainable nitrogen management in agricultural systems. J. Agric. Food Chem. 2020, 68, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.M.; Evers, J.B.; Bijma, P.; van Apeldoorn, D.F.; Smulders, M.J.M.; Kuyper, T.W.; Mommer, L.; Bonnema, G. Breeding beyond monoculture: Putting the “intercrop” into crops. Front. Plant Sci. 2021, 12, 734164. [Google Scholar] [CrossRef]
- Shah, K.K.; Modi, B.; Pandey, H.P.; Subedi, A.; Aryal, G.; Pandey, M.; Shrestha, J. Diversified crop rotation: An approach for sustainable agriculture production. Adv. Agric. 2021, 2021, 8924087. [Google Scholar] [CrossRef]
- Huss, C.P.; Holmes, K.D.; Blubaugh, C.K. Benefits and risks of intercropping for crop resilience and pest management. J. Econ. Entomol. 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Bai, N.; Lv, W.; Chu, X.; Fan, H.; Li, S.; Zheng, X.; Zhang, J.; Zhang, H.; Zhang, H. Comparison of soil microbial community and physicochemical properties between rice-fallow and rice-bean (green manure) rotation. Soil Sci. Soc. Am. J. 2022, 86, 593–603. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, X.; Duan, J.; Koide, R.T.; Xu, L.; Chu, J. Variation in microbial CAZyme families across degradation severity in a steppe grassland in northern China. Front. Environ. Sci. 2023, 11, 1080505. [Google Scholar] [CrossRef]
- Ju, J.; Gu, Q.; Zhou, H.; Zhang, H.; Mao, W.; Yang, H.; Mi, W.; Zhao, H. Effects of organic fertilizer combined with chemical fertilizer on nutrients, enzyme activities, and rice yield in reclaimed soil. Commun. Soil Sci. Plant Anal. 2022, 53, 3060–3071. [Google Scholar] [CrossRef]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Agricultural Chemical Analysis Method; China Agricultural Scientech Press: Beijing, China, 1999. [Google Scholar]
- Edwards, A.H. The semi-micro Kjeldahl method for the determination of nitrogen in coal. J. Appl. Chem. 1954, 4, 330–340. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Wen, Y.; You, J.; Zhu, J.; Hu, H.; Gao, J.; Huang, J. Long-term green manure application improves soil K availability in red paddy soil of subtropical China. J. Soil Sediment. 2021, 21, 63–72. [Google Scholar] [CrossRef]
- Bascomb, C.L. Rapid method for the determination of the cation exchange capacity of calcareous and non-calcareous soils. J. Sci. Food Agric. 1964, 15, 821–823. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Zhang, D.Q.; Huang, B.; Song, Z.X.; Ren, L.R.; Hao, B.Q.; Liu, J.; Zhu, J.H.; Fang, W.S.; Yan, D.D.; et al. Organic fertilizer improves soil fertility and restores the bacterial community after 1,3-dichloropropene fumigation. Sci. Total Environ. 2020, 738, 140345. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.J.; Lu, Y.; Xu, Z.G.; Fu, S.L. Enzyme activities of urban soils under different land use in the Shenzhen city, China. Plant Soil Environ. 2008, 4, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Yin, C.; Fan, X.; Gao, Z.; Chen, H.; Tan, L.; Chang, S.X.; Zhao, Y.; Liang, Y. Procyanidin inhibited N2O emissions from paddy soils by affecting nitrate reductase activity and nirS- and nirK-denitrifier populations. Biol. Fertil. Soils 2021, 57, 935–947. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, B.; Dong, Z.; Liu, Y.; Wang, C.; Ye, C. Soil N2O and NOx emissions are directly linked with N-cycling enzymatic activities. Appl. Soil Ecol. 2019, 139, 15–24. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Wu, P.; Entwistle, S.; Li, X.; Yohe, T.; Yi, H.; Yang, Z.; Yin, Y. dbCAN-seq: A database of carbohydrate-active enzyme (CAZyme) sequence and annotation. Nucleic Acids Res. 2017, 46, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Henseler, J.; Sarstedt, M. Goodness-of-fit indices for partial least squares path modeling. Comput. Stat. 2013, 28, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, R.; Gao, J.; Wang, X.; Fan, F.; Ma, X.; Yin, H.; Zhang, C.; Feng, K.; Deng, Y. Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol. Biolchem. 2017, 104, 208–217. [Google Scholar] [CrossRef]
- Hou, P.; Xue, L.; Wang, J.; Petropoulos, E.; Deng, X.; Qiao, J.; Xue, L.; Yang, L. Continuous milk vetch amendment in rice-fallow rotation improves soil fertility and maintains rice yield without increasing CH4 emissions: Evidence from a long-term experiment. Agric. Ecosyst. Environ. 2022, 325, 107774. [Google Scholar] [CrossRef]
- Bruun, T.B.; Mertz, O.; Elberling, B. Linking yields of upland rice in shifting cultivation to fallow length and soil properties. Agric. Ecosyst. Environ. 2006, 113, 139–149. [Google Scholar] [CrossRef]
- Urmi, T.A.; Rahman, M.M.; Islam, M.M.; Islam, M.A.; Jahan, N.A.; Mia, M.A.B.; Akhter, S.; Siddiqui, M.H.; Kalaji, H.M. Integrated nutrient management for rice yield, soil fertility, and carbon sequestration. Plants 2022, 11, 138. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, L.; Wang, W.; Xu, Y.; Zhang, W.; Zhang, H.; Liu, L.; Wang, Z.; Gu, J.; Yang, J. Effects of application of rapeseed cake as organic fertilizer on rice quality at high yield level. J. Sci. Food Agric. 2022, 102, 1832–1841. [Google Scholar] [CrossRef]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility—A review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbonhydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Ramulu, H.G.; Drula, E.; Coutinho, P.M.; Henrissat, B. The Carbonhydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [Green Version]
- Ping, Q.; Zheng, M.; Dai, X.; Li, Y. Metagenomic characterization of the enhanced performance of anaerobic fermentation of waste activated sludge with CaO2 addition at ambient temperature: Fatty acid biosynthesis metabolic pathway and CAZymes. Water Res. 2020, 170, 115309. [Google Scholar] [CrossRef] [PubMed]
- Uluisik, S.; Seymour, G.B. Pectate lyases: Their role in plants and importance in fruit ripening. Food Chem. 2020, 309, 125559. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, D.; Chen, Y.; Wen, H.; Pan, J.; Xiao, T.; Lv, D.; He, H.; Pan, J.; Ci, R.; et al. Mapping and identification of CsSF4, a gene encoding a UDP-N-acetyl glucosamine required for fruit elongation in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2023, 136, 54. [Google Scholar] [CrossRef]
- Ahangar, M.S.; Furze, C.M.; Guy, C.S.; Cooper, C.; Maskew, K.S.; Graham, B.; Cameron, A.D.; Fullam, E. Structural and functional determination of homologs of the Mycobacterium tuberculosis N-acetylglucosamine-6- phosphate deacetylase (NagA). J. Biol. Chem. 2018, 293, 9770–9783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Fang, W.; Wang, Q.; Zubair, M.; Zhang, G.; Ma, W.; Ca, Y.; Zhang, P. Metagenomic analysis of community, enzymes and metabolic pathways during corn straw fermentation with rumen microorganisms for volatile fatty acid production. Bioresour. Technol. 2021, 342, 126004. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Ghosh, T.S.; Mande, S.S. Global profiling of carbohydrate active enzymes in human gut microbiome. PLoS ONE 2015, 10, e0142038. [Google Scholar] [CrossRef] [Green Version]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic efficiency of animal-derived organic fertilizers and their effects on biology and fertility of soil: A review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Mohammadi, K.; Heidari, G.; Khalesro, S.; Sohrabi, Y. Soil management, microorganisms and organic matter interactions: A review. Afr. J. Biotechnol. 2011, 10, 19840–19849. [Google Scholar] [CrossRef] [Green Version]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical fertilizers and their impact on soil health. Microbiota Biofertil. 2021, 2, 1–20. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyaov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Lashermes, G.; Sinasbaugh, R.L. A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition. Soil Biol. Biochem. 2012, 53, 133–141. [Google Scholar] [CrossRef]

| Treatment | N (kg ha−1) | P (kg ha−1) | K (kg ha−1) | Total (kg ha−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Slow-Release Fertilizer | Organic Fertilizer | Slow-Release Fertilizer | Organic Fertilizer | Slow-Release Fertilizer | Organic Fertilizer | N | P | K | |
| CK1 | 0 | 0 | 75 | 0 | 150 | 0 | 0 | 75 | 150 |
| CK2 | 300 | 0 | 75 | 0 | 150 | 0 | 300 | 75 | 150 |
| Chicken | 180 | 120 | 0 | 90 | 97 | 53 | 300 | 90 | 150 |
| Vermicompost | 180 | 120 | 0 | 102 | 90 | 60 | 300 | 102 | 150 |
| Rapeseed | 180 | 120 | 9 | 66 | 104 | 46 | 300 | 75 | 150 |
| Characteristics | Sums of Sqs | Mean Sqs | F_Model | R2 | p Value |
|---|---|---|---|---|---|
| Cropping systems | 0.18 | 0.09 | 20.48 | 0.60 | 0.001 *** |
| RW (C-O) | 0.02 | 0.02 | 7.91 | 0.50 | 0.014 * |
| RW (O-O) | 0.01 | 0.00 | 0.70 | 0.19 | 0.54 |
| RG (C-O) | 0.04 | 0.04 | 21.41 | 0.73 | 0.007 ** |
| RG (O-O) | 0.02 | 0.01 | 2.67 | 0.47 | 0.13 |
| RF (C-O) | 0.02 | 0.02 | 20.17 | 0.72 | 0.004 ** |
| RF (O-O) | 0.00 | 0.00 | 0.58 | 0.16 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Zhang, T.; Wang, G.; Ju, J.; Mao, W.; Zhao, H. Response of Rice Grain Yield and Soil Fertility to Fertilization Management under Three Rice-Based Cropping Systems in Reclaimed Soil. Agronomy 2023, 13, 1840. https://doi.org/10.3390/agronomy13071840
Liu P, Zhang T, Wang G, Ju J, Mao W, Zhao H. Response of Rice Grain Yield and Soil Fertility to Fertilization Management under Three Rice-Based Cropping Systems in Reclaimed Soil. Agronomy. 2023; 13(7):1840. https://doi.org/10.3390/agronomy13071840
Chicago/Turabian StyleLiu, Ping, Tingyu Zhang, Guiliang Wang, Jing Ju, Wei Mao, and Haitao Zhao. 2023. "Response of Rice Grain Yield and Soil Fertility to Fertilization Management under Three Rice-Based Cropping Systems in Reclaimed Soil" Agronomy 13, no. 7: 1840. https://doi.org/10.3390/agronomy13071840






