Overexpression of the Peanut AhDGAT3 Gene Increases the Oil Content in Soybean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Isolation and Sequence Analysis of AhDGAT Gene in Peanut
2.3. Arabidopsis and Soybean Transformation of AhDGAT3
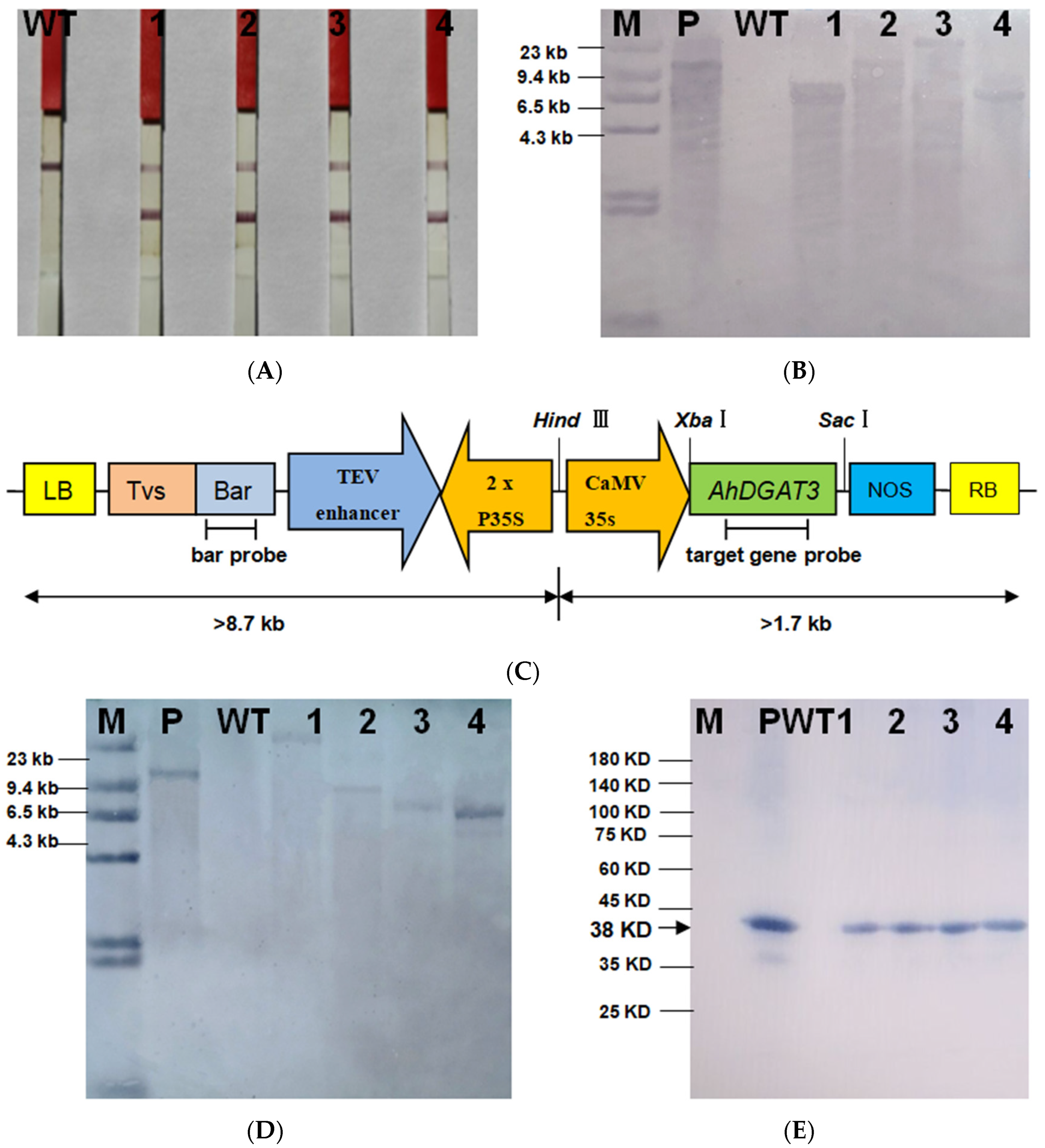
2.4. Molecular Detection of AhDGAT3 Transgenic Soybean
2.5. Genome Re-Sequencing
2.6. Quantitative RT-PCR Analysis
2.7. Measurement of Fatty Acid Content in Arabidopsis and Soybean
2.8. The Agronomic Trait Analysis of WT and Transgenic Soybeans
2.9. Statistical Analysis
3. Results
3.1. Isolation and Characterization of AhDGAT3 Gene
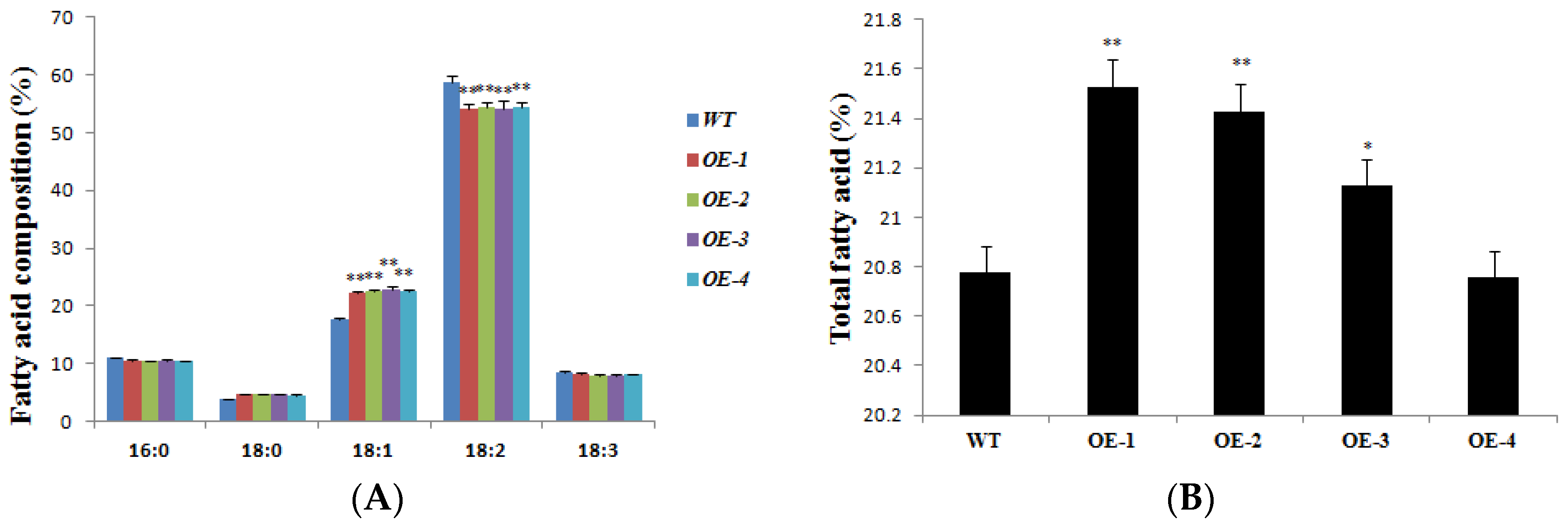
3.2. Oil Content in Seeds of Different Genotype Arabidopsis Plants
3.3. Overexpression AhDGAT3 Gene in Soybean
3.4. Genome Re-Sequencing of AhDGAT3 Transgenic Soybean
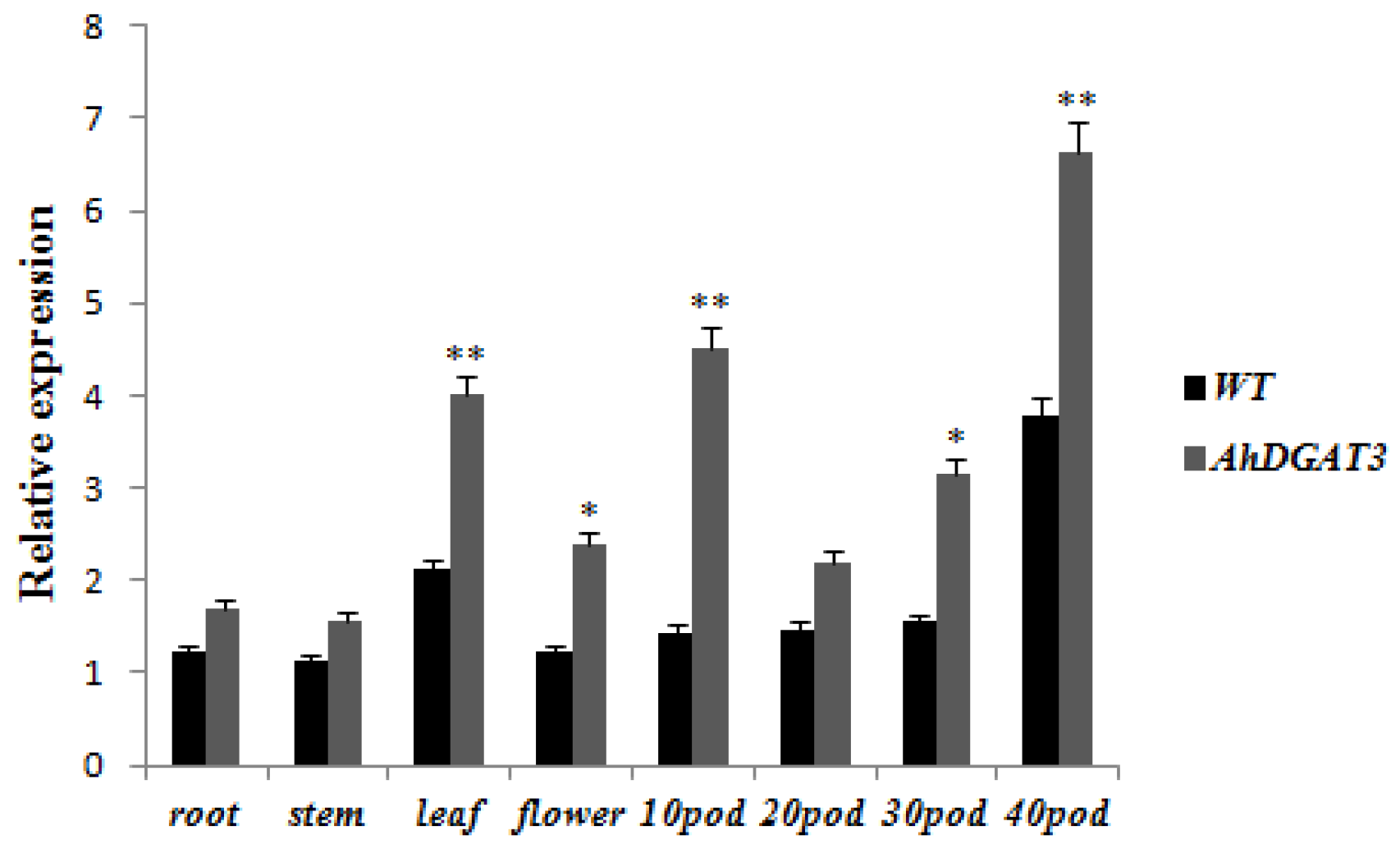
3.5. Expression Analysis of AhDGAT3 in WT and Transgenic Soybean
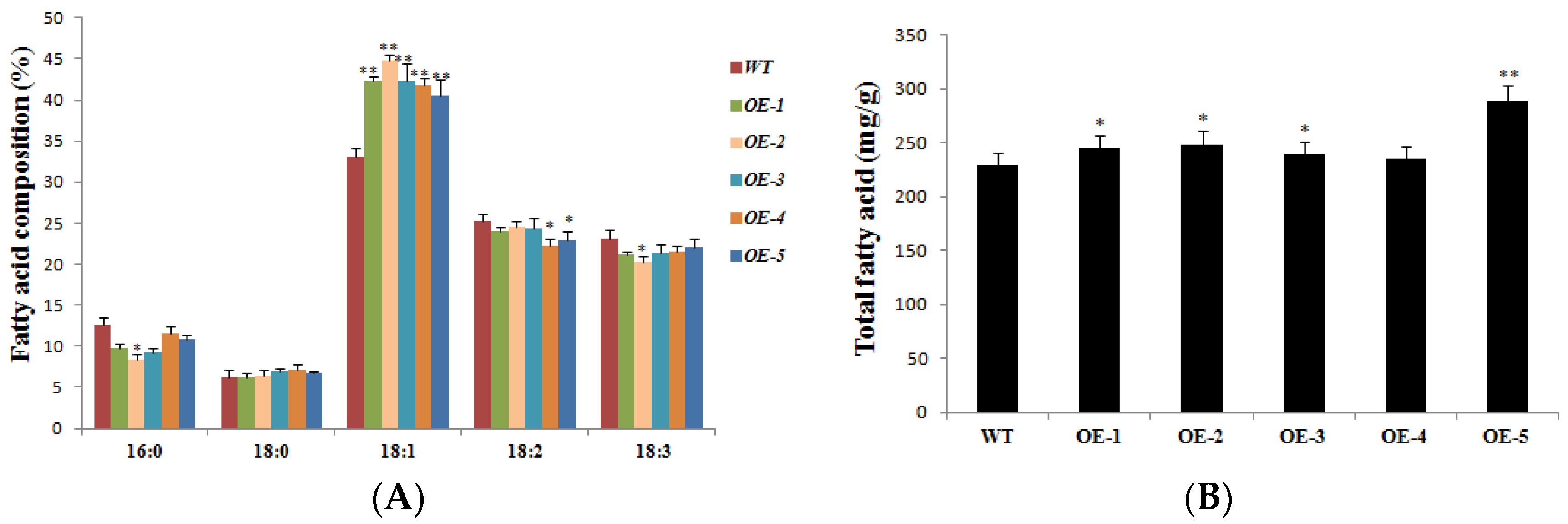
3.6. AhDGAT3 Over-Expression Enhances Fatty Acid Content in Transgenic Soybean
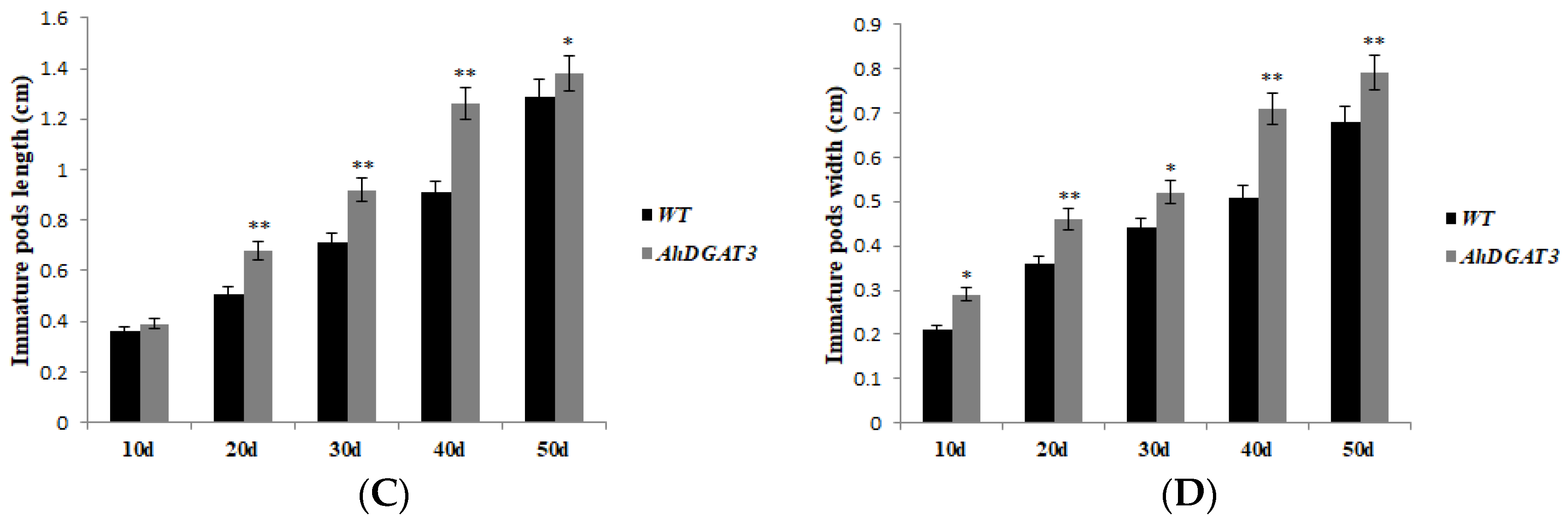
3.7. AhDGAT3 Overexpression Improved the Agronomic Traits of Transgenic Soybean
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, B.S. Analysis on the competitiveness of peanut oil industry in China. J. Peanut 2003, 32, 11–15. [Google Scholar]
- Li, X.D.; Cao, Y.L.; Hu, Y.P.; Xiao, L.; Wu, Y.; Wu, G.; Lu, C. Study on fatty acid accumulation patterns in peanut seed development. Chin. J. Oil Crops 2009, 31, 157–162. [Google Scholar]
- Mekhedov, S.; De Ilárduya, O.M.; Ohlrogge, J. Toward a functional catalog of the plant genome. A survey of genes for lipid biosynthesis. Plant Physiol. 2000, 122, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Barthole, G.; Lepiniec, L.; Rogowsky, P.M.; Baud, S. Controlling lipid accumulation in cereal grains. Plant Sci. 2012, 185–186, 33–39. [Google Scholar] [CrossRef]
- Liu, Z.J. Cloning of Genes Involved in Seed Oil Content and Transgenic Research of Cotton. Ph.D. Thesis, China Agricultural University, Beijing, China, 2013. [Google Scholar]
- Yen, C.E.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. DGAT enzymes and triacylglyrol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Smith, S.J.; Zheng, Y.W. Identification of a gene encoding an acyl-Co A: Diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef]
- Hobbs, H.D.; Chaofu, L.; Hills, M. Cloning of a c DNA encoding acyltransferase from Arabidopsis thaliana and its functional expression. FEBS Lett. 1999, 452, 145–149. [Google Scholar] [CrossRef]
- Xu, J.; Francis, T.; Mietkiewska, E.; Giblin, E.M.; Barton, D.L.; Zhang, Y.; Zhang, M.; Taylor, D.C. Cloning and characterization of an acyl Co A-dependent diacylglycerol acyltransferase 1 (DGAT1) gene from Tropaeolummajus, and a study of the functional motifs of the DGAT protein using site-directed mutagenesis to modify enzyme activity and oil content. Plant Biotechnol. J. 2008, 6, 799–818. [Google Scholar] [CrossRef]
- He, X.H.; Grace, Q.C.; Lin, J.T.; McKeon, T.A. Regulation of diacylglycerol acyltransferase in developing seeds of castor. Lipids 2004, 39, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Allen, W.B.; Roesler, K.; Williams, M.E.; Zhang, S.; Li, J.; Glassman, K.; Ranch, J.; Nubel, D.; Solawetz, W.; et al. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat. Genet. 2008, 40, 367–372. [Google Scholar] [CrossRef]
- Bouvier-Navé, P.; Benveniste, P.; Oelkers, P.; Sturley, S.L.; Schaller, H. Expression in yeast and tobacco of plant c DNAs encoding acyl-CoA: Diacylglycerol acyltransferase. Eur. J. Biochem. 2000, 267, 85–96. [Google Scholar] [CrossRef]
- Nykiforuk, C.L.; Laroche, A.; Weselake, R.J. Isolation and characterization of a c DNA encoding a second putative diacylglycerol acyltransferase from a microspore-derived cell suspension culture of Brassica napus L. cv Jet Neuf. Plant Physiol. 1999, 121, 1957–1959. [Google Scholar]
- Salanoubat, M.; Lemcke, K.; Rieger, M.; Ansorge, W.; Unseld, M.; Fartmann, B.; Valle, G.; Blöcker, H.; Perez-Alonso, M.; Obermaier, B.; et al. Sequence and analysis of chromosome 3 of the plant Arabidopsis thaliana. Nature 2000, 408, 820–822. [Google Scholar]
- Lardizabal, K.D.; Mai, J.T.; Wagner, N.W.; Wyrick, A.; Voelker, T.; Hawkins, D.J. DGAT2 is a new diacylglycerol acyltransferase gene family: Purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J. Biol. Chem. 2001, 276, 38862–38869. [Google Scholar] [CrossRef] [PubMed]
- Kroon, J.T.; Wei, W.; Simon, W.J.; Slabas, A.R. Identification and functional expression of a type 2 acyl-Co A: Diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry 2006, 67, 2541–2549. [Google Scholar] [CrossRef]
- Shockey, J.M.; Gidda, S.K.; Chapital, D.C.; Kuan, J.-C.; Dhanoa, P.K.; Bland, J.M.; Rothstein, S.J.; Mullen, R.T.; Dyer, J.M. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 2006, 18, 2294–2313. [Google Scholar] [CrossRef]
- Saha, S.; Enugutti, B.; Rajakumari, S.; Rajasekharan, R. Cytosolic triacylglycerol biosynthetic pathway in oilseeds. Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol. 2006, 141, 1533–1543. [Google Scholar] [CrossRef]
- Hernandez, M.L.; Whitehead, L.; He, Z.; Gazda, V.; Gilday, A.; Kozhevnikova, E. A cytosolic acyltransferase contributes to triacylglycerol synthesis in sucrose-rescued Arabidopsis seed oil catabolism mutants. Plant Physiol. 2012, 160, 215–225. [Google Scholar] [CrossRef]
- Zheng, L.; Shockey, J.; Guo, F.; Shi, L.; Li, X.; Shan, L.; Wan, S.; Peng, Z. Discovery of a new mechanism for regulation of plant triacylglycerol metabolism: The peanut diacylglycerol acyltransferase-1 gene family transcriptome is highly enriched in alternative splicing variants. J. Plant Physiol. 2017, 219, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kamisaka, Y.; Mishra, S.; Nakahara, T. Purification and characterization of diacylglycerol acyltransferase from the lipid body fraction of an oleagious fungus. J. Biol. Chem. 1997, 121, 1107–1114. [Google Scholar]
- Lardizabal, K.; Effertz, R.; Levering, C.; Mai, J.; Pedroso, M.C.; Jury, T.; Aasen, E.; Gruys, K.; Bennett, K. Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol. 2008, 148, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Wang, J.J.; Zhang, G.Y. Two types of soybean diacylglycerol acyltransferases are differentially involved in triacylglycerol biosynthesis and response to environmental stresses and hormones. Sci. Rep. 2016, 6, 28541. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, F.; Liu, Y.; Wang, Y.; Gao, H.; Zhao, S.; Wang, Q.; Li, J. Quantitative proteomic and lipidomics analyses of high oil content GmDGAT1-2 transgenic soybean, illustrates the regulatory mechanism of lipoxygenase and oleosin. Plant Cell Rep. 2021, 40, 2303–2323. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Liu, B.; Bi, Y.; Hou, W.; Wu, C. Validation of Internal Control for Gene Expression Study in Soybean by Quantitative Real-Time PCR. BMC Mol. Biol. 2008, 9, 59. [Google Scholar]
- Hu, R.; Fan, C.; Li, H.; Zhang, Q. Evaluation of Putative Reference Genes for Gene Expression Normalization in Soybean by Quantitative Real-Time RT-PCR. BMC Mol. Biol. 2009, 10, 93. [Google Scholar] [CrossRef]
- Le, D.T.; Aldrich, D.L.; Valliyodan, B.; Watanabe, Y.; Ha, C.V.; Nishiyama, R.; Guttikonda, S.K.; Quach, T.N.; Gutierrez-Gonzalez, J.J.; Tran, L.S.P.; et al. Evaluation of Candidate Reference Genes for Normalization of Quantitative RT-PCR in Soybean Tissues under Various Abiotic Stress Conditions. Edite par Christian Schönbach. PLoS ONE 2012, 7, e46487. [Google Scholar] [CrossRef]
- Browse, J.; McCourt, P.J.; Somerville, C.R. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal. Biochem. 1986, 152, 141–145. [Google Scholar] [CrossRef]
- Broun, P.; Gettner, S.; Somerville, C. Genetic engineering of plant lipids. Annu. Rev. Nutr. 1999, 19, 197–216. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipidmetabolism. Arab. Book 2010, 8, e0133. [Google Scholar] [CrossRef]
- Sun, Q.X. Cloning and Characterization of Genes Involved in the Biosynthesis of Very Long Chain Polyunsaturated Fatty Acid and the Reconstitution of This Pathway in Crop Plants. Ph.D. Thesis, Shandong Agricultural University, Taian, China, 2011. [Google Scholar]
- Jako, C.; Kumar, A.; Wei, Y.D.; Zou, J.; Barton, D.L.; Giblin, E.M.; Covello, P.S.; Taylor, D.C. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol. 2001, 126, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Ohlrogge, J. Supply of triacylglycerol in developing embryos. Plant Physiol. 1999, 120, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.L.; Isleib, T.G. Genetic analysis of fatty acids in American peanuts. Chin. J. Oil Crops 2000, 22, 35–37. [Google Scholar]
- Zou, J.; Wei, Y.D.; Jako, C.; Kumar, A.; Selvaraj, G.; Taylor, D.C. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 1999, 19, 645–653. [Google Scholar] [CrossRef]
- Feussner, I.; Kuhn, H.; Wasternack, C. Lipoxygenase-dependent degradation of storage lipids. Trends Plant Sci. 2001, 6, 268–273. [Google Scholar] [CrossRef] [PubMed]


| Purpose | Gene Name (Amplification Length) | Accession Number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|---|
| RT-PCR | AhDGAT3 (1438 bp) | XM_016339125 | AATAGAAATAGAAATGTGATAATGG | ACAAATCAGGCTCTGGAAGTT |
| qRT-PCR | AhDGAT3 (140 bp) | XM_016339125 | AGAATGGAACCGCTATGT | CTCTGCCCTTACTTGCTC |
| β-Tubulin (185 bp) | GMU12286 | GGAAGGCTTTCTTGCATTGGTA | AGTGGCATCCTGGTACTGC | |
| Actin (155 bp) | J01298 | GTCCTTTCAGGAGGTACAACC | CCTTGAAGTATCCTATTGAGC | |
| Detection | Bar (220 bp) | GTCTGCACCATCGTCAACCACTACA | AGACGTACACGGTCGACTCGGCCGT | |
| AhDGAT3 (304 bp) | XM_016339125 | AGAATGGAACCGCTATGT | CTCTGCCCTTACTTGCTC |
| Material | JACK | OE-1 (ng/g) | OE-2 (ng/g) | OE-3 (ng/g) | OE-4 (ng/g) |
|---|---|---|---|---|---|
| Nodules | NA | 196.2 ± 10.2 | 220.6 ± 14.3 | 200.5 ± 13.8 | 233.9 ± 14.9 |
| Root | NA | 236.1 ± 19.2 | 389.5 ± 8.7 | 248.2 ± 1.5 | 415.5 ± 20.4 |
| Stem | NA | 203.7 ± 14.9 | 208.7 ± 6.9 | 200.6 ± 7.9 | 317.1 ± 11.7 |
| Leave | NA | 483.7 ± 16.7 | 523.7 ± 14.4 | 511.3 ± 10.2 | 655.1 ± 11.4 |
| Flower | NA | 288.3 ± 27.2 | 385.1 ± 17.7 | 348.6 ± 11.5 | 417.6 ± 10.5 |
| 10-day Pod | NA | 803.8 ± 14.8 | 841.7 ± 9.2 | 816.9 ± 3.8 | 869.5 ± 6.9 |
| 20-day Pod | NA | 676.1 ± 22.9 | 706.6 ± 10.5 | 796.1 ± 4.6 | 787.1 ± 27.1 |
| 30-day Pod | NA | 700.6 ± 15.9 | 804.1 ± 5.4 | 753.5 ± 32.3 | 883.7 ± 8.8 |
| 40-day Pod | NA | 913.2 ± 5 | 985.4 ± 3.8 | 951.4 ± 20.3 | 1014.4 ± 11.2 |
| Seed | NA | 949.3 ± 17.9 | 1096.9 ± 11.1 | 1004.6 ± 16.9 | 1166.1 ± 22.1 |
| Sample | Chromosome | Integration Sites | Integrated Way |
|---|---|---|---|
| R01 | 3 | 36,280,392 | Single copy |
| R02 | 10 | 19,795,197 | Single copy |
| R03 | 11 | 32,500,049 | Single copy |
| R04 | 14 | 8,451,776 | Single copy |
| Material | WT | OE-1 | OE-2 | OE-3 |
|---|---|---|---|---|
| Plant height (cm) | 101.4 ± 0.14 a | 114.1 ± 0.17 b | 109.2 ± 0.21 b | 105.1 ± 0.27 a |
| Effective branch number | 4 ± 0.21 a | 8 ± 0.16 b | 7 ± 0.28 b | 6 ± 0.12 b |
| Pod number per plant | 78 ± 0.14 a | 188 ± 0.11 b | 168 ± 0.18 b | 161 ± 0.27 b |
| Seed number per plant | 155 ± 0.16 a | 316 ± 0.29 b | 293 ± 0.19 b | 289 ± 0.31 b |
| 100-seed weight (g) | 13.5 ± 0.21 a | 16.3 ± 0.22 b | 15.6 ± 0.24 a | 16.9 ± 0.17 b |
| Seed weight per plant (g) | 25.7 ± 0.17 a | 57.2 ± 0.24 b | 49.7 ± 0.26 b | 45.7 ± 0.26 b |
| Main stem node number | 18 ± 0.25 a | 22 ± 0.26 b | 21 ± 0.21 b | 21 ± 0.24 b |
| Podding height (g) | 8.4 ± 0.29 a | 10.1 ± 0.31 a | 9.9 ± 0.31 a | 10.1 ± 0.27 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yan, F.; Liang, Z.; Wang, Y.; Li, J.; Zhao, L.; Yang, X.; Wang, Q.; Liu, J. Overexpression of the Peanut AhDGAT3 Gene Increases the Oil Content in Soybean. Agronomy 2023, 13, 2333. https://doi.org/10.3390/agronomy13092333
Xu Y, Yan F, Liang Z, Wang Y, Li J, Zhao L, Yang X, Wang Q, Liu J. Overexpression of the Peanut AhDGAT3 Gene Increases the Oil Content in Soybean. Agronomy. 2023; 13(9):2333. https://doi.org/10.3390/agronomy13092333
Chicago/Turabian StyleXu, Yang, Fan Yan, Zhengwei Liang, Ying Wang, Jingwen Li, Lei Zhao, Xuguang Yang, Qingyu Wang, and Jingya Liu. 2023. "Overexpression of the Peanut AhDGAT3 Gene Increases the Oil Content in Soybean" Agronomy 13, no. 9: 2333. https://doi.org/10.3390/agronomy13092333





