Opportunities and Challenges of Castor Bean (Ricinus communis L.) Genetic Improvement
Abstract
:1. Introduction
2. Castor Bean Chemical Profile
2.1. Oil Lipid Profile
2.2. Noxious Compounds: Ricine and Its Homologue
3. Uses
4. Agronomy
5. Genetic Resources
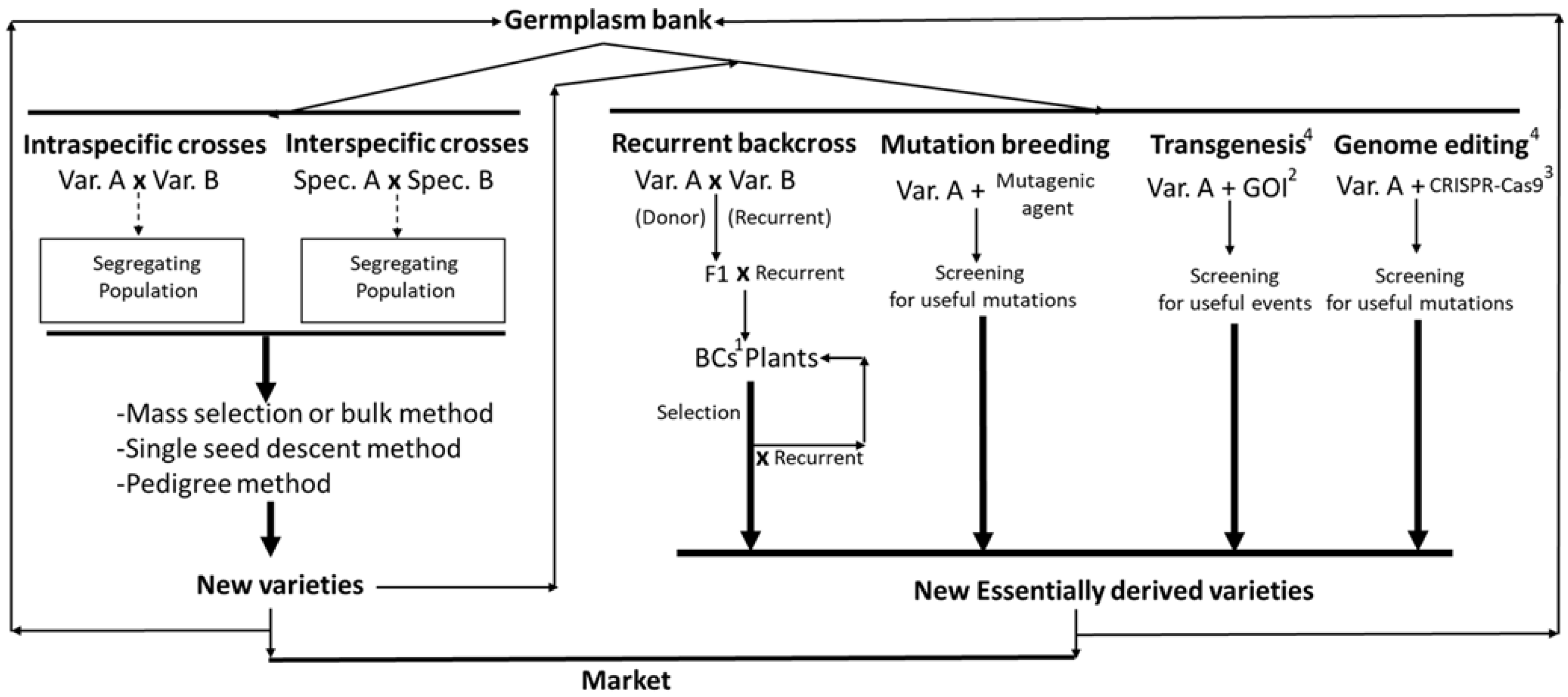
6. Genetic Improvement
6.1. Classical Breeding
6.2. New Breeding Techniques for Oleaginous Crops: GMO Technology and Genome Editing
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, E.A. Oilseed Crops; Blackwell Science: Oxford, UK, 2000; ISBN 0-632-05259-7. [Google Scholar]
- Chakrabarty, S.; Kalam Mohammad Aminul Islam, A.; Yaakob, Z.; Kalam Mohammad Mominul Islam, A. Castor (Ricinus communis): An Underutilized Oil Crop in the South East Asia. In Agroecosystems—Very Complex Environmental Systems; IntechOpen: London, UK, 2021. [Google Scholar]
- Xu, W.; Wu, D.; Yang, T.; Sun, C.; Wang, Z.; Han, B.; Wu, S.; Yu, A.; Chapman, M.A.; Muraguri, S.; et al. Genomic Insights into the Origin, Domestication and Genetic Basis of Agronomic Traits of Castor Bean. Genome Biol. 2021, 22, 113. [Google Scholar] [CrossRef]
- Zimmerman, L.H.; Smith, J.D. Production of F1 Seed in Castorbeans by Use of Sex Genes Sensitive to Environment. Crop Sci. 1966, 6, 406–409. [Google Scholar] [CrossRef]
- Shifriss, O. Growth and Sexuality of Ricinus communis L. in a Constant Environment. Am. Nat. 1964, 98, 187–189. [Google Scholar] [CrossRef]
- Yeboah, A.; Ying, S.; Lu, J.; Xie, Y.; Amoanimaa-Dede, H.; Boateng, K.G.A.; Chen, M.; Yin, X. Castor Oil (Ricinus communis): A Review on the Chemical Composition and Physicochemical Properties. Food Sci. Technol. 2021, 41, 399–413. [Google Scholar] [CrossRef]
- Castor|Kaiima. Available online: https://kaiima.com/castor/ (accessed on 10 May 2023).
- da Silva Ramos, L.C.; Tango, J.S.; Savi, A.; Leal, N.R. Variability for Oil and Fatty Acid Composition in Castorbean Varieties. J. Am. Oil Chem. Soc. 1984, 61, 1841–1843. [Google Scholar] [CrossRef]
- Ogunniyi, D.S. Castor Oil: A Vital Industrial Raw Material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, H.; Meier, M.A.R. Castor Oil as a Renewable Resource for the Chemical Industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Tumer, N. Ricin Toxins. Toxins 2020, 12, 13. [Google Scholar] [CrossRef] [Green Version]
- Nagler-Anderson, C. Man the Barrier! STRATEGIC DEFENCES in the Intestinal Mucosa. Nat. Rev. Immunol. 2001, 1, 59–67. [Google Scholar] [CrossRef]
- Lappi, D.A.; Kapmeyer, W.; Beglau, J.M.; Kaplan, N.O. The Disulfide Bond Connecting the Chains of Ricin. Proc. Natl. Acad. Sci. USA 1978, 75, 1096. [Google Scholar] [CrossRef]
- Endo, Y.; Tsurugi, K. RNA N-Glycosidase Activity of Ricin A-Chain. Mechanism of Action of the Toxic Lectin Ricin on Eukaryotic Ribosomes. J. Biol. Chem. 1987, 262, 8128–8130. [Google Scholar] [CrossRef] [PubMed]
- Parikh, B.A.; Tortora, A.; Li, X.P.; Tumer, N.E. Ricin Inhibits Activation of the Unfolded Protein Response by Preventing Splicing of the HAC1 mRNA. J. Biol. Chem. 2008, 283, 6145–6153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsnes, S.; Fernandez-Puentes, C.; Carrasco, L.; Vazquez, D. Ribosome Inactivation by the Toxic Lectins Abrin and Ricin. Kinetics of the Enzymic Activity of the Toxin A-Chains. Eur. J. Biochem. 1975, 60, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Severino, L.S.; Auld, D.L.; Baldanzi, M.; Cândido, M.J.D.; Chen, G.; Crosby, W.; Tan, D.; He, X.; Lakshmamma, P.; Lavanya, C.; et al. A Review on the Challenges for Increased Production of Castor. Agron. J. 2012, 104, 853–880. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Pan, C.; Fan, W.; Liu, W.; Zhao, H.; Li, D.; Wang, S.; Hu, L.; He, B.; Qian, K.; et al. A Chromosome-Level Genome Assembly of Wild Castor Provides New Insights into Its Adaptive Evolution in Tropical Desert. Genom. Proteom. Bioinform. 2022, 20, 42–59. [Google Scholar] [CrossRef]
- Ready, M.P.; Kim, Y.; Robertus, J.D. Site-Directed Mutagenesis of Ricin A-Chain and Implications for the Mechanism of Action. Proteins Struct. Funct. Bioinform. 1991, 10, 270–278. [Google Scholar] [CrossRef]
- Madeira, J.V.; Macedo, J.A.; Macedo, G.A. Detoxification of Castor Bean Residues and the Simultaneous Production of Tannase and Phytase by Solid-State Fermentation Using Paecilomyces variotii. Bioresour. Technol. 2011, 102, 7343–7348. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, K.V.; Deus-de-Oliveira, N.; Godoy, M.G.; Guimarães, Z.A.S.; Nascimento, V.V.; de Melo, E.J.T.; Freire, D.M.G.; Dansa-Petretski, M.; Machado, O.L.T. Simultaneous Allergen Inactivation and Detoxification of Castor Bean Cake by Treatment with Calcium Compounds. Braz. J. Med. Biol. Res. 2012, 45, 1002. [Google Scholar] [CrossRef] [Green Version]
- Harley, S.M.; Beevers, H. Lectins in Castor Bean Seedlings. Plant Physiol. 1986, 80, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sotelo-Leyva, C.; Salinas-Sánchez, D.O.; Peña-Chora, G.; Trejo-Loyo, A.G.; González-Cortázar, M.; Zamilpa, A. Insecticidal Compounds in Ricinus communis L. (Euphorbiaceae) to Control Melanaphis sacchari Zehntner (Hemiptera: Aphididae). Fla. Entomol. 2020, 103, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Salem, N.; Bachrouch, O.; Sriti, J.; Msaada, K.; Khammassi, S.; Hammami, M.; Selmi, S.; Boushih, E.; Koorani, S.; Abderraba, M.; et al. Fumigant and Repellent Potentials of Ricinus communis and Mentha pulegium Essential Oils against Tribolium castaneum and Lasioderma serricorne. Int. J. Food Prop. 2018, 20, S2899–S2913. [Google Scholar] [CrossRef] [Green Version]
- Salinas-Sánchez, D.O.; Flores-Franco, G.; Aviles-Montes, D.; Valladares-Cisneros, M.G.; Ataide, D.M.A.; Mendoza-Catalan, M.A.; Sotelo-Leyva, C. Bioactivity of a Linoleic Acid-Rich Fraction of Ricinus communis L. (Euphorbiaceae) Leaves against the Yellow Sugarcane Aphid, Sipha flava (Hemiptera: Aphididae). J. Food Prot. 2021, 84, 1524–1527. [Google Scholar] [CrossRef]
- Elhaj, W.E.; Osman, A.A.; Elawad, L.M.E. Efficacy of Ricinus communis L., Cassia occidentalis L. and Bacillus thuringiensis against Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). J. Agron. Res. 2021, 3, 46–53. [Google Scholar] [CrossRef]
- Kouakou, Y.Y.F.R.; Kra, K.D.; Diallo, H.A. Effects of Two-Dried Castor Leaf Formulations on the Population Dynamics and Pathological Activities of Root-Lesion and Root-Knot Nematodes on Water Yam. Int. J. Plant Soil Sci. 2023, 35, 33–41. [Google Scholar] [CrossRef]
- Yao, Y.; Regis, F.; Kra, K.D.; Assiri, K.P. Nematicidal Effectiveness of Products Stemming from Dried Leaves of Castor-Oil Plant (Ricinus communis L.) on Meloidogyne and Pratylenchus, Yam Pathogenic Nematodes in Côte d’ Ivoire. Int. J. Agron. Agric. Res. 2017, 11, 57–68. [Google Scholar]
- Izidoro, A., Jr.; Silva, E.J.; Tarini, G.; Bordin, J.C.; Silva, B.A.; Ambrosano, L.; Dias-Arieira, C.R. Aqueous Extract of Castor Bean Seed Cake for the Control of Pratylenchus Brachyurus in Soybean. Nematropica 2021, 51, 1–8. [Google Scholar]
- Adomako, J.; Kwoseh, C. Effect of Castor Bean (Ricinus communis L.) Aqueous Extracts on the Performance of Root-Knot Nematodes (Meloideogyne spp.) on Tomato (Solanum lycopersicum L.). J. Sci. Technol. 2013, 33, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.A.; Kinneary, J.F. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. J. Hazard. Mater. 1996, 30, 373. [Google Scholar]
- Oprea, S. Synthesis and Properties of Polyurethane Elastomers with Castor Oil as Crosslinker. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 313–320. [Google Scholar] [CrossRef]
- Yao, L.; Hammond, E.G.; Wang, T.; Bhuyan, S.; Sundararajan, S. Synthesis and Physical Properties of Potential Biolubricants Based on Ricinoleic Acid. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 937–945. [Google Scholar] [CrossRef]
- Zeng, Q. The Lubrication Performance and Viscosity Behavior of Castor Oil under High Temperature. Green Mater. 2021, 10, 51–58. [Google Scholar] [CrossRef]
- Sen, B.; Gupta, M.K.; Mia, M.; Pimenov, D.Y.; Mikolajczyk, T. Performance Assessment of Minimum Quantity Castor-Palm Oil Mixtures in Hard-Milling Operation. Materials 2021, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Gallego, R.; Romero, M.A.; González-Delgado, J.A.; Arteaga, J.F.; Valencia, C.; Franco, J.M. Impact of Natural Sources-Derived Antioxidants on the Oxidative Stability and Rheological Properties of Castor Oil Based-Lubricating Greases. Ind. Crop. Prod. 2016, 87, 297–303. [Google Scholar] [CrossRef]
- Morris, B. Phytochemical Traits in the Genetic Resources of Castor Bean. Curr. Top. Plant Biol. 2004, 5, 63–67. [Google Scholar]
- Pecina-Quintero, V.; Anaya-López, J.L.; Núñez-Colín, C.A.; Zamarripa-Colmenero, A.; Montes-García, N.; Solís-Bonilla, J.L.; Aguilar-Rangel, M.R. Assessing the Genetic Diversity of Castor Bean from Chiapas, México Using SSR and AFLP Markers. Ind. Crop. Prod. 2013, 41, 134–143. [Google Scholar] [CrossRef]
- Gowda, N.K.S.; Pal, D.T.; Bellur, S.R.; Bharadwaj, U.; Sridhar, M.; Satyanarayana, M.L.; Prasad, C.S.; Ramachandra, K.S.; Sampath, K.T. Evaluation of Castor (Ricinus communis) Seed Cake in the Total Mixed Ration for Sheep. J. Sci. Food Agric. 2009, 89, 216–220. [Google Scholar] [CrossRef]
- Diniz, L.L.; Valadares Filho, S.C.; Campos, J.M.S.; Valadares, R.F.D.; Da Silva, L.D.; Monnerat, J.P.I.S.; Benedeti, P.B.; De Oliveira, A.S.; Pina, D.S. Effects of Castor Meal on the Growth Performance and Carcass Characteristics of Beef Cattle. Asian-Australas. J. Anim. Sci. 2010, 23, 1308–1318. [Google Scholar] [CrossRef]
- Diniz, L.L.; Filho, S.C.V.; de Oliveira, A.S.; Pina, D.S.; da Silva, L.D.; Benedeti, P.B.; Baião, G.F.; Campos, J.M.S.; Valadares, R.F.D. Castor Bean Meal for Cattle Finishing: 1—Nutritional Parameters. Livest. Sci. 2011, 135, 153–167. [Google Scholar] [CrossRef]
- Gupta, A.P.; Antil, R.S.; Narwal, R.P. Utilization of Deoiled Castor Cake for Crop Production. Arch. Agron. Soil Sci. 2006, 50, 389–395. [Google Scholar] [CrossRef]
- Lima, R.L.S.; Severino, L.S.; Sampaio, L.R.; Sofiatti, V.; Gomes, J.A.; Beltrão, N.E.M. Blends of Castor Meal and Castor Husks for Optimized Use as Organic Fertilizer. Ind. Crop. Prod. 2011, 33, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.D.L.S.D.; Severino, L.S.; Silva, M.I.D.L.; Vale, L.S.D.; Beltrão, N.E.D.M. Recipients Volume and Substrate Composition for Castor Seedlings Production. Web Sci. 2006, 30, 480–486. [Google Scholar] [CrossRef]
- Lima, R.D.L.S.D.; Severino, L.S.; Silva, M.I.D.L.; Jerônimo, J.F.; Vale, L.S.D.; Beltrão, N.E.D.M. Substrates for Castor Seedlings Production Composed by Blends of Five Organic Materials. Web Sci. 2006, 30, 474–479. [Google Scholar] [CrossRef]
- Milfont, M.; Freitas, B.; Rizzardo, R.; Guimarães, M. Honey Production by Africanized Honey Bees in Castor Bean Cropping. Cencia Rural 2009, 39, 1195–1200. [Google Scholar] [CrossRef] [Green Version]
- Romeiro, S.; Lagôa, A.M.M.A.; Furlani, P.R.; De Abreu, C.A.; De Abreu, M.F.; Erismann, N.M. Lead Uptake and Tolerance of Ricinus communis L. Braz. J. Plant Physiol. 2006, 18, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Cai, Q. Zinc Tolerance and Accumulation in Eight Oil Crops. J. Plant Nutr. 2010, 33, 982–997. [Google Scholar] [CrossRef]
- Niu, Z.X.; Sun, L.N.; Sun, T.H.; Li, Y.S.; Hong, W. Evaluation of Phytoextracting Cadmium and Lead by Sunflower, Ricinus, Alfalfa and Mustard in Hydroponic Culture. J. Environ. Sci. 2007, 19, 961–967. [Google Scholar] [CrossRef]
- Zeng, F.; Mallhi, Z.I.; Khan, N.; Rizwan, M.; Ali, S.; Ahmad, A.; Hussain, A.; Alsahli, A.A.; Alyemeni, M.N. Combined Citric Acid and Glutathione Augments Lead (Pb) Stress Tolerance and Phytoremediation of Castorbean through Antioxidant Machinery and pb Uptake. Sustainability 2021, 13, 4073. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Pivetta, L.A. Mechanical and Biological Approaches to Alleviate Soil Compaction in Tropical Soils: Assessed by Root Growth and Activity (rb Uptake) of Soybean and Maize Grown in Rotation with Cover Crops. Soil Use Manag. 2017, 33, 141–152. [Google Scholar] [CrossRef]
- Orji, O.A.; Eke, I.P. Effect of Mulch Materials on Soil Physico-Chemical Properties and the Performance of Castor Bean Plant (Ricinus communis) in Rivers State, Nigeria. Indian J. Anim. Res. 2018, 52, 649–654. [Google Scholar] [CrossRef]
- Zhang, D.M.; Xu, H.G.; Wang, L.; Li, Y.J.; Sun, P.H.; Wu, X.M.; Wang, G.J.; Chen, W.M.; Ye, W.C. Betulinic Acid and Its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a Novel Anti-Inflammatory and Anti-Cancer Dietary Triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 10 May 2023).
- Mubofu, E.B. Castor Oil as a Potential Renewable Resource for the Production of Functional Materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Neto, S.S.; De Paula Manjavachi, M.K.; Zeffa, D.M.; Sartori, M.M.P.; Zanotto, M.D. Morphological Characterization and Selection of Castor Bean Accessions for Mechanized Production. Pesqui. Agropecu. Trop. 2019, 49, 1–9. [Google Scholar] [CrossRef]
- Latterini, F.; Stefanoni, W.; Cavalaris, C.; Karamoutis, C.; Pari, L.; Alexopoulou, E. Effectiveness of Three Terminating Products on Reducing the Residual Moisture in Dwarf Castor Plants: A Preliminary Study of Direct Mechanical Harvesting in Central Greece. Agronomy 2022, 12, 146. [Google Scholar] [CrossRef]
- Stefanoni, W.; Latterini, F.; Malkogiannidis, V.; Salpiggidis, V.; Alexopoulou, E.; Pari, L. Mechanical Harvesting of Castor Bean (Ricinus communis L.) with a Combine Harvester Equipped with Two Different Headers: A Comparison of Working Performance. Energies 2022, 15, 2999. [Google Scholar] [CrossRef]
- Canecchio Filho, V.; Freire, E.S. Adubação da Mamoneira: I—Experiências Preliminares. Bragantia 1958, 17, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Manickam, S.; Kalaiselvan, P.; Subramaniyan, K.; Venkatachalam, S.R. Role of Herbicide in Castor Based Intercropping System. J. Phytol. Res. 2009, 22, 291–294. [Google Scholar]
- Sofiatti, V.; Severino, L.S.; Silva, F.M.O.; Silva, V.N.B.; Brito, G.G. Pre and Postemergence Herbicides for weed Control in Castor Crop. Ind. Crop. Prod. 2012, 37, 235–237. [Google Scholar] [CrossRef]
- Soares, J.D. Gray Mold of Castor: A Review. In Plant Pathology; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Manjula, K.; Sarma, P.S.; Nageshwar Rao, T.; Thatikunta, R. In Vitro Screening of Castor Genotypes for Stress Tolerance Seed Physiology View Project Horticulture View Project. J. Oilseeds Res. 2001, 18, 292–293. [Google Scholar]
- Lakshmamma, P.; Lakshmi, P.; Lavanya, C.; Anjani, K. Growth and Yield of Different Castor Genotypes Varying in Drought Tolerance. Ann. Arid Zone 2009, 48, 35–39. [Google Scholar]
- Zhong, W.; Hartung, W.; Komor, E.; Schobert, C. Phloem Transport of Abscisic Acid in Ricinus communis L. Seedlings. Plant Cell Environ. 1996, 19, 471–477. [Google Scholar] [CrossRef]
- Babita, M.; Maheswari, M.; Rao, L.M.; Shanker, A.K.; Rao, D.G. Osmotic Adjustment, Drought Tolerance and Yield in Castor (Ricinus communis L.) Hybrids. Environ. Exp. Bot. 2010, 69, 243–249. [Google Scholar] [CrossRef]
- Sausen, T.L.; Rosa, L.M.G. Growth and Limitations to Carbon Assimilation in Ricinus communis (Euphorbiaceae) under Soil Water Stress Conditions. Acta Bot. Bras. 2010, 24, 648–654. [Google Scholar] [CrossRef]
- Funk, J.L.; Zachary, V.A. Physiological Responses to Short-Term Water and Light Stress in Native and Invasive Plant Species in Southern California. Biol. Invasions 2010, 12, 1685–1694. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; Silva, J.V.; Endres, L.; Ferreira, V.M.; de Albuquerque Câmara, C.; Cabral, F.F.; Oliveira, J.F.; de Carvalho, L.W.T.; dos Santos, J.M.; dos Santos Filho, B.G. Leaf Gas Exchange, Chloroplastic Pigments and Dry Matter Accumulation in Castor Bean (Ricinus communis L.) Seedlings Subjected to Salt Stress Conditions. Ind. Crop. Prod. 2008, 27, 385–392. [Google Scholar] [CrossRef]
- Silva, S.M.S.; Alves, A.N.; Gheyi, H.R.; Beltrão, N.E.D.M.; Severino, L.S.; Soares, F.A.L. Growth and Production of Two Cultivars Of Castor Bean under Saline Stress|Desenvolvimento e Producao de Duas Cultivares de Mamoneira Sob Estresse Salino. Rev. Bras. Eng. Agric. Ambient. 2008, 12, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf Chlorophyll Fluorescence, Hyperspectral Reflectance, Pigments Content, Malondialdehyde and Proline Accumulation Responses of Castor Bean (Ricinus communis L.) Seedlings to Salt Stress Levels. Ind. Crop. Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased Root Hydraulic Conductivity Reduces Leaf Water Potential, Initiates Stomatal Closure and Slows Leaf Expansion in Flooded Plants of Castor Oil (Ricinus communis) Despite Diminished Delivery of ABA from the Roots to Shoots in Xylem Sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Lourdes, R.D.E.; Lima, S.D.E.; Severino, L.I.V.S.; Ferreira, G.B. Soil Exchangeable Aluminum Influencing the Growth and Leaf Tissue Macronutrients Content of Castor Plants. Rev. Caatinga 2014, 27, 10–15. [Google Scholar]
- de Freitas, L.B.; Fernandes, D.M.; Maia, S.C.M.; Pivetta, L.G.; Zanotto, M.D. Aluminum Tolerance in Castor Bean Lines. Pesqui. Agropecu. Trop. 2018, 48, 299–305. [Google Scholar] [CrossRef]
- Chan, A.P.; Crabtree, J.; Zhao, Q.; Lorenzi, H.; Orvis, J.; Puiu, D.; Melake-Berhan, A.; Jones, K.M.; Redman, J.; Chen, G.; et al. Draft Genome Sequence of the Oilseed Species Ricinus communis. Nat. Biotechnol. 2010, 28, 951–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinkerton, S.D.; Rolfe, R.; Auld, D.L.; Ghetie, V.; Lauterbach, B.F. Selection of Castor for Divergent Concentrations of Ricin and Ricinus communis Agglutinin. Crop Sci. 1999, 39, 353–357. [Google Scholar] [CrossRef]
- Baldanzi, M.; Pugliesi, C. Selection for Non-Branching in Castor, Ricinus communis L. Plant Breed. 1998, 117, 392–394. [Google Scholar] [CrossRef]
- Baldanzi, M.; Fambrini, M.; Pugliesi, C. Redesign of the Castorbean Plant Body Plan for Optimal Combine Harvesting. Ann. Appl. Biol. 2003, 142, 299–306. [Google Scholar] [CrossRef]
- Anjani, K. Castor Genetic Resources: A Primary Gene Pool for Exploitation. Ind. Crop. Prod. 2012, 35, 1–14. [Google Scholar] [CrossRef]
- Anjani, K.; Raoof, M.A.; Prasad, M.S.L.; Duraimurugan, P.; Lucose, C.; Yadav, P.; Prasad, R.D.; Lal, J.J.; Sarada, C. Trait-Specific Accessions in Global Castor (Ricinus communis L.) Germplasm Core Set for Utilization in Castor Improvement. Ind. Crop. Prod. 2018, 112, 766–774. [Google Scholar] [CrossRef]
- dos S. de Oliveira, F.; Bassegio, D.; Ramos, A.R.; da Silva, G.H.; da Silva, J.; Zanotto, M.D.; Fernandes, D.M. Phosphate Fertilization of Low Size Castor Bean in Conventional and Narrow Cultivation in Second Cropping Season. J. Exp. Agric. Int. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- da Silva, J.; Ramos, A.R.; Amorim, D.J.; Zanotto, M.D.; Sartori, M.M.P. Productive Potential and Castor Bean Selection of the FCA-PB Cultivar Progenies. Rev. Ceres 2020, 67, 42–51. [Google Scholar] [CrossRef]
- Severino, L.S.; Auld, D.L.; Vale, L.S.; Marques, L.F. Plant Density Does Not Influence Every Castor Plant Equally. Ind. Crop. Prod. 2017, 107, 588–594. [Google Scholar] [CrossRef]
- Rojas-Barros, P.; De Haro, A.; Muñoz, J.; Fernández-Martínez, J.M. Isolation of a Natural Mutant in Castor with High Oleic/Low Ricinoleic Acid Content in the Oil. Crop Sci. 2004, 44, 76–80. [Google Scholar] [CrossRef]
- Venegas-Calerón, M.; Sánchez, R.; Salas, J.J.; Garcés, R.; Martínez-Force, E. Molecular and Biochemical Characterization of the OLE-1 High-Oleic Castor Seed (Ricinus communis L.) Mutant. Planta 2016, 244, 245–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, C.N.; Hendon, B.R.; Mishra, D.; Rieff, J.M.; Lowery, C.C.; Lambert, K.C.; Witt, T.W.; Oswalt, S.J.; Bechere, E.; Smith, C.W.; et al. Cotton (Gossypium hirsutum L.) Mutants with Reduced Levels of Palmitic Acid (C16:0) in Seed Lipids. Euphytica 2019, 215, 112. [Google Scholar] [CrossRef]
- Hendon, B.R.; Bechere, E.; Witt, T.W.; Kelly, B.R.; Mishra, D.; Auld, D.L. Genetic Improvement of Naked-Tufted Seed Mutants in Upland Cotton (Gossypium hirsutum L.). Euphytica 2019, 215, 81. [Google Scholar] [CrossRef]
- Bechere, E.; Zeng, L.; Auld, D. Registration of Five Upland Cotton Mutant Germplasm Lines with Superior Fiber Length, Strength, and Uniformity. J. Plant Regist. 2018, 12, 107–111. [Google Scholar] [CrossRef]
- Percy, R.; Hendon, B.; Bechere, E.; Auld, D. Qualitative Genetics and Utilization of Mutants. Cotton 2015, 57, 155–185. [Google Scholar] [CrossRef]
- Brown, N.; Wayne Smith, C.; Hague, S.; Auld, D.; Hequet, E.; Joy, K.; Jones, D. Within-Boll Yield Characteristics and Their Correlation with Fiber Quality Parameters Following Mutagenesis of Upland Cotton, TAM 94L-25. Crop Sci. 2015, 55, 1513–1523. [Google Scholar] [CrossRef]
- Lavanya, C.; Gopinath, V. Inheritance Studies for Morphological Characters and Sex Expression in Pistillate Lines of Castor (Ricinus communis L.). Indian J. Genet. Plant Breed. 2008, 68, 275–282. [Google Scholar]
- Ahmed, H.M.; Sarwar, G.; ul Haq, M.A. Genetic Variability and Interdependence of Morphological Traits in Castorbean (Ricinus communis L) Mutants. Songklanakarin J. Sci. Technol. 2012, 34, 279–286. [Google Scholar]
- Allan, G.; Williams, A.; Rabinowicz, P.D.; Chan, A.P.; Ravel, J.; Keim, P. Worldwide Genotyping of Castor Bean Germplasm (Ricinus communis L.) Using AFLPs and SSRs. Genet. Resour. Crop Evol. 2008, 55, 365–378. [Google Scholar] [CrossRef]
- Milani, M.; de Medeiros Nbreg, M.B. Castor Breeding. In Plant Breeding from Laboratories to Fields; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Moshkin, V.A.; Dvoryadkina, A.G. Cytology and Genetics of Qualitative Characteristics; Castor: New Delhi, India, 1986. [Google Scholar]
- Severino, L.S. Single-Seed Selection of Fast-Germinating Genotypes of Castor (Ricinus communis). Ind. Crop. Prod. 2023, 194, 116307. [Google Scholar] [CrossRef]
- Hu, W.; Chen, L.; Qiu, X.; Lu, H.; Wei, J.; Bai, Y.; He, N.; Hu, R.; Sun, L.; Zhang, H.; et al. Morphological, Physiological and Proteomic Analyses Provide Insights into the Improvement of Castor Bean Productivity of a Dwarf Variety in Comparing with a High-Stalk Variety. Front. Plant Sci. 2016, 7, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur, E.; Kurczyńska, E.U.; Friml, J. Cellular Events during Interfascicular Cambium Ontogenesis in Inflorescence Stems of Arabidopsis. Protoplasma 2014, 251, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H. A Growing Stem Inhibits Bud Outgrowth—The Overlooked Theory of Apical Dominance. Front. Plant Sci. 2017, 8, 1874. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A Role for Flavin Monooxygenase-like Enzymes in Auxin Biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, A.; Li, F.; Xu, W.; Han, B.; Cheng, X.; Liu, A. Bulked Segregant Analysis Reveals Candidate Genes Responsible for Dwarf Formation in Woody Oilseed Crop Castor Bean. Sci. Rep. 2021, 11, 6277. [Google Scholar] [CrossRef]
- Mori, K.K.; Patel, J.; Rani, K.; Mori, V.K.; Kumar, M.; Ajay, B.C. Deciphering Higher Order Non-Allelic Interactions for Quantitative Characters through Twelve Generation Mean Analysis in Castor (Ricinus communis L.). Genet. Resour. Crop Evol. 2022, 69, 1759–1785. [Google Scholar] [CrossRef]
- Solanki, S.S.; Deora, V.S.; Singh, D.P. Combining Ability of New Castor (Ricinus communis L.) Pistillate Lines: MCP-1-1. J. Oilseeds Res. 2004, 21, 274–276. [Google Scholar]
- Golakia, P.R.; Poshiya, V.K.; Monpara, B.A. Identification of Superior Donor Parents for Earliness through Combining Ability in Castor. Int. J. Res. Plant Sci. 2015, 5, 26–31. [Google Scholar]
- Virani, H.P.; Dhedhi, K.K.; Dhaduk, H.L. Gene Effects of Seed Yield and Component Traits in Castor Crosses. PKV Res. J. 2013, 37, 8–13. [Google Scholar]
- Lavanya, C.; Chandramohan, Y. Combining Ability and Heterosis for Seed Yield and Yield Components in Castor. J. Oilseeds Res. 2003, 20, 220–224. [Google Scholar]
- Ramana, P.V.; Lavanya, C.; Ratnasree, P. Combining Ability and Heterosis Studies under Rainfed Conditions in Castor (Ricinus communis L.). Indian J. Genet. Plant Breed. 2005, 65, 325–326. [Google Scholar]
- Lavanya, C.; Solanki, S.S. Crop Improvement of Castor. The Challenges Ahead. Research and Development in Castor. Present Status and Future Strategies. Indian Soc. Oilseeds Res. Hyderabad 2010, 36–55. Available online: https://scholar.google.co.in/citations?view_op=view_citation&hl=en&user=8KzYu1cAAAAJ&citation_for_view=8KzYu1cAAAAJ:hC7cP41nSMkC (accessed on 14 May 2023).
- Sailaja, M.; Tarakeswari, M.; Sujatha, M. Stable Genetic Transformation of Castor (Ricinus communis L.) via Particle Gun-Mediated Gene Transfer Using Embryo Axes from Mature Seeds. Plant Cell Rep. 2008, 27, 1509–1519. [Google Scholar] [CrossRef]
- Bertozzo, F.; Machado, I.S. Meios de Cultura No Desenvolvimento de Ápices Caulinares de Mamoneira (Ricinus communis L.) In Vitro. Ciência Agrotecnol. 2010, 34, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Sujatha, M.; Sailaja, M. Stable Genetic Transformation of Castor (Ricinus communis L.) via Agrobacterium Tumefaciens-Mediated Gene Transfer Using Embryo Axes from Mature Seeds. Plant Cell Rep. 2005, 23, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, B.; Yang, Y.; Mei, J.; Zhao, X.; Guo, X.; Huang, X.; Tang, D.; Liu, X. Piercing and Vacuum Infiltration of the Mature Embryo: A Simplified Method for Agrobacterium-Mediated Transformation of Indica Rice. Plant Cell Rep. 2009, 28, 1065–1074. [Google Scholar] [CrossRef]
- Sánchez-Álvarez, A.; Ruíz-López, N.; Moreno-Pérez, A.J.; Martínez-Force, E.; Garcés, R.; Salas, J.J. Agrobacterium-Mediated Transient Gene Expression in Developing Ricinus communis Seeds: A First Step in Making the Castor Oil Plant a Chemical Biofactory. Front. Plant Sci. 2019, 10, 1410. [Google Scholar]
- Auld, D.L.; Pinkerton, S.D.; Boroda, E.; Lombard, K.A.; Murphy, C.K.; Kenworthy, K.E.; Becker, W.D.; Rolfe, R.D.; Ghetie, V. Registration of TTU-LRC Castor Germplasm with Reduced Levels of Ricin and RCA120. Crop Sci. 2003, 43, 746–747. [Google Scholar] [CrossRef]
- Auld, D.L.; Rolfe, R.D.; McKeon, T.A. Development of Castor with Reduced Toxicity. J. New Seeds 2008, 3, 61–69. [Google Scholar] [CrossRef]
- Sousa, N.L.; Cabral, G.B.; Vieira, P.M.; Baldoni, A.B.; Aragão, F.J.L. Bio-Detoxification of Ricin in Castor Bean (Ricinus communis L.) Seeds. Sci. Rep. 2017, 7, 15385. [Google Scholar] [CrossRef] [Green Version]
- Guhling, O.; Hobl, B.; Yeats, T.; Jetter, R. Cloning and Characterization of a Lupeol Synthase Involved in the Synthesis of Epicuticular Wax Crystals on Stem and Hypocotyl Surfaces of Ricinus communis. Arch. Biochem. Biophys. 2006, 448, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, R.; Lu, W.; Zhou, Z.; Jiang, X.; Zhao, H.; Yang, B.; Lü, S. Transcriptome Analysis Identifies Key Genes Involved in the Regulation of Epidermal Lupeol Biosynthesis in Ricinus communis. Ind. Crop. Prod. 2021, 160, 113100. [Google Scholar] [CrossRef]
- Li, D.; Pan, C.; Lu, J.; Zaman, W.; Zhao, H.; Zhang, J.; Lü, S. Lupeol Accumulation Correlates with Auxin in the Epidermis of Castor. Molecules 2021, 26, 2978. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, F.M.; Brigelius-Flohé, R.; Aumann, K.D.; Roveri, A.; Schomburg, D.; Flohé, L. Diversity of glutathione peroxidases. Methods Enzymol. 1995, 252, 38–48. [Google Scholar] [CrossRef]
- Bela, K.; Horváth, E.; Gallé, Á.; Szabados, L.; Tari, I.; Csiszár, J. Plant Glutathione Peroxidases: Emerging Role of the Antioxidant Enzymes in Plant Development and Stress Responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef]
- Passaia, G.; Margis-Pinheiro, M. Glutathione Peroxidases as Redox Sensor Proteins in Plant Cells. Plant Sci. 2015, 234, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Roxas, V.P.; Lodhi, S.A.; Garrett, D.K.; Mahan, J.R.; Allen, R.D. Stress Tolerance in Transgenic Tobacco Seedlings That Overexpress Glutathione S-Transferase/Glutathione Peroxidase. Plant Cell Physiol. 2000, 41, 1229–1234. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, K.; Miyao, K.; Gaber, A.; Takeda, T.; Kanaboshi, H.; Miyasaka, H.; Shigeoka, S. Enhancement of Stress Tolerance in Transgenic Tobacco Plants Overexpressing Chlamydomonas Glutathione Peroxidase in Chloroplasts or Cytosol. Plant J. 2004, 37, 21–33. [Google Scholar] [CrossRef]
- Navrot, N.; Collin, V.; Gualberto, J.; Gelhaye, E.; Hirasawa, M.; Rey, P.; Knaff, D.B.; Issakidis, E.; Jacquot, J.P.; Rouhier, N. Plant Glutathione Peroxidases Are Functional Peroxiredoxins Distributed in Several Subcellular Compartments and Regulated during Biotic and Abiotic Stresses. Plant Physiol. 2006, 142, 1364–1379. [Google Scholar] [CrossRef] [Green Version]
- Passaia, G.; Spagnolo Fonini, L.; Caverzan, A.; Jardim-Messeder, D.; Christoff, A.P.; Gaeta, M.L.; de Araujo Mariath, J.E.; Margis, R.; Margis-Pinheiro, M. The Mitochondrial Glutathione Peroxidase GPX3 Is Essential for H2O2 Homeostasis and Root and Shoot Development in Rice. Plant Sci. 2013, 208, 93–101. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jang, M.G.; Noh, H.Y.; Lee, H.J.; Sukweenadhi, J.; Kim, J.H.; Kim, S.Y.; Kwon, W.S.; Yang, D.C. Molecular Characterization of Two Glutathione Peroxidase Genes of Panax Ginseng and Their Expression Analysis against Environmental Stresses. Gene 2014, 535, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, T.; Manna, M.; Kaul, T.; Pandey, S.; Reddy, C.S.; Reddy, M.K. Genome-Wide Dissection of Arabidopsis and Rice for the Identification and Expression Analysis of Glutathione Peroxidases Reveals Their Stress-Specific and Overlapping Response Patterns. Plant Mol. Biol. Report. 2015, 33, 1413–1427. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; An, Y.Q.C.; Zhang, H.; Meng, D.; Jin, Y.; Huo, H.; Yu, L.; Zhang, J. Identification of Glutathione Peroxidase Gene Family in Ricinus communis and Functional Characterization of RcGPX4 in Cold Tolerance. Front. Plant Sci. 2021, 12, 707127. [Google Scholar] [CrossRef]
- Li, B.; He, L.; Guo, S.; Li, J.; Yang, Y.; Yan, B.; Sun, J.; Li, J. Proteomics Reveal Cucumber Spd-Responses under Normal Condition and Salt Stress. Plant Physiol. Biochem. PPB 2013, 67, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Xu, J.; Chen, T.; Tao, A.; Qi, J. Proteomic Changes in Kenaf (Hibiscus cannabinus L.) Leaves under Salt Stress. Ind. Crop. Prod. 2016, 91, 255–263. [Google Scholar] [CrossRef]
- Veeranagamallaiah, G.; Jyothsnakumari, G.; Thippeswamy, M.; Chandra Obul Reddy, P.; Surabhi, G.K.; Sriranganayakulu, G.; Mahesh, Y.; Rajasekhar, B.; Madhurarekha, C.; Sudhakar, C. Proteomic Analysis of Salt Stress Responses in Foxtail Millet (Setaria italica L. cv. Prasad) Seedlings. Plant Sci. 2008, 175, 631–641. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, X.; Salvato, F.; Wang, Y.; Yan, X.; Zhou, Z.; Lin, J. Salt-Adaptive Strategies in Oil Seed Crop Ricinus communis Early Seedlings (Cotyledon vs. True Leaf) Revealed from Proteomics Analysis. Ecotoxicol. Environ. Saf. 2019, 171, 12–25. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Yang, F.; Zhou, W.; Mao, S.; Lin, J.; Yan, X. Untargeted LC-MS-Based Metabolomics Revealed Specific Metabolic Changes in Cotyledons and Roots of Ricinus communis during Early Seedling Establishment under Salt Stress. Plant Physiol. Biochem. 2021, 163, 108–118. [Google Scholar] [CrossRef]
- Djakovic-Petrovic, T.; De Wit, M.; Voesenek, L.A.C.J.; Pierik, R. DELLA Protein Function in Growth Responses to Canopy Signals. Plant J. 2007, 51, 117–126. [Google Scholar] [CrossRef]
- Lei, P.; Liu, Z.; Hu, Y.; Kim, H.C.; Liu, S.; Liu, J.; Xu, L.; Li, J.; Zhao, Y.; Yu, Z.; et al. Transcriptome Analysis of Salt Stress Responsiveness in the Seedlings of Wild and Cultivated Ricinus communis L. J. Biotechnol. 2021, 327, 106–116. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wen, X. The Effect of Temperature on the Germination Rates on Castor Bean. Seeds 2008, 27, 67–69. [Google Scholar]
- Wang, X.; Li, M.; Liu, X.; Zhang, L.; Duan, Q.; Zhang, J. Quantitative Proteomic Analysis of Castor (Ricinus communis L.) Seeds during Early Imbibition Provided Novel Insights into Cold Stress Response. Int. J. Mol. Sci. 2019, 20, 355. [Google Scholar] [CrossRef] [Green Version]
- Ghidoli, M.; Frazzini, S.; De Benedetti, S.; Sangiorgio, S.; Landoni, M.; Scarafoni, A.; Rossi, L.; Pilu, R. Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges. Agronomy 2023, 12, 1562. [Google Scholar] [CrossRef]
- Al Amin, N.; Ahmad, N.; Wu, N.; Pu, X.; Ma, T.; Du, Y.; Bo, X.; Wang, N.; Sharif, R.; Wang, P. CRISPR-Cas9 Mediated Targeted Disruption of FAD2-2 Microsomal Omega-6 Desaturase in Soybean (Glycine max.L). BMC Biotechnol. 2019, 19, 9. [Google Scholar] [CrossRef]
- Wang, J.; Kuang, H.; Zhang, Z.; Yang, Y.; Yan, L.; Zhang, M.; Song, S.; Guan, Y. Generation of Seed Lipoxygenase-Free Soybean Using CRISPR-Cas9. Crop J. 2020, 8, 432–439. [Google Scholar] [CrossRef]
- Ma, J.; Sun, S.; Whelan, J.; Shou, H. CRISPR/Cas9-Mediated Knockout of GmFATB1 Significantly Reduced the Amount of Saturated Fatty Acids in Soybean Seeds. Int. J. Mol. Sci. 2021, 22, 3877. [Google Scholar] [CrossRef] [PubMed]
- Okuzaki, A.; Ogawa, T.; Koizuka, C.; Kaneko, K.; Inaba, M.; Imamura, J.; Koizuka, N. CRISPR/Cas9-Mediated Genome Editing of the Fatty Acid Desaturase 2 Gene in Brassica napus. Plant Physiol. Biochem. PPB 2018, 131, 63–69. [Google Scholar] [CrossRef]
- Zhang, K.; Nie, L.; Cheng, Q.; Yin, Y.; Chen, K.; Qi, F.; Zou, D.; Liu, H.; Zhao, W.; Wang, B.; et al. Effective Editing for Lysophosphatidic Acid Acyltransferase 2/5 in Allotetraploid Rapeseed (Brassica napus L.) Using CRISPR-Cas9 System. Biotechnol. Biofuels 2019, 12, 225. [Google Scholar] [CrossRef]
- Xie, T.; Chen, X.; Guo, T.; Rong, H.; Chen, Z.; Sun, Q.; Batley, J.; Jiang, J.; Wang, Y. Targeted Knockout of BnTT2 Homologues for Yellow-Seeded Brassica napus with Reduced Flavonoids and Improved Fatty Acid Composition. J. Agric. Food Chem. 2020, 68, 5676–5690. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Yu, P.; Tian, X.; Guo, L.; Tu, J.; Shen, J.; Yi, B.; Fu, T.; Wen, J.; Liu, K.; et al. DELLA Proteins BnaA6.RGA and BnaC7.RGA Negatively Regulate Fatty Acid Biosynthesis by Interacting with BnaLEC1s in Brassica napus. Plant Biotechnol. J. 2021, 19, 2011–2026. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, M.; Li, H.; Wang, L.; Liu, R.; Liu, Z.; Zhang, X.; Jin, S. High-Oleic Acid Content, Nontransgenic Allotetraploid Cotton (Gossypium hirsutum L.) Generated by Knockout of GhFAD2 Genes with CRISPR/Cas9 System. Plant Biotechnol. J. 2021, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhu, J.; Gong, L.; He, L.; Lee, C.; Han, S.; Chen, C.; He, G. Mutagenesis of FAD2 Genes in Peanut with CRISPR/Cas9 Based Gene Editing. BMC Biotechnol. 2019, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Morineau, C.; Bellec, Y.; Tellier, F.; Gissot, L.; Kelemen, Z.; Nogué, F.; Faure, J.D. Selective Gene Dosage by CRISPR-Cas9 Genome Editing in Hexaploid Camelina sativa. Plant Biotechnol. J. 2017, 15, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.Z.; Henry, I.M.; Lynagh, P.G.; Comai, L.; Cahoon, E.B.; Weeks, D.P. Significant Enhancement of Fatty Acid Composition in Seeds of the Allohexaploid, Camelina sativa, Using CRISPR/Cas9 Gene Editing. Plant Biotechnol. J. 2017, 15, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Lyzenga, W.J.; Harrington, M.; Bekkaoui, D.; Wigness, M.; Hegedus, D.D.; Rozwadowski, K.L. CRISPR/Cas9 Editing of Three CRUCIFERIN C Homoeologues Alters the Seed Protein Profile in Camelina sativa. BMC Plant Biol. 2019, 19, 292. [Google Scholar] [CrossRef] [Green Version]
- Ozseyhan, M.E.; Kang, J.; Mu, X.; Lu, C. Mutagenesis of the FAE1 Genes Significantly Changes Fatty Acid Composition in Seeds of Camelina sativa. Plant Physiol. Biochem. PPB 2018, 123, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aznar-Moreno, J.A.; Durrett, T.P. Simultaneous Targeting of Multiple Gene Homeologs to Alter Seed Oil Production in Camelina sativa. Plant Cell Physiol. 2017, 58, 1260–1267. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Zeng, W.; Chen, W.; Wu, Y.; Wen, G.; Chen, X.; Wang, Q.; Zhou, J.; Li, Y.; Yang, Z.; et al. HaCYC2c Regulating the Heteromorphous Development and Functional Differentiation of Florets by Recognizing HaNDUA2 in Sunflower. Plant Cell Rep. 2022, 41, 1025–1041. [Google Scholar] [CrossRef]
- Aznar-Moreno, J.A.; Venegas-Calerón, M.; Du, Z.Y.; Garcés, R.; Tanner, J.A.; Chye, M.L.; Martínez-Force, E.; Salas, J.J. Characterization and Function of a Sunflower (Helianthus annuus L.) Class II Acyl-CoA-Binding Protein. Plant Sci. 2020, 300, 110630. [Google Scholar] [CrossRef]
- CPVO. Essentially Derived Varieties. Available online: https://cpvo.europa.eu/sites/default/files/documents/articles/EDV_presentation_PlantumNL_March_2006_BK.pdf (accessed on 18 June 2023).

| Fatty Acid | Amount (%) | Chemical Structure |
|---|---|---|
| Ricinoleic (C18:1-OH) | 88–92 | 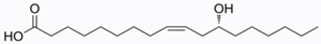 |
| Linoleic (C18:2) | 4.2–4.8 |  |
| Oleic (C18:1) | 2.3–3.4 |  |
| Palmitic (C16:0) | 0.8–1.1 | 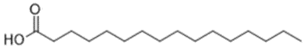 |
| Stearic (C18:0) | 0.6–1.1 |  |
| Institution | Country | Nr. of Accessions |
|---|---|---|
| National Bureau of Plant Genetic Resources | India | 4307 |
| Institute of Crop Science (CAAS) and Institute of Oil Crops Research (CAAS) | China | 3341 |
| Embrapa, Empresa Baiana de Desenvolvimento Agricola S.A. and Instituto Agroanomico de Campinas (I.A.C.) | Brazil | 1348 |
| USDA, ARS, PGRCU and USDA, ARS, NCGRP | United States | 1043 |
| C.I. La Selva-CORPOICA | Colombia | 424 |
| N.I. Vavilov All-Russian Scientific Research Institute of Plant Industry | Russia | 423 |
| Institute of Oil Crops | Ukraine | 255 |
| Biodiversity Conservation and Research Institute | Ethiopia | 232 |
| National Dryland Farming Research Station and National Genebank of Kenya, Crop Plant Genetic Resource Centre, KARI | Kenya | 173 |
| Maize Research Institute and Institute of Field and Vegetable Crops | Serbia | 112 |
| Agricoltural Research Station Teleorman | Romania | 66 |
| Total: | 11,300 |
| Plant | Genes | Mutant Phenotypes | Citations |
|---|---|---|---|
| soybean | FAD2-2 | Increase of oleic acid content and decrease of linoleic acid | [141] |
| GmLox1/2/3 | Free of lipoxygenase | [142] | |
| GmFATB1 | Significant reduction of saturated fatty acids content in seeds | [143] | |
| rapeseed | BnFAD2 | Significant increase in the content of oleic acid | [144] |
| BnLPAT2/5 | Seeds of the mutants were wizened and showed enlarged oil bodies, disrupted distribution of protein bodies, and increased accumulation of starch in mature seeds | [145] | |
| BnTT2 | Yellow-seeded rapeseed with reduced flavonoids and improved fatty acid composition | [146] | |
| BnaLEC1 | Reduced seed oil content and C18:1, increase of C18:2 | [147] | |
| cotton | GhFAD2 | High-oleic acid content, no transgenic allotetraploid cotton (Gossypium hirsutum L.) generated by knockout of GhFAD2 genes with CRISPR/Cas9 system | [148] |
| peanut | AhFAD2 | Mutagenesis of FAD2 genes in peanut with CRISPR/Cas9 based gene editing (NO plant regeneration) | [149] |
| false flax | CsFAD2 | Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa | [150] |
| CsFAD2 | Decreased polyunsaturated fatty acids and concomitant increase of oleic acid in the oil | [151] | |
| CsCRUC | CRISPR/Cas9 editing of three CRUCIFERIN C homeologs alters the seed protein profile in Camelina sativa | [152] | |
| CsFAE1 | Mutagenesis of the FAE1 genes significantly changes fatty acid composition in seeds of Camelina sativa | [153] | |
| CsDGAT1 | Simultaneous targeting of multiple gene homeologs to alter seed oil production in Camelina sativa | [154] | |
| CsPDAT1 | |||
| sunflower | HaCYC2c | Overexpression of HaCYC2c leads to the transformation of floral symmetry and influences the fertility of disc florets for the optimization of reproductive efficiency | [155] |
| HaACBP1 | HaACBP1 plays a role in the transport and trafficking of acyl-CoAs during seed development | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landoni, M.; Bertagnon, G.; Ghidoli, M.; Cassani, E.; Adani, F.; Pilu, R. Opportunities and Challenges of Castor Bean (Ricinus communis L.) Genetic Improvement. Agronomy 2023, 13, 2076. https://doi.org/10.3390/agronomy13082076
Landoni M, Bertagnon G, Ghidoli M, Cassani E, Adani F, Pilu R. Opportunities and Challenges of Castor Bean (Ricinus communis L.) Genetic Improvement. Agronomy. 2023; 13(8):2076. https://doi.org/10.3390/agronomy13082076
Chicago/Turabian StyleLandoni, Michela, Greta Bertagnon, Martina Ghidoli, Elena Cassani, Fabrizio Adani, and Roberto Pilu. 2023. "Opportunities and Challenges of Castor Bean (Ricinus communis L.) Genetic Improvement" Agronomy 13, no. 8: 2076. https://doi.org/10.3390/agronomy13082076





